Effects of Dietary Baicalin on Growth Performance, Serum Biochemical Parameters, Liver Health, Intestinal Health, and Microbiota of Yellow Catfish (Pelteobagrus fulvidraco)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Experimental Fish and Feeding Management
2.3. Sample Collection
2.4. Measurement Indicators and Methods
2.4.1. Growth Performance and Body Morphometric Indices
2.4.2. Proximate Composition of Diets and Whole Body
2.4.3. Serum, Hepatic, and Intestinal Biochemical Parameters
2.4.4. Histological Examination
2.4.5. Intestinal Microbiota Analysis Based on 16S rRNA
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Morphometric Indices
3.2. Serum Biochemical Indices
3.3. Antioxidant Capacity
3.4. Whole-Body Proximate Composition
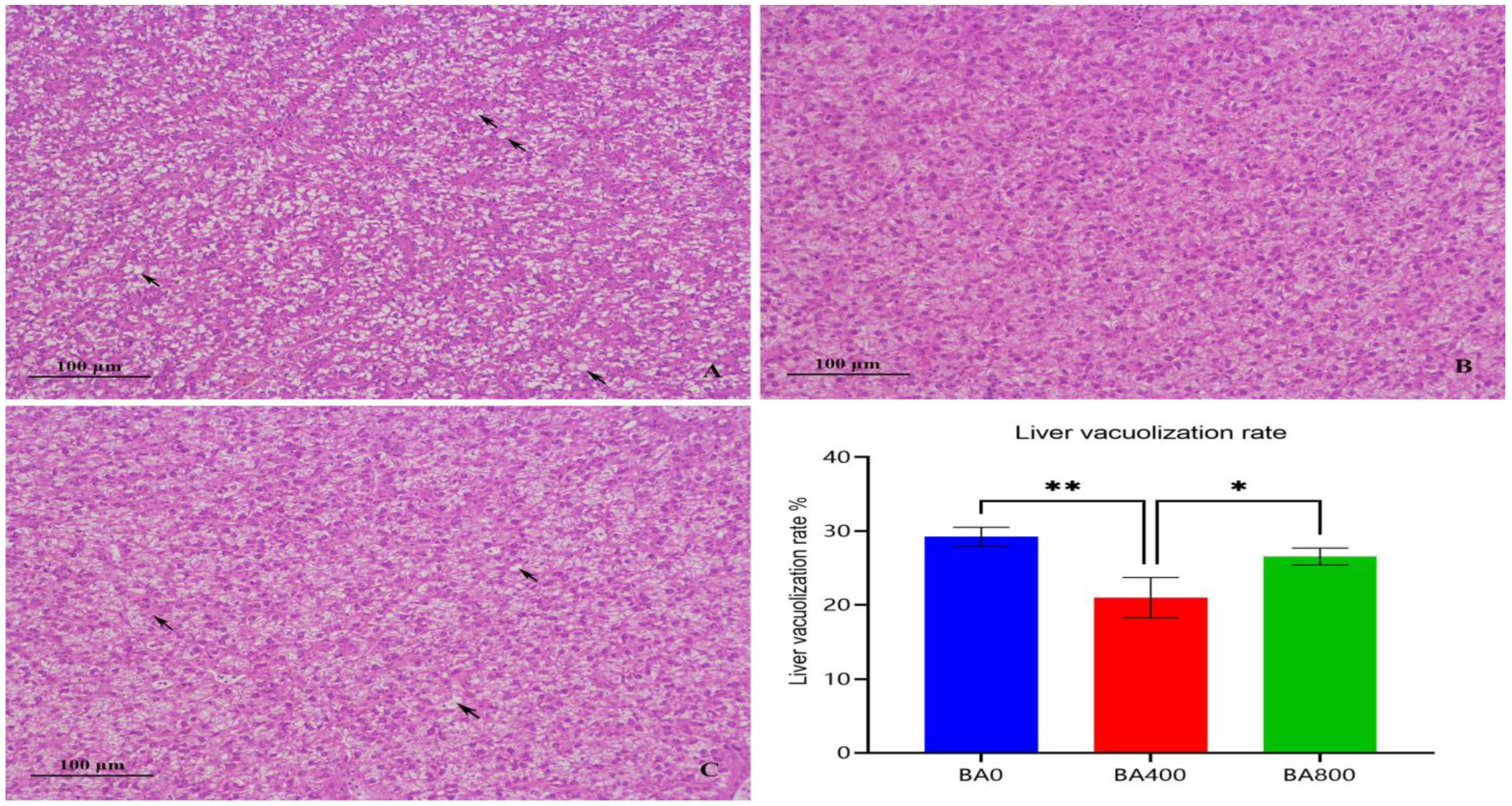
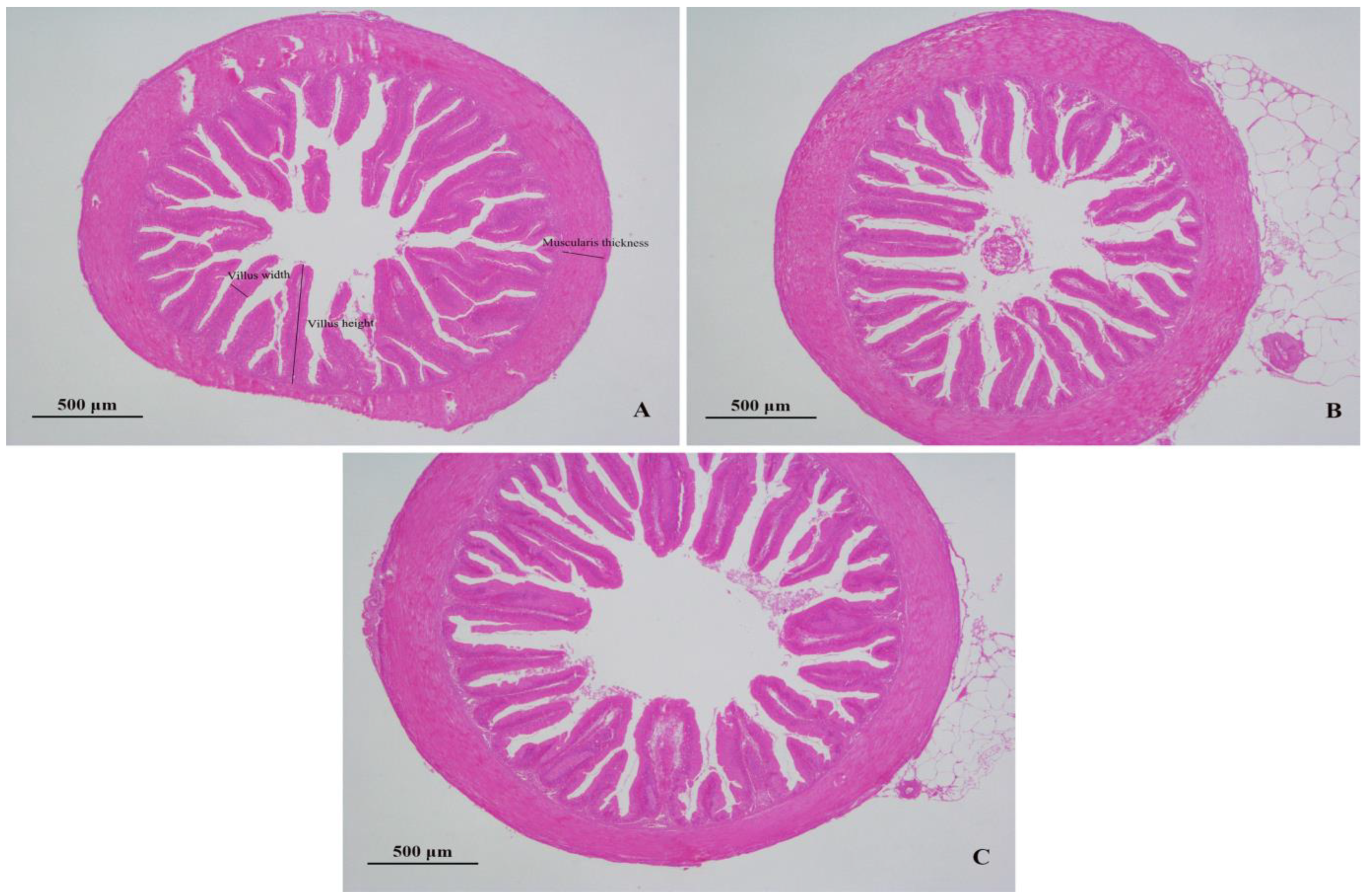
3.5. Liver and Intestinal Histomorphology
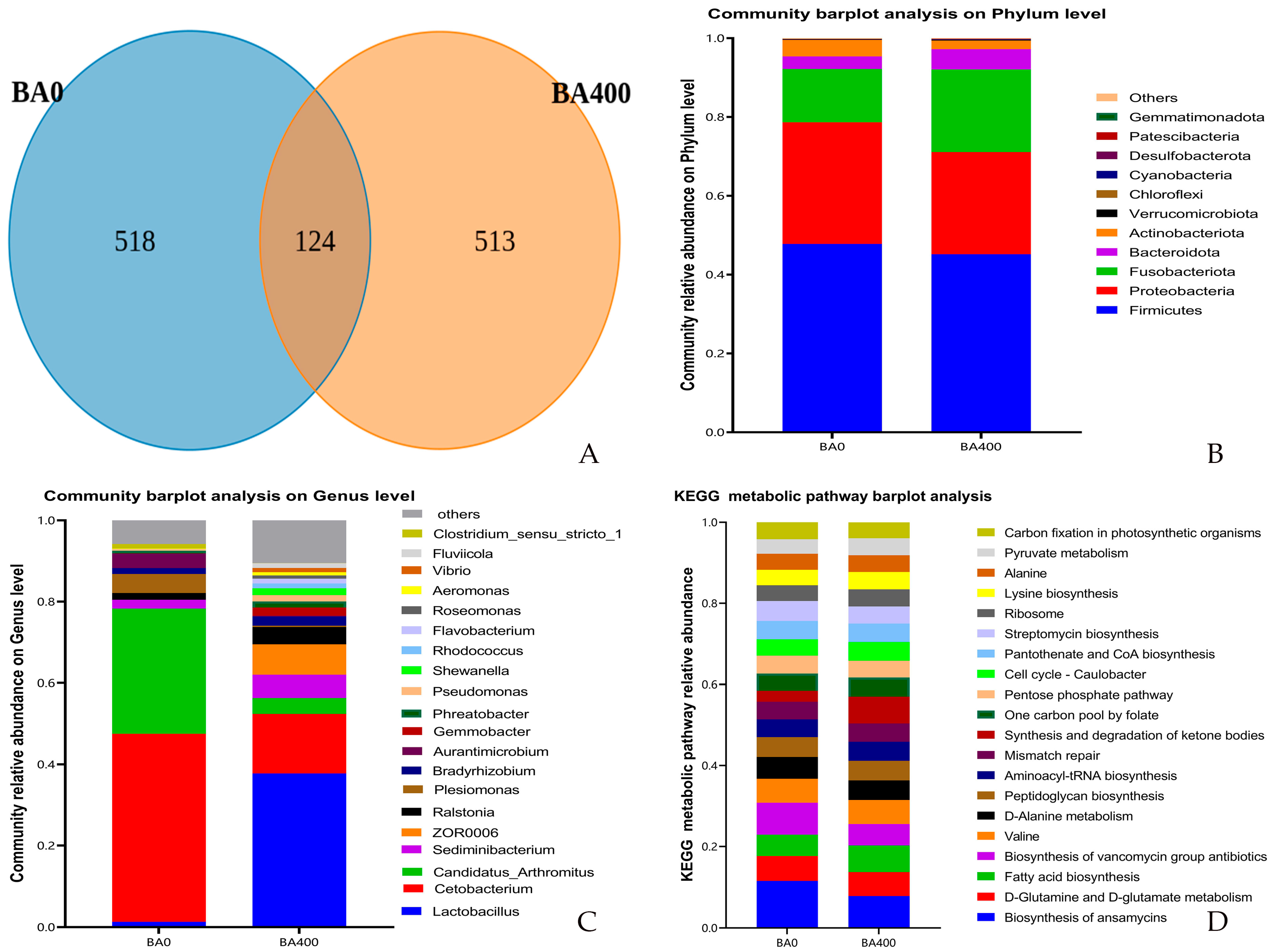
3.6. Intestinal Microbiota Analysis
4. Discussion
4.1. Growth Performance
4.2. Serum, Liver, and Intestinal Biochemical Indices
4.3. Liver and Intestinal Histology
4.4. Intestinal Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Hu, F.; Bi, Y.; Zheng, X.; Lu, M.; Diao, Q.; Tu, Y. Effect of baicalin supplementation on the growth, health, antioxidant and anti-inflammatory capacity, and immune function of preweaned calves. Anim. Feed. Sci. Technol. 2023, 298, 115598. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Y.; Miao, Z.; Lv, H.; Lv, Z.; Guo, Y.; Nie, W. Effects of baicalin and chlorogenic acid on growth performance, slaughter performance, antioxidant capacity, immune function and intestinal health of broilers. Poult. Sci. 2024, 103, 104251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Wang, F.; Yang, X.; Yao, F.; Ming, K.; Yuan, W.; Zeng, L.; Liu, J. Antiviral effect of baicalin phospholipid complex against duck hepatitis A virus type 1. Poult. Sci. 2018, 97, 2722–2732. [Google Scholar] [CrossRef]
- Zha, A.; Yuan, D.; Cui, Z.; Qi, M.; Liao, S.; Liao, P.; Tan, B. The evaluation of the antioxidant and intestinal protective effects of baicalin-copper in deoxynivalenol-challenged piglets. Oxidative Med. Cell. Longev. 2020, 2020, 5363546. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; Xu, P.; Yin, G. Effects of dietary baicalin supplementation on growth performance, antioxidative status and protection against oxidative stress-induced liver injury in GIFT tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108914. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xian, X.-R.; Guo, W.-L.; Zhong, Z.-H.; Wang, S.-F.; Cai, Y.; Sun, Y.; Chen, X.-f.; Wang, Y.-q.; Zhou, Y.-C. Baicalin attenuates Streptococcus agalactiae virulence and protects tilapia (Oreochromis niloticus) from group B streptococcal infection. Aquaculture 2020, 516, 734645. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, Y.F.; Shen, P.j.; Xu, Y.; Gao, Q.; Cheng, H.H.; Wang, W.D.; Gu, Z.m.; Chen, X.F. Baicalin protects giant freshwater prawn, Macrobrachium rosenbergii against Vibrio parahaemolyticus via modulation of the Toll signaling pathway. J. World Aquac. Soc. 2021, 52, 987–1000. [Google Scholar] [CrossRef]
- Yan, P.; Liu, J.; Huang, Y.; Yi, T.; Zhang, H.; Dai, G.; Wang, X.; Gao, Z.; He, B.; Guo, W. Baicalin enhances antioxidant, inflammatory defense, and microbial diversity of yellow catfish (Pelteobagrus fulvidraco) infected with Aeromonas hydrophila. Front. Microbiol. 2024, 15, 1465346. [Google Scholar] [CrossRef]
- Chen, G.-L. Effects of Baicalin Yeast Culture on Growth, Muscle Composition, Antioxidant Capacity, and Immune Performance in Giant Grouper (Epinephelus lanceolatus); Southwest University: Chongqing, China, 2020. [Google Scholar]
- Liu, F.; Shi, H.-Z.; Guo, Q.-S.; Yu, Y.-B.; Wang, A.-M.; Lv, F.; Shen, W.-B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish. Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, C.; Yang, L.; Wang, C.; Wang, B.; Wang, H.; Shu, Y.; Yan, Y. The growth-promoting and lipid-lowering effects of berberine are associated with the regulation of intestinal bacteria and bile acid profiles in yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2023, 33, 101848. [Google Scholar] [CrossRef]
- Yao, J.Y.; Xu, Y.; Sheng, P.C.; Yin, W.L.; Lin, L.Y.; Yuan, X.M.; Pan, X.Y.; Hao, G.J.; Shen, J.Y. Effects of three immunoenhancers on non-specific immune function in yellow catfish (Pelteobagrus fulvidraco). J. Anhui Agric. Sci. 2014, 42, 2921–2923. [Google Scholar]
- Wu, X.; Bai, D.Q.; Yang, G.; Ning, B.; Zhang, X.T.; Sun, L.Z. Effects of Ganoderma lucidum polysaccharides on immune cell activity in yellow catfish (Pelteobagrus fulvidraco). Acta Agric. Boreali-Sin. 2011, 26, 195–198. [Google Scholar]
- Xu, Z.; Yang, H.; Liang, G.Y.; Gao, B.W.; Li, X.Q.; Leng, X.J. Effects of baicalein on growth performance, serum antioxidant indices, and muscle quality of grass carp (Ctenopharyngodon idellus). J. Fish. China 2019, 43, 2383–2393. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Xia, Y.-T.; Wu, Q.-Y.; Cheng, E.H.-C.; Dong, T.T.-X.; Qin, Q.-W.; Wang, W.-X.; Tsim, K.W.-K. The inclusion of extract from aerial part of Scutellaria baicalensis in feeding of pearl gentian grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceo-latus♂) promotes growth and immunity. Fish Shellfish. Immunol. 2022, 127, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Leng, X.J.; You, Y.H.; Liu, J.W.; Chen, Y.F.; Li, X.Q. Effects of Scutellaria baicalensis on growth performance, serum and liver biochemical indices, and liver histology of channel catfish (Ictalurus punctatus). J. Dalian Ocean. Univ. 2024, 39, 739–745. [Google Scholar]
- Dičkancaitė, E.; Nemeikaitė, A.; Kalvelytė, A.; Čėnas, N. Prooxidant character of flavonoid cytotoxicity: Structure-activity relationships. IUBMB Life 1998, 45, 923–930. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free. Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Rader, B.A. Alkaline phosphatase, an unconventional immune protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef]
- Yan, J.; Luo, K.; Wang, B.; He, J.; Li, C.; Zhou, J.; Lai, W.; Zhou, X.; Ye, J.; Pan, M. Effects of dietary compound acidifier (Biomin) on the growth, anti-oxidation, immunity, and intestinal health of juvenile channel catfish (Ictalurus punctatus). Aquac. Rep. 2025, 40, 102577. [Google Scholar] [CrossRef]
- Chipman, D.M.; Sharon, N. Mechanism of Lysozyme Action: Lysozyme is the first enzyme for which the relation between structure and function has become clear. Science 1969, 165, 454–465. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Jiang, M.; Li, Z.; Zhu, G. Immunological regulatory effect of flavonoid baicalin on innate immune toll-like receptors. Pharmacol. Res. 2020, 158, 104890. [Google Scholar] [CrossRef]
- Peng-Fei, L.; Fu-Gen, H.; Bin-Bin, D.; Tian-Sheng, D.; Xiang-Lin, H.; Ming-Qin, Z. Purification and antioxidant activities of baicalin isolated from the root of huangqin (Scutellaria baicalensis gcorsi). J. Food Sci. Technol. 2013, 50, 615–619. [Google Scholar] [CrossRef]
- Xi, Y.; Wu, M.; Li, H.; Dong, S.; Luo, E.; Gu, M.; Shen, X.; Jiang, Y.; Liu, Y.; Liu, H. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: Dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell. Physiol. Biochem. 2015, 35, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Elshopakey, G.E.; Mahboub, H.H.; Sheraiba, N.I.; Abduljabbar, M.H.; Mahmoud, Y.K.; Abomughaid, M.M.; Ismail, A.K. Ammonia toxicity in Nile tilapia: Potential role of dietary baicalin on biochemical profile, antioxidant status and inflammatory gene expression. Aquac. Rep. 2023, 28, 101434. [Google Scholar] [CrossRef]
- Kim, K.T.; Jeon, G.H.; Cho, S.H.; Lim, S.G.; Kwon, M.G.; Yoo, J.H. Effects of dietary inclusion of various concentrations of Scutellaria baicalensis Georgi extract on growth, body composition, serum chemistry and challenge test of far eastern catfish (Silurus asotus). Aquac. Res. 2013, 44, 1502–1510. [Google Scholar] [CrossRef]
- Mosconi-Bac, N. Hepatic disturbances induced by an artificial feed in the sea bass (Dicentrarchus labrax) during the first year of life. Aquaculture 1987, 67, 93–99. [Google Scholar] [CrossRef]
- Wroblewski, F.; Ladue, J.S. Serum glutamic pyruvic transaminase in cardiac and hepatic disease. Proc. Soc. Exp. Biol. Med. 1956, 91, 569–571. [Google Scholar] [CrossRef]
- Guo, H.-x.; Liu, D.-h.; Ma, Y.; Liu, J.-f.; Wang, Y.; Du, Z.-Y.; Wang, X.; Shen, J.-K.; Peng, H.-L. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol. Sin. 2009, 30, 1505–1512. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, X.Q.; You, Y.H.; Cheng, Z.H.; Liu, H.N.; Leng, X.J. Effects of dietary sodium butyrate supplementation on growth performance, serum biochemical and antioxidant indices, and intestinal health of juvenile channel catfish (Ictalurus punctatus). Chin. J. Anim. Nutr. 2024, 36, 5895–5909. [Google Scholar]
- Wang, X.; Xie, L.; Long, J.; Liu, K.; Lu, J.; Liang, Y.; Cao, Y.; Dai, X.; Li, X. Therapeutic effect of baicalin on inflammatory bowel disease: A review. J. Ethnopharmacol. 2022, 283, 114749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Qu, P.; Huang-Fu, Y.-X.; Liu, D.; Wu, Y.; Liu, Y.; Chen, P.; Mai, K.; Zhang, W. Herbs mixture improves growth performance, intestine and liver histology, and immunity of juvenile large yellow croaker (Larimichthys crocea). Aquac. Rep. 2024, 36, 102136. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Simon, G.L.; Gorbach, S.L. Intestinal flora in health and disease. Gastroenterology 1984, 86, 174–193. [Google Scholar] [CrossRef]
- Chen, Y.X.; Wang, G.X.; Qiu, J.Q.; Liu, Y.; Shi, H.Q.; Dong, R.Q.; Peng, K. Curcumin alleviates the adverse effects of oxidized fish oil on the intestine of yellow catfish (Pelteobagrus fulvidraco). Chin. J. Anim. Nutr. 2024, 36, 5297–5307. [Google Scholar]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of Marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.; Eley, A. Fusobacteria: New taxonomy and related diseases. J. Med. Microbiol. 1993, 39, 246–254. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Zhu, H.; Qiang, J.; He, J.; Tao, Y.; Bao, J.; Xu, P. Physiological parameters and gut microbiome associated with different dietary lipid levels in hybrid yellow catfish (Tachysurus fulvidraco♀× Pseudobagrus vachellii♂). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100777. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Gatesoupe, F.-J. Lactic acid bacteria in fish: A review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Gallet, A.; Halary, S.; Duval, C.; Huet, H.; Duperron, S.; Marie, B. Disruption of fish gut microbiota composition and holobiont’s metabolome during a simulated Microcystis aeruginosa (Cyanobacteria) bloom. Microbiome 2023, 11, 108. [Google Scholar] [CrossRef]
- Xia, Y.-T.; Cheng, E.H.-C.; Wang, H.-Y.; Zhang, L.H.-L.; Lin, S.-Y.; Dong, T.T.-X.; Duan, R.; Qin, Q.-W.; Wang, W.-X.; Tsim, K.W.-K. The extract from aerial part of Scutellaria baicalensis regulates gut microbiota in rabbit fish: Replacement of antibiotic fighting against pathogenic bacteria. Aquaculture 2023, 565, 739140. [Google Scholar] [CrossRef]
- Du, Y.; Han, Y.; Zhang, R.; Zhang, Y.; Bao, S.; Cao, Y. Dietary baicalein improves growth performance, antioxidant activity, and intestinal flora of koi carp (Cyprinus carpio). Aquac. Rep. 2022, 27, 101421. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiong, X.; Danska, J.; Parkinson, J. Metatranscriptomic analysis of diverse microbial communities reveals core metabolic pathways and microbiome-specific functionality. Microbiome 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar]
- Ju, M.; Liu, Y.; Li, M.; Cheng, M.; Zhang, Y.; Deng, G.; Kang, X.; Liu, H. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur. J. Pharmacol. 2019, 857, 172457. [Google Scholar] [CrossRef]
- August, P.R.; Tang, L.; Yoon, Y.J.; Ning, S.; Müller, R.; Yu, T.-W.; Taylor, M.; Hoffmann, D.; Kim, C.-G.; Zhang, X. Biosynthesis of the ansamycin antibiotic rifamycin: Deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 1998, 5, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.S. Introduction to vancomycin. Rev. Infect. Dis. 1981, 3, S200–S204. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bajinka, O.; Jarju, P.O.; Tan, Y.; Taal, A.M.; Ozdemir, G. The varying effects of antibiotics on gut microbiota. AMB Express 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | BA0 | BA100 | BA200 | BA400 | BA800 |
|---|---|---|---|---|---|
| Fish meal | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Chicken meal | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Soybean meal | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Soybean protein concentrate | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Wheat flour | 22.96 | 22.95 | 22.94 | 22.92 | 22.88 |
| Corn gluten meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Cottonseed gluten meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soy lecithin | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| Baicalin | 0.00 | 0.01 | 0.02 | 0.04 | 0.08 |
| Ca(H2PO4) | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Vitamin and mineral premix 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin C phosphate | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition 2 | |||||
| Crude protein | 46.53 | 46.60 | 46.71 | 46.52 | 46.49 |
| Crude lipid | 8.95 | 8.98 | 8.97 | 8.97 | 8.97 |
| Crude ash | 8.63 | 8.98 | 8.52 | 8.90 | 8.69 |
| Moisture | 8.36 | 8.40 | 8.61 | 8.33 | 8.58 |
| Items | BA0 | BA100 | BA200 | BA400 | BA800 |
|---|---|---|---|---|---|
| Initial weight (g) | 11.23 ± 0.05 | 11.20 ± 0.04 | 11.20 ± 0.07 | 11.27 ± 0.08 | 11.09 ± 0.04 |
| Final weight (g) | 31.82 ± 0.25 a | 32.57 ± 0.80 a | 36.30 ± 1.14 bc | 39.10 ± 1.08 c | 33.23 ± 1.27 ab |
| Survival (%) | 95.56 ± 3.85 a | 100 ± 0.00 b | 100 ± 0.00 b | 100 ± 0.00 b | 100 ± 0.00 b |
| WG (%) | 183.28 ± 3.44 a | 190.77 ± 7.16 a | 228.79 ± 11.14 bc | 247.04 ± 9.62 c | 199.43 ± 12.74 ab |
| SGR (%) | 1.75 ± 0.05 a | 1.88 ± 0.06 a | 2.12 ± 0.06 bc | 2.22 ± 0.05 c | 1.96 ± 0.08 ab |
| FCR | 1.80 ± 0.02 a | 1.71 ± 0.07 a | 1.42 ± 0.06 b | 1.32 ± 0.06 b | 1.66 ± 0.09 a |
| CF (g/cm3) | 1.75 ± 0.28 | 1.84 ± 0.16 | 1.74 ± 0.15 | 1.82 ± 0.10 | 1.81 ± 0.29 |
| VSI (%) | 8.83 ± 0.82 | 7.87 ± 2.56 | 8.46 ± 1.18 | 7.93 ± 0.60 | 8.86 ± 1.44 |
| HSI (%) | 2.56 ± 0.33 | 2.52 ± 0.10 | 2.31 ± 0.32 | 2.36 ± 0.16 | 2.34 ± 0.29 |
| Items | BA0 | BA100 | BA200 | BA400 | BA800 |
|---|---|---|---|---|---|
| TCHO (mmol/L) | 8.11 ± 0.80 | 7.04 ± 0.04 | 7.68 ± 0.33 | 6.99 ± 0.52 | 7.95 ± 0.55 |
| TG (mmol/L) | 10.68 ± 1.11 ab | 9.11 ± 0.41 c | 9.41 ± 0.79 bc | 9.19 ± 0.79 c | 10.87 ± 0.84 a |
| ALP (U/L) | 67.32 ± 3.46 a | 66.51 ± 6.25 a | 73.94 ± 6.64 a | 90.43 ± 8.73 b | 74.06 ± 5.66 a |
| ACP (U/L) | 286.03 ± 17.10 a | 296.00 ± 18.12 ab | 299.24 ± 17.66 ab | 314.12 ± 15.11 b | 304.47 ± 17.51 ab |
| ALT (U/L) | 4.39 ± 0.32 | 4.67 ± 0.19 | 4.41 ± 0.16 | 4.55 ± 0.27 | 4.46 ± 0.14 |
| AST (U/L) | 140.38 ± 11.42 a | 101.43 ± 14.04 bc | 81.32 ± 1.67 c | 87.63 ± 1.67 c | 110.00 ± 12.38 b |
| TP (gprot/L) | 31.61 ± 1.10 | 29.82 ± 2.02 | 30.58 ± 1.58 | 30.32 ± 2.52 | 29.42 ± 1.92 |
| LZM (ug/mL) | 1.18 ± 0.19 a | 1.39 ± 0.17 a | 1.79 ± 0.19 b | 2.36 ± 0.23 c | 1.51 ± 0.26 ab |
| Items | BA0 | BA100 | BA200 | BA400 | BA800 |
|---|---|---|---|---|---|
| Liver | |||||
| T-AOC (mmol/mg prot) | 0.16 ± 0.05 a | 0.23 ± 0.04 bc | 0.26 ± 0.04 cd | 0.31 ± 0.05 d | 0.19 ± 0.04 ab |
| MDA (nmol/mgprot) | 4.44 ± 0.58 a | 2.96 ± 0.76 b | 2.50 ± 0.36 b | 2.35 ± 0.58 b | 2.91 ± 0.41 b |
| CAT (U/mgprot) | 8.70 ± 1.91 a | 11.93 ± 2.26 ab | 13.00 ± 1.90 bc | 15.71 ± 2.31 c | 11.56 ± 2.26 ab |
| SOD (U/mgprot) | 71.81 ± 10.11 a | 94.69 ± 10.01 b | 109.58 ± 4.38 c | 140.86 ± 5.33 d | 109.74 ± 6.08 c |
| Intestine | |||||
| T-AOC (μmol/mg prot) | 0.017 ± 0.004 a | 0.022 ± 0.003 ab | 0.026 ± 0.004 b | 0.025 ± 0.004 b | 0.019 ± 0.003 ab |
| MDA (nmol/mgprot) | 1.50 ± 0.26 a | 0.76 ± 0.20 b | 0.72 ± 0.14 b | 0.79 ± 0.19 b | 1.17 ± 0.28 ab |
| CAT (U/mgprot) | 18.08 ± 1.58 a | 25.55 ± 5.08 ab | 23.71 ± 4.98 ab | 31.21 ± 3.76 b | 20.84 ± 3.36 a |
| LZM (ug/mgprot) | 0.06 ± 0.01 | 0.064 ± 0.01 | 0.060 ± 0.01 | 0.079 ± 0.009 | 0.070 ± 0.01 |
| SOD (U/mgprot) | 6.99 ± 2.38 a | 8.12 ± 2.39 a | 14.43 ± 2.30 b | 16.56 ± 1.25 b | 9.84 ± 2.93 a |
| Items | BA0 | BA100 | BA200 | BA400 | BA800 |
|---|---|---|---|---|---|
| Moisture | 72.38 ± 0.40 | 72.12 ± 1.08 | 72.25 ± 0.70 | 72.00 ± 1.09 | 71.65 ± 0.78 |
| Crude ash | 4.06 ± 0.53 | 4.00 ± 0.23 | 4.08 ± 0.21 | 3.95 ± 0.05 | 4.07 ± 0.20 |
| Crude lipid | 6.48 ± 0.46 | 6.61 ± 0.46 | 6.51 ± 0.36 | 6.46 ± 0.59 | 6.37 ± 0.45 |
| Crude protein | 15.33 ± 0.64 | 15.64 ± 0.68 | 15.33 ± 0.73 | 15.60 ± 0.67 | 15.89 ± 0.26 |
| Items (μm) | BA0 | BA400 | BA800 |
|---|---|---|---|
| Villus height | 570.4 ± 20.13 a | 670.9 ± 51.51 b | 561.2 ± 31.65 a |
| Villus width | 105.6 ± 5.44 a | 138.4 ± 19.65 b | 120.8 ± 14.78 ab |
| Muscularis thickness | 167.5 ± 26.05 a | 222.6 ± 15.29 b | 197.1 ± 23.30 ab |
| Items | BA0 | BA400 |
|---|---|---|
| Shannon | 3.01 ± 0.12 a | 4.69 ± 0.007 b |
| Simpson | 0.64 ± 0.03 a | 0.89 ± 0.006 b |
| Chao1 | 265.53 ± 12.58 | 299.22 ± 26.85 |
| ACE | 266.25 ± 12.24 | 299.70 ± 26.89 |
| Coverage | 0.999 ± 0.00 | 0.999 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, X.; Fan, Y.; Xiao, Y.; Chen, Y.; Li, X.; Leng, X. Effects of Dietary Baicalin on Growth Performance, Serum Biochemical Parameters, Liver Health, Intestinal Health, and Microbiota of Yellow Catfish (Pelteobagrus fulvidraco). Animals 2025, 15, 2903. https://doi.org/10.3390/ani15192903
Liu H, Li X, Fan Y, Xiao Y, Chen Y, Li X, Leng X. Effects of Dietary Baicalin on Growth Performance, Serum Biochemical Parameters, Liver Health, Intestinal Health, and Microbiota of Yellow Catfish (Pelteobagrus fulvidraco). Animals. 2025; 15(19):2903. https://doi.org/10.3390/ani15192903
Chicago/Turabian StyleLiu, Haonan, Xinru Li, Yang Fan, Yang Xiao, Yunfeng Chen, Xiaoqin Li, and Xiangjun Leng. 2025. "Effects of Dietary Baicalin on Growth Performance, Serum Biochemical Parameters, Liver Health, Intestinal Health, and Microbiota of Yellow Catfish (Pelteobagrus fulvidraco)" Animals 15, no. 19: 2903. https://doi.org/10.3390/ani15192903
APA StyleLiu, H., Li, X., Fan, Y., Xiao, Y., Chen, Y., Li, X., & Leng, X. (2025). Effects of Dietary Baicalin on Growth Performance, Serum Biochemical Parameters, Liver Health, Intestinal Health, and Microbiota of Yellow Catfish (Pelteobagrus fulvidraco). Animals, 15(19), 2903. https://doi.org/10.3390/ani15192903






