Influence of Different Warming Methods in Rabbits Subjected to Prolonged Pneumoperitoneum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redondo, J.I.; Suesta, P.; Serra, I.; Soler, C.; Soler, G.; Gil, L.; Gomez-Villamandos, R.J. Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Vet. Rec. 2012, 171, 374. [Google Scholar] [CrossRef]
- Davidson, E.B.; Moll, H.D.; Payton, M.E. Comparison of laparoscopic ovariohysterectomy and ovariohysterectomy in dogs. Vet. Surg. 2004, 33, 62–69. [Google Scholar] [CrossRef]
- Wittenborn, J.; Clausen, A.; Zeppernick, F.; Stickeler, E.; Meinhold-Heerlein, I. Prevention of Intraoperative Hypothermia in Laparoscopy by the Use of Body-Temperature and Humidified CO2: A Pilot Study. Geburtshilfe Frauenheilkd. 2019, 79, 969–975. [Google Scholar] [CrossRef]
- Farrell, M.; Singh, A.; Mayhew, P.D.; Lillo-Araya, F.; Massari, F.; Richardson, D.; Collier, A.J. Bilateral, single-session, laparoscopic adrenalectomy was associated with favorable outcomes in a cohort of dogs. J. Am. Vet. Med. Assoc. 2023, 261, 1–5. [Google Scholar] [CrossRef] [PubMed]
- van Bokhorst, K.L.; Galac, S.; Kooistra, H.S.; de Grauw, J.C.; Teske, E.; Grinwis, G.C.M.; van Nimwegen, S.A. Laparoscopic vs. open adrenalectomy: Perioperative data and survival analysis in 70 dogs with an adrenal tumor. Front. Vet. Sci. 2023, 10, 1156801. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Mayhew, P.D.; Culp, W.T.N.; Brad Case, J.; Singh, A.; Fuller, M.C.; Della Maggiore, A. Outcome of laparoscopic adrenalectomy for resection of unilateral noninvasive adrenocortical tumors in 11 cats. Vet. Surg. 2017, 46, 714–721. [Google Scholar] [CrossRef]
- Smith, R.R.; Mayhew, P.D.; Berent, A.C. Laparoscopic adrenalectomy for management of a functional adrenal tumor in a cat. J. Am. Vet. Med. Assoc. 2012, 241, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.D. Advanced laparoscopic procedures (hepatobiliary, endocrine) in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Shariati, E.; Bakhtiari, J.; Khalaj, A.; Molazem, M.; Shariati, E.; Niasari-Naslaji, A. Clinical and paraclinical evaluation of partial nephrectomy using laparoscopy and open surgery in dogs: New suturing technique. Iran. J. Vet. Res. 2017, 18, 1–5. [Google Scholar]
- Rohrer, M.J.; Natale, A.M. Effect of hypothermia on the coagulation cascade. Crit. Care Med. 1992, 20, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, J.; Xing, H.; Luo, Y.; Song, Y.; Li, Y.; Li, B.; Lu, F.; Dong, Z. Reflective Blankets vs. Electric Warming in Preventing Hypothermia During Liposuction: A Randomized Controlled Trial. Aesthetic Surg. J. 2025, 45, 914–921. [Google Scholar] [CrossRef]
- Hoyos, A.E.; Perez Pachon, M.E.; Benavides, J.E.; Eljaiek, A.; Borras Osorio, M.; Ramirez, B. Effects of Optimal Temperature Control in Body Contouring Surgery: A Nonrandomized Controlled Clinical Trial. Aesthetic Surg. J. 2024, 44, NP790–NP797. [Google Scholar] [CrossRef]
- Huang, J.; Miao, Y.; Shen, X.; Hou, C.; Zhang, L.; Zhang, Z. Risk factors for intraoperative hypothermia in patients receiving lung transplants. J. Thorac. Dis. 2024, 16, 7607–7616. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, Y.; Liang, Y.; Xia, T.; Li, L.; Xiong, W.; Liu, B.; Feng, Y.; Liu, Y.; Jin, X.; et al. Perioperative active warming strategies in children: A protocol for a multicentre, prospective, randomized controlled trial. Front. Pediatr. 2023, 11, 1155666. [Google Scholar] [CrossRef]
- Russel, W.A.; Jimenez, A.G.; Paul, K.D.; Hoopes, B.C.; Ay, A. Body Temperature Regulation in Domestic Dogs After Agility Trials: The Effects of Season, Training, Body Characteristics, Age, and Genetics. J. Exp. Zool. A Ecol. Integr. Physiol. 2025, 343, 400–415. [Google Scholar] [CrossRef]
- Kudo, A.; Oboso, R.; Oshita, R.; Yamauchi, A.; Kamo, S.; Yoshida, H.; Kanai, E.; Takagi, S. Peripheral warming for prevention of hypothermia in small dogs during soft tissue surgery: A randomized controlled trial. Vet. Anaesth. Analg. 2024, 51, 658–666. [Google Scholar] [CrossRef]
- Meng-Meng, T.; Xue-Jun, X.; Xiao-Hong, B. Clinical effects of warmed humidified carbon dioxide insufflation in infants undergoing major laparoscopic surgery. Medicine 2019, 98, e16151. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Singh, A.; Valverde, A.; Blois, S.L.; Foster, R.A.; Kilkenny, J.J.; Linden, A.Z. Effect of pneumoperitoneum with warmed humidified or standard-temperature carbon dioxide during laparoscopy on core body temperature, cardiorespiratory and thromboelastography variables, systemic inflammation, peritoneal response, and signs of postoperative pain in healthy mature dogs. Am. J. Vet. Res. 2018, 79, 1321–1334. [Google Scholar]
- Ferreira, R.F.; Damasceno-Ferreira, J.A.; da Silva, P.; Abilio, E.J.; Benchimol de Souza, D. Effect of heated pneumoperitoneum on body temperature in dogs undergoing laparoscopic ovariectomy-A randomized controlled trial. Vet. Surg. 2025, 54, 945–951. [Google Scholar] [CrossRef]
- Meenakshi-Sundaram, B.; Furr, J.R.; Malm-Buatsi, E.; Boklage, B.; Nguyen, E.; Frimberger, D.; Palmer, B.W. Reduction in surgical fog with a warm humidified gas management protocol significantly shortens procedure time in pediatric robot-assisted laparoscopic procedures. J. Pediatr. Urol. 2017, 13, 489.e481–489.e485. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lin, C.Y.; Aljaberi, M.A.; Lee, C.H.; Chou, Y.H.; Griffiths, M.D. Effects of Forced-Air Warming Blanket on Anxiety Reduction and Thermal Comfort Improvement With Physical Indicators for Patients Undergoing Abdominal Surgery: A Quasi-experimental Study. J. PeriAnesthesia Nurs. 2025, 40, 1300–1306. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, L.; Wang, X.; Song, F.; Hou, H.; Qiu, Y. Effect of Forced-Air Warming Blanket on Perioperative Hypothermia in Elderly Patients Undergoing Laparoscopic Radical Resection of Colorectal Cancer. Ther. Hypothermia Temp. Manag. 2022, 12, 68–73. [Google Scholar] [CrossRef]
- Isaka, M.; Hayashida, R.; Tamashima, Y.; Araki, R. Surgical ventricular restoration for rabbit dilated cardiomyopathy model: Preliminary study. Res. Vet. Sci. 2021, 136, 373–376. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.Y.; Yoon, H.Y. A rabbit model of tracheal collapse for optimal self-expanding metal stents. J. Vet. Med. Sci. 2023, 85, 386–392. [Google Scholar] [CrossRef]
- Calasans-Maia, M.D.; Monteiro, M.L.; Ascoli, F.O.; Granjeiro, J.M. The rabbit as an animal model for experimental surgery. Acta Cir. Bras. 2009, 24, 325–328. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Machon, R.G.; Raffe, M.R.; Robinson, E.P. Warming with a forced air warming blanket minimizes anesthetic-induced hypothermia in cats. Vet. Surg. 1999, 28, 301–310. [Google Scholar] [CrossRef]
- Rastas, J.P.; Zhao, Q.; Johnson, R.A. Comparison of two active warming techniques on body temperature in healthy, anesthetized dogs premedicated with acepromazine or dexmedetomidine: A pilot study. PLoS ONE 2025, 20, e0317997. [Google Scholar] [CrossRef]
- Waterman, A. Accidental hypothermia during anaesthesia in dogs and cats. Vet. Rec. 1975, 96, 308–313. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative thermoregulation and heat balance. Lancet 2016, 387, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; McGuire, J.; Sessler, A.M. Perioperative thermal insulation. Anesthesiology 1991, 74, 875–879. [Google Scholar] [CrossRef]
- Lennon, R.L.; Hosking, M.P.; Conover, M.A.; Perkins, W.J. Evaluation of a forced-air system for warming hypothermic postoperative patients. Anesth. Analg. 1990, 70, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Moayeri, A. Skin-surface warming: Heat flux and central temperature. Anesthesiology 1990, 73, 218–224. [Google Scholar] [CrossRef]
- Yi, J.; Lei, Y.; Xu, S.; Si, Y.; Li, S.; Xia, Z.; Shi, Y.; Gu, X.; Yu, J.; Xu, G.; et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS ONE 2017, 12, e0177221. [Google Scholar] [CrossRef]
- Redondo, J.I.; Suesta, P.; Gil, L.; Soler, G.; Serra, I.; Soler, C. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet. Rec. 2012, 170, 206. [Google Scholar] [CrossRef]
- Corriveau, K.M.; Giuffrida, M.A.; Mayhew, P.D.; Runge, J.J. Outcome of laparoscopic ovariectomy and laparoscopic-assisted ovariohysterectomy in dogs: 278 cases (2003–2013). J. Am. Vet. Med. Assoc. 2017, 251, 443–450. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Brown, D.C. Comparison of three techniques for ovarian pedicle hemostasis during laparoscopic-assisted ovariohysterectomy. Vet. Surg. 2007, 36, 541–547. [Google Scholar] [CrossRef]
- Noll, E.; Diemunsch, S.; Pottecher, J.; Rameaux, J.P.; Diana, M.; Sauleau, E.; Ruetzler, K.; Diemunsch, P. Prevention of laparoscopic surgery induced hypothermia with warmed humidified insufflation: Is the experimental combination of a warming blanket synergistic? PLoS ONE 2018, 13, e0199369. [Google Scholar] [CrossRef] [PubMed]


| CT | HP | FA | HP + FA | p Value | |
|---|---|---|---|---|---|
| Body weight (Kg) | 3.74 ± 0.76 | 3.88 ± 0.68 | 3.90 ± 0.79 | 3.77 ± 0.64 | 0.9524 |
| Rectal temperature—I0 (°C) | 39.26 ± 0.30 | 39.44 ± 0.36 | 39.42 ± 0.36 | 39.36 ± 0.43 | 0.7270 |
| Rectal temperature—I1 (°C) | 37.17 ± 1.34 | 37.97 ± 0.27 | 37.72 ± 0.64 | 37.53 ± 0.78 | 0.2609 |
| Δt-r (I0–I1) (°C) | 2.09 ± 1.19 | 1.48 ± 0.42 | 1.70 ± 0.54 | 1.82 ± 0.64 | 0.3923 |
| Esophageal temperature—I1 (°C) | 37.08 ± 1.22 | 37.91 ± 0.36 | 37.62 ± 0.61 | 37.56 ± 0.78 | 0.1991 |
| CO2 volume (L) | 12.9 ± 6.43 | 10.7 ± 18.6 | 28.3 ± 39.9 | 3.61 ± 1.02 | 0.1606 |
| Initial Temperature (I0 or I1) | Final Temperature (I9 or I10) | p Value | |
|---|---|---|---|
| Group CT | |||
| I0 vs. I10 (°C) | 39.26 ± 0.30 | 32.96 ± 1.61 | <0.0001 |
| I0 vs. I9 (°C) | 39.26 ± 0.30 | 33.39 ± 1.39 | <0.0001 |
| I1 vs. I10 (°C) | 37.17 ± 1.34 | 32.96 ± 1.61 | <0.0001 |
| I1 vs. I9 (°C) | 37.17 ± 1.34 | 33.39 ± 1.39 | <0.0001 |
| Group HP | |||
| I0 vs. I10 (°C) | 39.44 ± 0.36 | 34.28 ± 0.57 | <0.0001 |
| I0 vs. I9 (°C) | 39.44 ± 0.36 | 34.51 ± 0.53 | <0.0001 |
| I1 vs. I10 (°C) | 37.97 ± 0.27 | 34.28 ± 0.57 | <0.0001 |
| I1 vs. I9 (°C) | 37.97 ± 0.27 | 34.51 ± 0.53 | <0.0001 |
| Group FA | |||
| I0 vs. I10 (°C) | 39.42 ± 0.36 | 36.89 ± 0.62 | <0.0001 |
| I0 vs. I9 (°C) | 39.42 ± 0.36 | 36.85 ± 0.56 | <0.0001 |
| I1 vs. I10 (°C) | 37.72 ± 0.64 | 36.89 ± 0.62 | 0.0126 |
| I1 vs. I9 (°C) | 37.72 ± 0.64 | 36.85 ± 0.56 | 0.0070 |
| Group HP + FA | |||
| I0 vs. I10 (°C) | 39.36 ± 0.43 | 36.71 ± 1.27 | <0.0001 |
| I0 vs. I9 (°C) | 39.36 ± 0.43 | 36.68 ± 1.29 | <0.0001 |
| I1 vs. I10 (°C) | 37.53 ± 0.78 | 36.71 ± 1.27 | 0.0025 |
| I1 vs. I9 (°C) | 37.53 ± 0.78 | 36.68 ± 1.29 | 0.0022 |
| CT | HP | FA | HP + FA | p Value | |
|---|---|---|---|---|---|
| Rectal temperature—I9 (°C) | 33.39 ± 1.39 | 34.51 ± 0.53 | 36.85 ± 0.56 a,b | 36.68 ± 1.29 a,b | <0.0001 |
| Rectal temperature—I10 (°C) | 32.96 ± 1.61 | 34.28 ± 0.57 | 36.89 ± 0.62 a,b | 36.71 ± 1.27 a,b | <0.0001 |
| Esophageal temperature—I9 (°C) | 33.48 ± 1.22 | 34.60 ± 0.47 | 36.96 ± 0.78 a,b | 36.88 ± 1.25 a,b | <0.0001 |
| Δt-r (I1–I9) (°C) | 3.78 ± 0.89 | 3.45 ± 0.48 | 0.87 ± 0.90 a,b | 0.85 ± 0.65 a,b | <0.0001 |
| Δt-r (I1–I10) (°C) | 4.21 ± 1.06 | 3.69 ± 0.50 | 0.83 ± 0.98 a,b | 0.82 ± 0.64 a,b | <0.0001 |
| Δt-e (I1–I9) (°C) | 3.60 ± 0.95 | 3.31 ± 0.26 | 0.66 ± 0.94 a,b | 0.67 ± 0.65 a,b | <0.0001 |
| Δt-r (I9–I10) (°C) | 0.43 ± 0.33 | 0.23 ± 0.18 | −0.04 ± 0.10 a,b | −0.03 ± 0.10 a,b | <0.0001 |
| CT | HP | FA | HP + FA | |||||
|---|---|---|---|---|---|---|---|---|
| Pearsons’s Correlation | R2 | p Value | R2 | p Value | R2 | p Value | R2 | p Value |
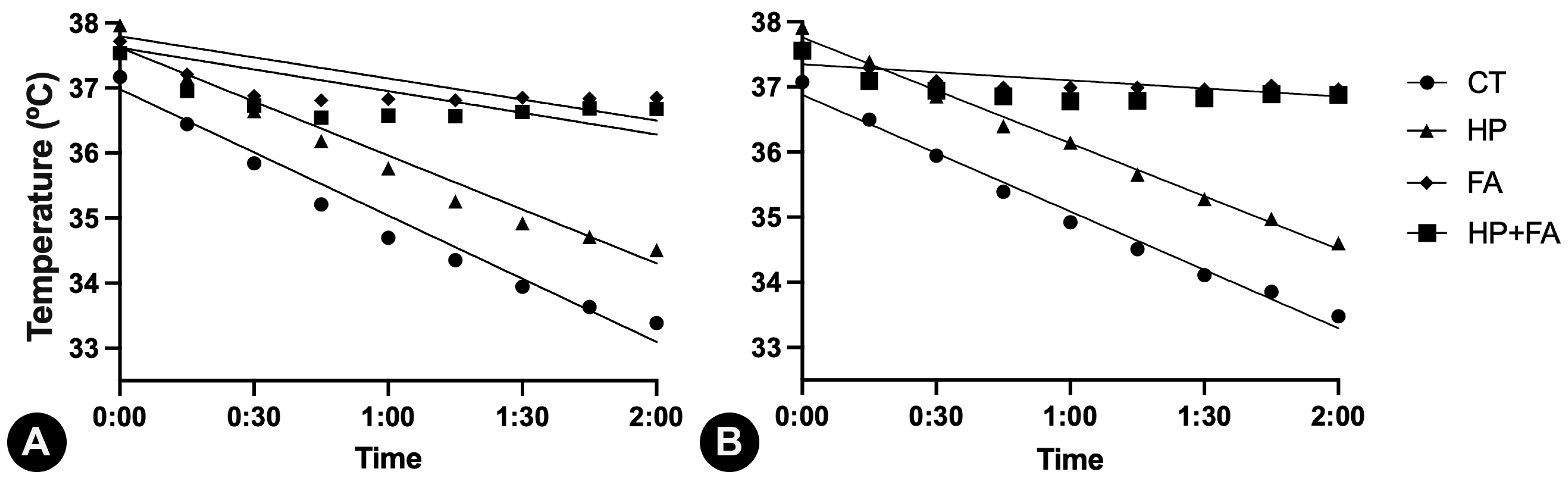
| Time (min) × Rectal temperature (°C) | 0.9818 | <0.0001 | 0.9782 | <0.0001 | 0.6249 | 0.0019 | 0.5966 | 0.0027 |
| Time (min) × Esophageal temperature (°C) | 0.9866 | <0.0001 | 0.9928 | <0.0001 | 0.5905 | 0.0078 | 0.4598 | 0.0223 |
| Linear regression | ||||||||
| Time (min) × Rectal temperature (°C) | 0.6729 | <0.0001 | 0.9013 | <0.0001 | 0.4046 | <0.0001 | 0.2301 | <0.0001 |
| Equation | RT = −0.03237 × t + 36.98 | RT = −0.02766 × t + 37.62 | RT = −0.01079 × t + 37.80 | RT = −0.0111 × X + 37.62 | ||||
| Time (min) × Esophageal temperature (°C) | 0.4938 | <0.0001 | 0.8220 | <0.0001 | 0.0584 | 0.0217 | 0.0231 | 0.1757 |
| Equation | ET = −0.02986 × t + 36.88 | ET = −0.02709 × t + 37.76 | ET = −0.00412 × t + 37.35 | * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curopos, R.N.A.; Damasceno-Ferreira, J.A.; Sampaio, F.J.B.; Benchimol de Souza, D. Influence of Different Warming Methods in Rabbits Subjected to Prolonged Pneumoperitoneum. Animals 2025, 15, 2891. https://doi.org/10.3390/ani15192891
Curopos RNA, Damasceno-Ferreira JA, Sampaio FJB, Benchimol de Souza D. Influence of Different Warming Methods in Rabbits Subjected to Prolonged Pneumoperitoneum. Animals. 2025; 15(19):2891. https://doi.org/10.3390/ani15192891
Chicago/Turabian StyleCuropos, Rodrigo N. A., José A. Damasceno-Ferreira, Francisco J. B. Sampaio, and Diogo Benchimol de Souza. 2025. "Influence of Different Warming Methods in Rabbits Subjected to Prolonged Pneumoperitoneum" Animals 15, no. 19: 2891. https://doi.org/10.3390/ani15192891
APA StyleCuropos, R. N. A., Damasceno-Ferreira, J. A., Sampaio, F. J. B., & Benchimol de Souza, D. (2025). Influence of Different Warming Methods in Rabbits Subjected to Prolonged Pneumoperitoneum. Animals, 15(19), 2891. https://doi.org/10.3390/ani15192891






