Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin Improves Growth and Survival in Suckling Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Medium-Chain Triglyceride Emulsion Formulations
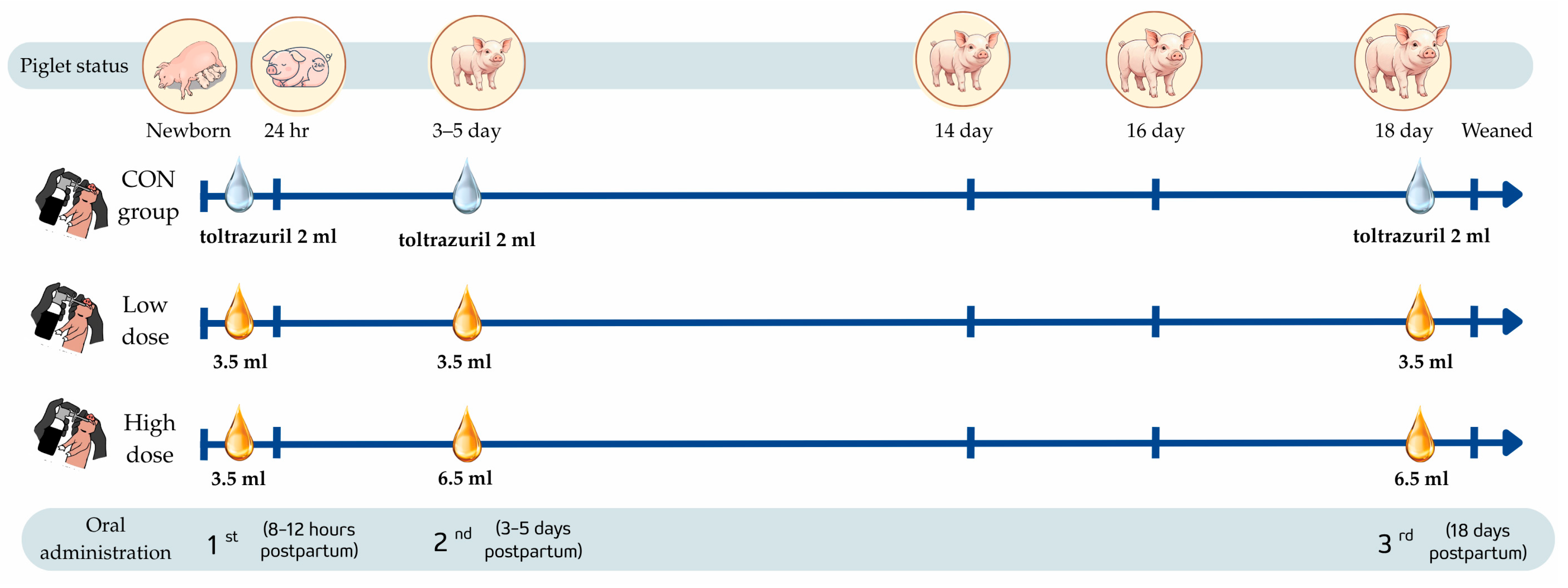
2.2. Animals and Experimental Design
2.3. Productivity and Health Measurements
- (1)
- Piglet performance—litter size at birth, number of live-born piglets, and litter size at 5, 14, and 18 days postpartum.
- (2)
- (3)
- Health outcomes—pre-weaning mortality rate, recorded from birth to 24 h and throughout the suckling period, with incidence and causes of death documented.
- (4)
2.4. Hematological and Biochemical Assessments
2.5. Mortality and Welfare-Related Assessments
2.5.1. Mortality Classification
- (1)
- Milk starvation—characterized by emaciation and milk deprivation, evidenced by frothing at the mouth and severe wasting.
- (2)
- Weak state—referring to piglets that died due to poor vigor and chronic weakness, typically manifested as small body size, visible skeletal structures, or ongoing disease.
- (3)
- Crushing—diagnosed when piglets were found flattened beneath the sow with distinctive purplish swelling at compression sites.
- (4)
- Diarrhea—identified by persistent enteric distress, including yellow staining around the anus, fecal contamination, and malodor.
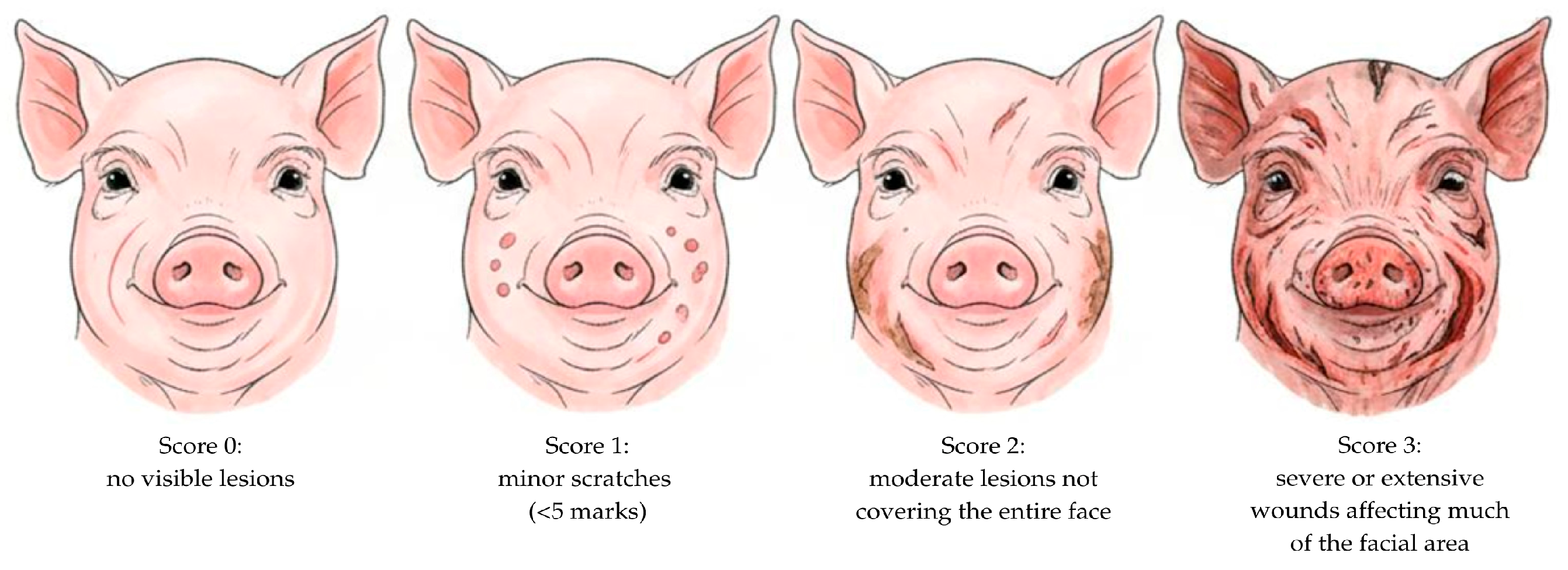
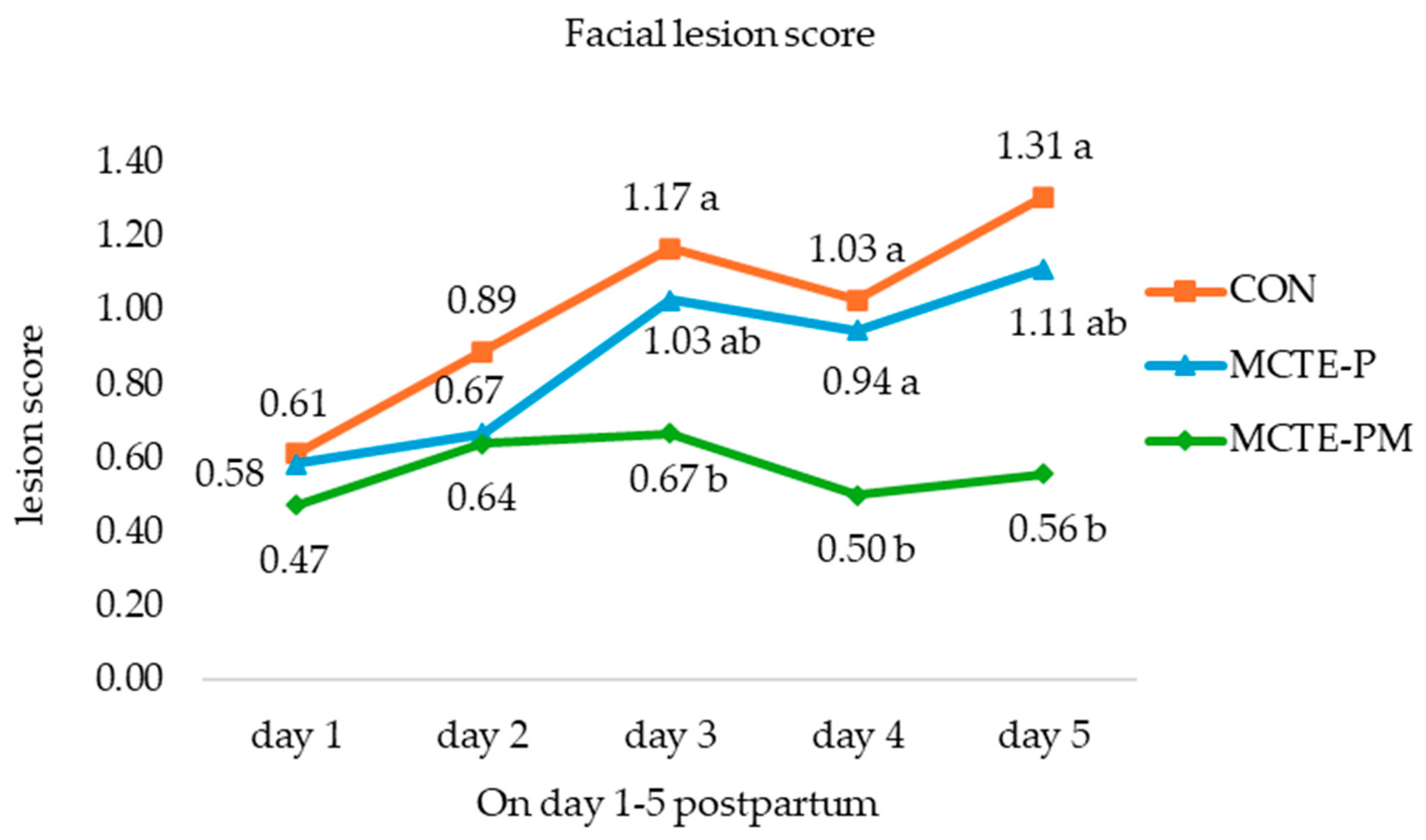
2.5.2. Behavioral and Lesion Assessments
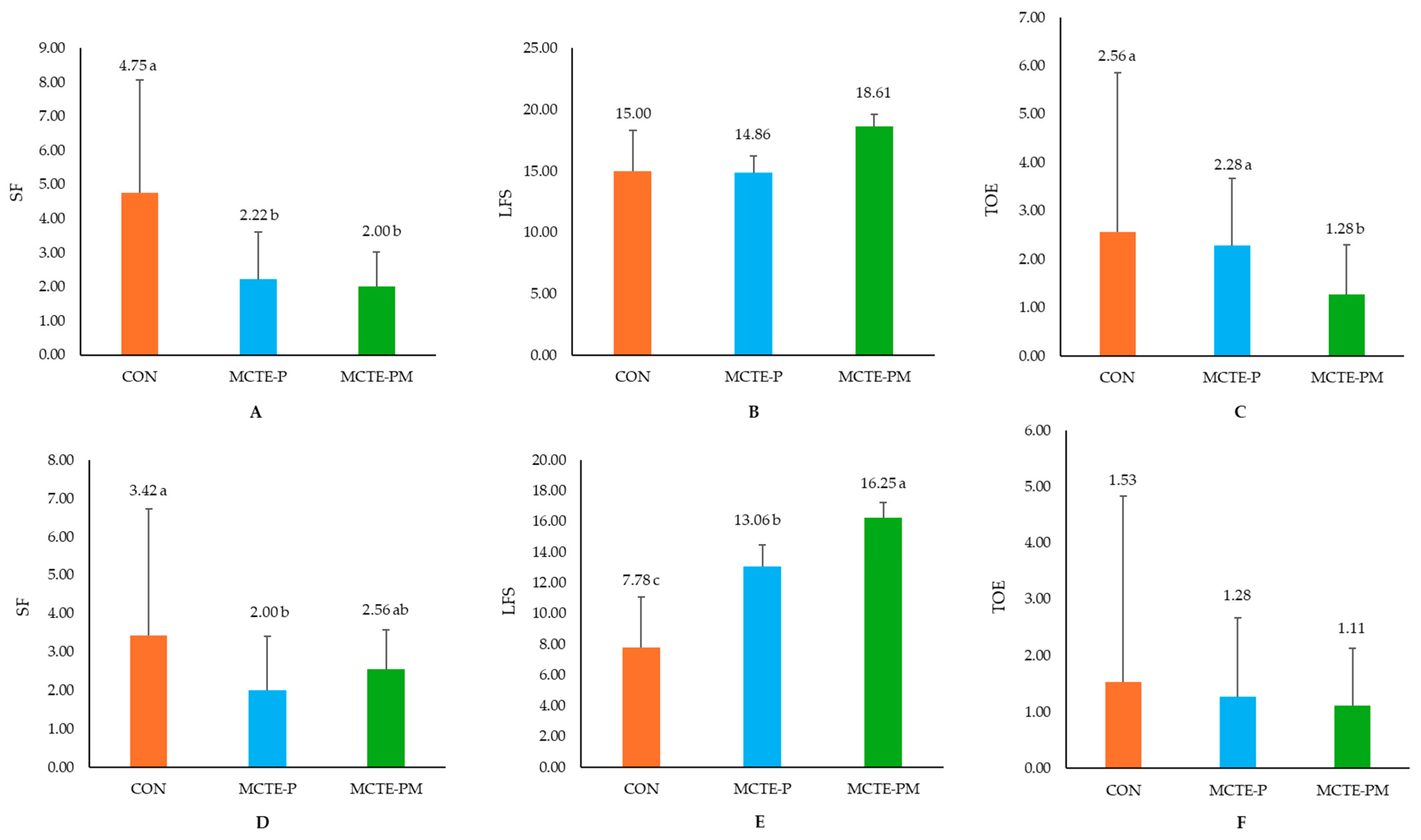
- (1)
- Suckling frequency (SF), defined as the number of approaches each piglet made to the sow’s udder within a two-hour period after treatment [22].
- (2)
- Latency to first suckling (LFS), measured as the time interval (minutes) from release after supplementation until the piglet successfully suckled, providing an index of neonatal vigor and colostrum acquisition [21].
- (3)
- Teat competition and establishment (TOE), quantified by recording the frequency of aggressive interactions or attempts to secure a teat within the same two-hour window [38].
2.6. Statistical Analysis
3. Results
3.1. Trial 1: Effects of MCT Emulsion Formulations and Dosage Levels on Piglet Growth Performance, Colostrum and Milk Intake, and Hematological Parameters
3.1.1. Piglet Performance
3.1.2. Mortality Rate & Causes
3.1.3. Hematological and Biochemical Profiles
3.2. Trial 2: Comparative Efficacy of MCTE-P and Monolaurin-Fortified MCT Emulsions (MCTE-PM) on Piglet Performance and Physiological Parameters
3.2.1. Piglet Performance
3.2.2. Mortality Rate & Causes
3.2.3. Piglet Behavior
3.2.4. Hematological and Biochemical Profiles
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCTE | Medium-Chain Triglyceride Emulsion |
| L-MCTE | Low Dose Medium-Chain Triglyceride Emulsion |
| H-MCTE | High Dose Medium-Chain Triglyceride Emulsion |
| MCTE-P | Medium-Chain Triglyceride Emulsion Supplemented with Phytocannabinoids |
| L-MCTE-P | Low Dose Medium-Chain Triglyceride Emulsion with Phytocannabinoids |
| H-MCTE-P | High Dose Medium-Chain Triglyceride Emulsion with Phytocannabinoids |
| MCTE-PM | Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin |
| MCFA | Medium-Chain Fatty Acids |
| MCT | Medium-chain triglycerides |
| PKO | Palm Kernel Oil |
| MCHC | Mean corpuscular hemoglobin concentration |
References
- Nuntapaitoon, M.; Tummaruk, P. Piglet Preweaning Mortality in a Commercial Swine Herd in Thailand. Trop. Anim. Health Prod. 2015, 47, 1539–1546. [Google Scholar] [CrossRef]
- Mbuthia, J.M.; Kasper, C.; Zenk, M.; Bee, G.; Metges, C.C.; Daş, G. Predicting Piglet Survival until Weaning Using Birth Weight and Within-Litter Birth Weight Variation as Easily Measured Proxy Predictors. Animal 2025, 19, 101479. [Google Scholar] [CrossRef]
- Feldpausch, J.A.; Jourquin, J.; Bergstrom, J.R.; Bargen, J.L.; Bokenkroger, C.D.; Davis, D.L.; Gonzalez, J.M.; Nelssen, J.L.; Puls, C.L.; Trout, W.E.; et al. Birth Weight Threshold for Identifying Piglets at Risk for Preweaning Mortality. Transl. Anim. Sci. 2019, 3, 633–640. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-Infectious Causes of Pre-Weaning Mortality in Piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Rosvold, E.M.; Kielland, C.; Ocepek, M.; Framstad, T.; Fredriksen, B.; Andersen-Ranberg, I.; Næss, G.; Andersen, I.L. Management Routines Influencing Piglet Survival in Loose-Housed Sow Herds. Livest. Sci. 2017, 196, 1–6. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal Piglet Survival: Impact of Sow Nutrition around Parturition on Fetal Glycogen Deposition and Production and Composition of Colostrum and Transient Milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Chiengsorn, K.; Prasongsoo, S.; Bunchasak, C.; Kayan, A.; Poeikhampha, T. Dietary MCFAs and Lauric Acid as Antibiotic Alternatives: Effects on Growth, Immunity and Gut Health in Weaned Pigs. Adv. Anim. Vet. Sci. 2025, 13, 1856–1863. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, L.; Zhang, L.; Wang, T. Effect of Medium-Chain Triglycerides on Growth Performance, Nutrient Digestibility, Plasma Metabolites and Antioxidant Capacity in Weanling Pigs. Anim. Nutr. 2015, 1, 12–18. [Google Scholar] [CrossRef]
- Gebhardt, J.T.; Thomson, K.A.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; DeRouchey, J.M.; Goodband, R.D.; Jones, C.K.; Cochrane, R.A.; Niederwerder, M.C.; et al. Effect of Dietary Medium-Chain Fatty Acids on Nursery Pig Growth Performance, Fecal Microbial Composition, and Mitigation Properties against Porcine Epidemic Diarrhea Virus Following Storage. J. Anim. Sci. 2020, 98, skz358. [Google Scholar] [CrossRef]
- Laowansiri, M.; Suwanchote, S.; Wannigama, D.L.; Badavath, V.N.; Hongsing, P.; Edwards, S.W.; Suratannon, N.; Chatchatee, P.; Lertpichitkul, P.; Rerknimitr, P.; et al. Monolaurin Inhibits Antibiotic-Resistant Staphylococcus Aureus in Patients with Atopic Dermatitis. Sci. Rep. 2025, 15, 23180. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nitti, F.; Jumina, J.; Detha, A.I.R. Antimicrobial Properties of Lauric Acid and Monolaurin in Virgin Coconut Oil: A Review. Chem. Bio. Eng. Rev. 2022, 9, 442–461. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Morikawa, T.; Kawai, T.; Nonomura, Y. Selective Bactericidal Activity of Divalent Metal Salts of Lauric Acid. ACS Omega 2017, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, R.A.; Dritz, S.S.; Woodworth, J.C.; Stark, C.R.; Saensukjaroenphon, M.; Gebhardt, J.T.; Bai, J.; Hesse, R.A.; Poulsen, E.G.; Chen, Q.; et al. Assessing the Effects of Medium-Chain Fatty Acids and Fat Sources on PEDV Infectivity. Transl. Anim. Sci. 2020, 4, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-Chain Fatty Acids and Monoglycerides as Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, D.; Ji, C.; Wu, T.; Wang, M.; Guo, S.; Wang, L.; Zhao, D.; Hou, Y. Monolaurin Confers a Protective Effect Against Porcine Epidemic Diarrhea Virus Infection in Piglets by Regulating the Interferon Pathway. Front. Immunol. 2022, 12, 797476. [Google Scholar] [CrossRef]
- Jackman, J.A.; Lavergne, T.A.; Elrod, C.C. Antimicrobial Monoglycerides for Swine and Poultry Applications. Front. Anim. Sci. 2022, 3, 1019320. [Google Scholar] [CrossRef]
- Rebucci, R.; Comi, M.; Ghiringhelli, M.; Giorgi, S.; Cheli, F.; Bontempo, V. Lauric Acid Saponified with Calcium Ameliorates Indices of Intestinal Function and Gut Health in Weaned Piglets. Ital. J. Anim. Sci. 2021, 20, 1479–1490. [Google Scholar] [CrossRef]
- Lerner, A.B.; Cochrane, R.A.; Gebhardt, J.T.; Dritz, S.S.; Jones, C.K.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Bai, J.; Porter, E.; et al. Effects of Medium Chain Fatty Acid Application in Swine Feed on Porcine Epidemic Diarrhea Virus. Kans. Agric. Exp. Stn. Res. Rep. 2019, 5, 1. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Tomaszewska-Zaremba, D.; Gajewska, A.; Misztal, T. Anti-Inflammatory Effects of Cannabinoids in Therapy of Neurodegenerative Disorders and Inflammatory Diseases of the CNS. Int. J. Mol. Sci. 2025, 26, 6570. [Google Scholar] [CrossRef]
- Gimsa, U.; Brückmann, R.; Tuchscherer, A.; Tuchscherer, M.; Kanitz, E. Early-Life Maternal Deprivation Affects the Mother-Offspring Relationship in Domestic Pigs, as Well as the Neuroendocrine Development and Coping Behavior of Piglets. Front. Behav. Neurosci. 2022, 16, 980350. [Google Scholar] [CrossRef]
- Puppe, B.; Tuchscherer, A. The Development of Suckling Frequency in Pigs from Birth to Weaning of Their Piglets: A Sociobiological Approach. Anim. Sci. 2000, 71, 273–279. [Google Scholar] [CrossRef]
- Salvo, A.; Chiaradia, E.; Sforna, M.; Della Rocca, G. Endocannabinoid System and Phytocannabinoids in the Main Species of Veterinary Interest: A Comparative Review. Vet. Res. Commun. 2024, 48, 2915–2941. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; De Nunzio, V.; Tutino, V.; Notarnicola, M. The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. Int. J. Mol. Sci. 2025, 26, 1306. [Google Scholar] [CrossRef] [PubMed]
- Kongkeaw, A.; Charoensook, R.; Incharoen, T.; Hwanhlem, N.; Tartrakoon, W. Optimization of Phytocannabinoid Extraction from Hemp (Cannabis sativa L.) with Decarboxylation-Based Crude Palm Kernel Oil and Its Potential as an Energy Supplement Product for Suckling Piglets. Int. J. Agric. Technol. 2025, 21, 903–926. [Google Scholar] [CrossRef]
- Somrup, S.; Saracharoen, P. Efficacy of Oral Toltrazuril in Newborn Piglet. ASEAN J. Sci. Technol. Rep. 2022, 25, 36–40. [Google Scholar] [CrossRef]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef]
- Chisoro, P.; Krogh, U.; Theil, P.K.; Eskildsen, M. Characteristics of Sows’ Milk and Piglet Nutrient Utilization during a 28-d Lactation Period. J. Anim. Sci. 2023, 101, skad261. [Google Scholar] [CrossRef]
- Peltoniemi, O.; Yun, J.; Björkman, S.; Han, T. Coping with Large Litters: The Management of Neonatal Piglets and Sow Reproduction. J. Anim. Sci. Technol. 2021, 63, 1–15. [Google Scholar] [CrossRef]
- Miguel, J.; Mitjana, O.; Tejedor, M.T.; Martínez, A.; Falceto, M.V. Supplementing Colostrum from Multiparous Sows: Effects on Performance and Health in Piglets from Gilts in Farm Conditions. Animals 2021, 11, 2563. [Google Scholar] [CrossRef] [PubMed]
- Thongkhuy, S.; Chuaychu, S.B.; Burarnrak, P.; Ruangjoy, P.; Juthamanee, P.; Nuntapaitoon, M.; Tummaruk, P. Effect of Backfat Thickness during Late Gestation on Farrowing Duration, Piglet Birth Weight, Colostrum Yield, Milk Yield and Reproductive Performance of Sows. Livest. Sci. 2020, 234, 103983. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Simionatto, M.; Cruz, B.R.; Bittencourt, J.I.M.; Krum, E.A.; Moss, M.F.; Borato, D.C.K. Stability of Complete Blood Count in Different Storage Conditions Using the ABX PENTRA 60 Analyzer. Int. J. Lab. Hematol. 2018, 40, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.; Nogueira, L.S.; Rios, D.R.A. Hematological Parameters: Is There a Difference between Those Released by the Hematological Analyzer and to the Customer? Einstein São Paulo 2023, 21, eAO0501. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Campbell, T.W. Veterinary Hematology, Clinical Chemistry, and Cytology; Wiley-Blackwell: Hoboken, NJ, USA, 2022; ISBN 978-1119500734. [Google Scholar]
- Tóthová, C.; Link, R.; Kyzeková, P.; Nagy, O. Serum Protein Electrophoretic Pattern in Piglets during the Early Postnatal Period. Sci. Rep. 2021, 11, 17539. [Google Scholar] [CrossRef]
- Garcia Moreira, V.; Beridze Vaktangova, N.; Martinez Gago, M.D.; Laborda Gonzalez, B.; Garcia Alonso, S.; Fernandez Rodriguez, E. Overestimation of Albumin Measured by Bromocresol Green vs Bromocresol Purple Method: Influence of Acute-Phase Globulins. Lab. Med. 2018, 49, 355–361. [Google Scholar] [CrossRef]
- Wilk, I.; Kernberger-Fischer, I.; Gerritzen, M.A.; Kongsted, H.; Schrader, L. Review on Euthanasia of Suckling Piglets on Farm. 2021. Available online: https://library.wur.nl/WebQuery/wurpubs/591049 (accessed on 30 June 2024).
- Skok, J.; Prevolnik, M.; Urek, T.; Mesarec, N.; Škorjanc, D. Behavioural Patterns Established during Suckling Reappear When Piglets Are Forced to Form a New Dominance Hierarchy. Appl. Anim. Behav. Sci. 2014, 161, 42–50. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, N.; Wang, J.; Cao, Y.; Johnston, L.J.; Ma, Y. Effects of Medium- and Short-Chain Fatty Acids on Growth Performance, Nutrient Digestibility, Gut Microbiota and Immune Function in Weaned Piglets. Animals 2024, 15, 37. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Pazos, M.R.; Mohammed, N.; Lafuente, H.; Santos, M.; Martínez-Pinilla, E.; Moreno, E.; Valdizan, E.; Romero, J.; Pazos, A.; Franco, R.; et al. Mechanisms of Cannabidiol Neuroprotection in Hypoxic–Ischemic Newborn Pigs: Role of 5HT1A and CB2 Receptors. Neuropharmacology 2013, 71, 282–291. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Ji, C.; Hu, Y.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Hou, Y. Effects of Monolaurin on Intestinal Barrier, Blood Biochemical Profile, Immunity and Antioxidant Function in Porcine Epidemic Diarrhoea Virus-Infected Piglets. Br. J. Nutr. 2024, 131, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Amdi, C.; Larsen, C.; Jensen, K.M.R.; Tange, E.Ø.; Sato, H.; Williams, A.R. Intrauterine Growth Restriction in Piglets Modulates Postnatal Immune Function and Hepatic Transcriptional Responses Independently of Energy Intake. Front. Physiol. 2023, 14, 1254958. [Google Scholar] [CrossRef]
- Fabà, L.; Martín-Orúe, S.M.; Hulshof, T.G.; Pérez, J.F.; Wellington, M.O.; Van Hees, H.M.J. Impact of Initial Postweaning Feed Intake on Weanling Piglet Metabolism, Gut Health, and Immunity. J. Anim. Sci. 2025, 103, skaf099. [Google Scholar] [CrossRef]
- Pearodwong, P.; Tummaruk, P. Oral Administration of Iron and Toltrazuril in Combination Improves Survival Rate and Growth Rate and Reduces Anaemia in Piglets. Thai J. Vet. Med. 2022, 52, 465–471. [Google Scholar] [CrossRef]
- Ghany, S.S.H.A.E.; Ibrahem, R.A.; EL-Gendy, A.O.; El-Baky, R.M.A.; Mustafa, A.; Azmy, A.F. Novel Synergistic Interactions between Monolaurin, a Mono-Acyl Glycerol and β Lactam Antibiotics against Staphylococcus Aureus: An in Vitro Study. BMC Infect. Dis. 2024, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Yang, X.; Zhao, H.; Chen, H.; Bei, W. Effects of Combined Application of Benzoic Acid and 1-Monolaurin on Growth Performance, Nutrient Digestibility, Gut Microbiome and Inflammatory Factor Levels in Weaned Piglets. Porc. Health Manag. 2023, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Hassan Abd El-Ghany, S.S.; Azmy, A.F.; Osama EL-Gendy, A.; Abd El-Baky, R.M.; Mustafa, A.; Abourehab, M.A.S.; El-Beeh, M.E.; Ibrahem, R.A. Antimicrobial and Antibiofilm Activity of Monolaurin against Methicillin-Resistant Staphylococcus aureus Isolated from Wound Infections. Int. J. Microbiol. 2024, 2024, 7518368. [Google Scholar] [CrossRef]
- Cortes, L.M.; Brodsky, D.; Chen, C.; Pridgen, T.; Odle, J.; Snider, D.B.; Cruse, G.; Putikova, A.; Masuda, M.Y.; Doyle, A.D.; et al. Immunologic and Pathologic Characterization of a Novel Swine Biomedical Research Model for Eosinophilic Esophagitis. Front. Allergy 2022, 3, 1029184. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.K.; Kuehn, L.A.; Wells, J.E.; Rempel, L.A.; Chitko-McKown, C.G.; Keel, B.N.; Oliver, W.T. Hematology Parameters as Potential Indicators of Feed Efficiency in Pigs. Transl. Anim. Sci. 2021, 5, txab219. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.; Zhao, D.; Dai, H. Pharmacological Applications and Action Mechanisms of Phytochemicals as Alternatives to Antibiotics in Pig Production. Front. Immunol. 2021, 12, 798553. [Google Scholar] [CrossRef] [PubMed]
- Landa, L.; Sulcova, A.; Gbelec, P. The Use of Cannabinoids in Animals and Therapeutic Implications for Veterinary Medicine: A Review. Veterinární Medicína 2016, 61, 111–122. [Google Scholar] [CrossRef]
| Item 1 | MCTE | MCTE-P | MCTE-PM |
|---|---|---|---|
| Fatty acid composition (g/100 g) | |||
| Caproic acid (C6:0) | 0.09 | 0.09 | 0.09 |
| Caprylic acid (C8:0) | 1.38 | 1.26 | 1.12 |
| Capric acid (C10:0) | 1.37 | 1.25 | 1.21 |
| Lauric acid (C12:0) | 33.94 | 41.42 | 41.23 |
| Medium-chain fatty acids (MCFA) | 36.80 | 44.04 | 43.67 |
| Myristic acid (C14:0) | 5.97 | 5.93 | 5.48 |
| Palmitic acid (C16:0) | 7.62 | 9.08 | 8.22 |
| Stearic acid (C18:0) | 1.43 | 1.45 | 1.42 |
| Arachidic acid (C20:0) | 0.16 | 0.19 | 0.15 |
| Behenic acid (C22:0) | 0.07 | 0.09 | 0.07 |
| Lignoceric acid (C24:0) | 0.07 | 0.08 | 0.06 |
| Saturated Fatty acid (g/100 g) | 52.10 | 60.84 | 59.05 |
| Palmitoleic acid (C16:1n7) | 0.05 | 0.05 | 0.05 |
| Trans-9-Elaidic acid (C18:1n9-t) | 0.03 | 0.06 | 0.05 |
| cis-9-0leic acid (C18:1n9-c) | 13.21 | 14.92 | 13.81 |
| cis-11-Eicosenoic acid(C20:1n11-c) | 0.13 | 0.15 | 0.12 |
| Nervonic acid (C24:1n9) | 0.03 | 0.05 | 0.04 |
| Monounsaturated fatty acid (g/100 g) | 13.46 | 15.12 | 14.08 |
| cis-9,12-Linoleic acid (C18:2n6) | 7.31 | 7.71 | 7.04 |
| gamma-Linoleic acid (C18:3n6) | 0.03 | 0.04 | 0.03 |
| alpha-Linoleic acid (C18:3n3) | 0.40 | 0.59 | 0.48 |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.06 | 0.03 | 0.03 |
| Arachidonic acid (C20:4n6) | 0.01 | 0.01 | 0.01 |
| Polyunsaturated Fatty acid (g/100 g) | 7.66 | 7.59 | 7.38 |
| Unsaturated fat (g/100 g) | 21.32 | 22.42 | 21.64 |
| Omega 3 (mg/100 g) | 442.00 | 498.42 | 482.64 |
| Omega 6 (mg/100 g) | 7358.63 | 7761.14 | 7478.79 |
| Omaga 9 (mg/100 g) | 13,242.06 | 14,270.29 | 12,841.89 |
| Chemical composition | |||
| Ash | 0.12 | 0.12 | 0.10 |
| Calories from Fat (kcal/100 g) | 661.59 | 788.94 | 748.78 |
| Carbohydrates (g/100 g) | <0.01 | <0.01 | <0.01 |
| Fat (g/100 g) | 73.51 | 87.65 | 82.42 |
| Water Content (%) | 15.23 | 15.42 | 15.32 |
| Iodine Value (%) | 47.85 | 41.89 | 42.30 |
| Peroxide Value (mEq Peroxide/kg) | 3.7 | 4.73 | 5.23 |
| Parameters | CON | MCTE | MCTE-P | SEM | p-Value from Orthogonal Contrast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-MCTE | H-MCTE | L-MCTE-P | H-MCTE-P | CON vs. Emulsion | CON vs. MCTE vs. MCTE-P | A | B | A × B | |||
| Number of sows | 15 | 15 | 15 | 15 | 15 | ||||||
| Newborn piglets | |||||||||||
| Number of piglets | 229 | 197 | 220 | 208 | 209 | ||||||
| Litter size (piglet/sow) | 15.27 | 13.13 | 14.67 | 13.87 | 13.93 | 0.31 | 0.074 | 0.224 | 0.353 | 0.244 | 0.285 |
| Birth weight (kg) | 1.37 | 1.31 | 1.31 | 1.25 | 1.28 | 0.02 | 0.108 | 0.465 | 0.320 | 0.728 | 0.706 |
| Live born piglets (24 h) | |||||||||||
| Number of piglets | 202 | 188 | 199 | 191 | 196 | ||||||
| Weight (kg) | 1.49 | 1.43 | 1.42 | 1.36 | 1.42 | 0.02 | 0.105 | 0.419 | 0.414 | 0.634 | 0.422 |
| Weight gain (kg) | 0.09 b | 0.11 ab | 0.09 b | 0.13 a | 0.13 a | 0.01 | 0.020 | 0.001 | 0.001 | 0.303 | 0.139 |
| Colostrum intake (mL/piglet) | 287.69 b | 310.51 ab | 279.93 b | 325.04 ab | 334.63 a | 5.61 | 0.079 | 0.006 | 0.003 | 0.355 | 0.080 |
| Piglets at 5 days old | |||||||||||
| Number of piglets | 183 | 150 | 160 | 171 | 167 | ||||||
| Weight (kg) | 2.13 | 1.87 | 1.91 | 1.74 | 1.96 | 0.05 | 0.038 | 0.173 | 0.706 | 0.203 | 0.415 |
| ADG (g/day/piglet) | 156.29 | 100.72 | 132.87 | 104.90 | 117.07 | 8.55 | 0.046 | 0.228 | 0.686 | 0.127 | 0.488 |
| Milk intakes (mL/piglet) | 567.49 | 501.84 | 586.41 | 530.86 | 545.59 | 17.63 | 0.554 | 0.611 | 0.883 | 0.218 | 0.385 |
| Piglets at 14 days old | |||||||||||
| Number of piglets | 167 | 146 | 143 | 165 | 165 | ||||||
| Weight (kg) | 3.21 | 3.57 | 3.44 | 3.33 | 3.52 | 0.08 | 0.197 | 0.604 | 0.647 | 0.855 | 0.364 |
| ADG (g/day/piglet) | 120.97 b | 164.24 ab | 155.47 ab | 177.06 a | 170.98 a | 7.19 | 0.010 | 0.106 | 0.366 | 0.635 | 0.931 |
| Weaned pigs (at 18 days old) | |||||||||||
| Number of piglets | 143 | 127 | 127 | 148 | 157 | ||||||
| Litter size (piglet/sow) | 9.50 | 8.43 | 8.52 | 9.88 | 10.49 | 0.18 | 0.446 | 0.754 | 0.285 | 0.680 | 0.804 |
| Weight (kg) | 4.27 | 4.67 | 4.20 | 4.29 | 4.21 | 0.11 | 0.783 | 0.664 | 0.424 | 0.253 | 0.402 |
| ADG (g/day/piglet) | 163.16 | 170.67 | 164.91 | 171.16 | 179.93 | 6.34 | 0.595 | 0.569 | 0.363 | 0.172 | 0.589 |
| Mortality rate & causes | |||||||||||
| Mortality rate (%) | 29.44 ab | 32.69 ab | 35.82 a | 22.65 bc | 19.69 c | 1.68 | 0.744 | 0.016 | 0.001 | 0.787 | 0.238 |
| Mortality causes | |||||||||||
| Milk starvation (%) | 15.40 a | 10.95 ab | 10.20 ab | 6.14 ab | 5.36 b | 0.96 | 0.037 | 0.073 | 0.050 | 0.371 | 0.606 |
| Weak state (%) | 1.33 b | 6.07 a | 6.30 a | 3.35 ab | 2.40 b | 0.55 | 0.002 | 0.009 | 0.010 | 0.772 | 0.636 |
| Crushing (%) | 2.47 c | 7.34 b | 13.31 a | 10.20 ab | 8.76 b | 0.77 | 0.001 | 0.001 | 0.599 | 0.162 | 0.024 |
| Diarrhea (%) | 10.24 a | 8.33 a | 6.01 ab | 2.96 b | 3.17 b | 0.74 | 0.015 | 0.008 | 0.002 | 0.417 | 0.331 |
| Parameters | CON | MCTE | MCTE-P | SEM | p-Value from Orthogonal Contrast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-MCTE | H-MCTE | L-MCTE-P | H- MCTE-P | CON vs. Emulsion | CON vs. MCTE vs. MCTE-P | A | B | A × B | |||
| Number of piglets | 10 | 10 | 10 | 10 | 10 | ||||||
| Complete Blood Count (CBC) | |||||||||||
| WBC (cell × 104/ mm3) | 1.28 | 1.16 | 1.37 | 1.14 | 1.31 | 0.057 | 0.830 | 0.680 | 0.760 | 0.163 | 0.886 |
| RBC (cell × 106/mm3) | 6.69 | 6.14 | 6.24 | 6.67 | 6.45 | 0.087 | 0.148 | 0.156 | 0.053 | 0.747 | 0.399 |
| Hemoglobin (g/dL) | 11.75 | 11.07 | 10.87 | 11.47 | 11.36 | 0.22 | 0.304 | 0.742 | 0.367 | 0.752 | 0.927 |
| Hct (%) | 36.20 | 34.00 | 34.00 | 38.60 | 37.70 | 0.67 | 0.941 | 0.089 | 0.005 | 0.746 | 0.746 |
| Platelet count (cell × 104/mm3) | 58.43 | 62.21 | 62.59 | 59.95 | 47.72 | 2.58 | 0.962 | 0.356 | 0.163 | 0.331 | 0.301 |
| MCV (fL) | 53.90 | 55.00 | 54.10 | 57.40 | 58.20 | 0.67 | 0.179 | 0.142 | 0.020 | 0.970 | 0.530 |
| MCH (pg) | 17.60 | 18.00 | 17.40 | 17.30 | 17.70 | 0.22 | 1.000 | 0.874 | 0.692 | 0.843 | 0.326 |
| MCHC (g/dL) | 32.70 a | 32.70 a | 32.10 a | 29.90 b | 30.10 b | 0.22 | 0.005 | <0.001 | <0.001 | 0.468 | 0.151 |
| RDW (%) | 17.05 b | 17.58 b | 17.15 b | 19.77 a | 21.01 a | 0.34 | 0.029 | <0.001 | <0.001 | 0.509 | 0.178 |
| Neutrophil (%) | 38.00 | 30.50 | 32.20 | 28.80 | 29.80 | 1.85 | 0.098 | 0.557 | 0.608 | 0.735 | 0.930 |
| Lymphocyte (%) | 51.40 | 61.50 | 58.70 | 61.90 | 62.80 | 1.91 | 0.039 | 0.323 | 0.580 | 0.815 | 0.649 |
| Monocyte (%) | 6.80 | 6.00 | 7.00 | 7.60 | 6.50 | 0.29 | 0.973 | 0.512 | 0.401 | 0.939 | 0.114 |
| Eosinophil (%) | 3.80 a | 2.00 ab | 2.10 ab | 1.70 b | 0.90 b | 0.25 | <0.001 | 0.004 | 0.142 | 0.488 | 0.374 |
| Biochemical tests | |||||||||||
| Total protein (g/dL) | 4.12 c | 4.80 b | 4.28 c | 5.40 a | 5.29 a | 0.10 | <0.001 | <0.001 | <0.001 | 0.053 | 0.200 |
| Albumin (g/dL) | 3.33 | 3.62 | 3.60 | 3.69 | 3.65 | 0.05 | 0.011 | 0.154 | 0.579 | 0.781 | 0.926 |
| Globulin (g/dL) | 0.79 b | 1.18 bc | 0.68 b | 1.71 a | 1.64 ab | 0.09 | <0.001 | <0.001 | <0.001 | 0.043 | 0.122 |
| Parameters | CON | MCTE-P | MCTE-PM | SEM | p-Value |
|---|---|---|---|---|---|
| Number of sows | 12 | 12 | 12 | ||
| Newborn piglets | |||||
| Number of piglets | 183 | 158 | 168 | ||
| Litter size (piglet/sow) | 15.25 | 13.17 | 14.00 | 0.45 | 0.159 |
| Birth weight (kg) | 1.35 | 1.26 | 1.34 | 0.03 | 0.459 |
| Live born piglets (24 h) | |||||
| Number of piglets | 163 | 149 | 160 | ||
| Litter size (piglet/sow) | 13.61 | 12.44 | 13.32 | 0.46 | 0.613 |
| Weight (kg) | 1.47 | 1.38 | 1.47 | 0.03 | 0.377 |
| Weight gain (kg) | 0.08 b | 0.13 a | 0.13 a | 0.01 | 0.001 |
| Colostrum intake (mL/piglet) | 276.76 b | 328.73 a | 336.73 a | 8.65 | 0.006 |
| Piglets at 5 days old | |||||
| Number of piglets | 133 | 124 | 144 | ||
| Litter size (pigs/sow) | 11.08 | 10.33 | 12.00 | 0.25 | 0.652 |
| Weight (kg) | 1.86 | 1.85 | 1.90 | 0.12 | 0.158 |
| ADG (g/day/piglet) | 100.89 | 108.47 | 109.00 | 12.35 | 0.113 |
| Milk intakes (mL/piglet) | 523.14 | 527.05 | 514.97 | 523.14 | 0.279 |
| Piglets at 14 days old | |||||
| Number of piglets | 126 | 122 | 137 | ||
| Litter size (piglet/sow) | 10.50 b | 10.20 b | 11.42 a | 0.29 | 0.031 |
| Weight (kg) | 3.13 | 3.37 | 3.53 | 0.12 | 0.425 |
| ADG (g/day/piglet) | 107.81 c | 170.58 a | 155.57 b | 11.62 | 0.043 |
| Weaned pigs (at 18 days old) | |||||
| Number of piglets | 115 | 114 | 137 | ||
| Litter size (piglet/sow) | 9.60 b | 9.50 b | 11.42 a | 0.50 | 0.052 |
| Weight (kg) | 4.05 | 4.36 | 4.44 | 0.17 | 0.623 |
| ADG (g/day/piglet) | 151.88 b | 173.67 a | 172.38 a | 9.18 | 0.046 |
| Mortality rate & causes | |||||
| Mortality rate (%) | 29.40 a | 23.63 ab | 14.27 b | 2.36 | 0.012 |
| Mortality causes | |||||
| Milk starvation (%) | 14.40 a | 9.23 ab | 4.30 b | 1.51 | 0.002 |
| Weak state (%) | 1.68 | 3.40 | 1.78 | 0.57 | 0.304 |
| Crushing (%) | 2.92 b | 9.30 a | 7.33 a | 1.02 | 0.022 |
| Diarrhea (%) | 10.40 a | 1.70 b | 0.86 b | 1.11 | 0.001 |
| Parameters | CON | MCTE-P | MCTE-PM | SEM | p-Value |
|---|---|---|---|---|---|
| Number of piglets | 8 | 8 | 8 | ||
| Complete Blood Count (CBC) | |||||
| WBC (cell × 104/ mm3) | 1.15 | 1.24 | 1.03 | 0.06 | 0.303 |
| RBC (cell ×106/mm3) | 6.82 a | 6.80 a | 5.98 b | 0.12 | 0.003 |
| Hemoglobin (g/dL) | 11.83 | 11.66 | 11.06 | 0.28 | 0.536 |
| Hct (%) | 36.00 | 39.13 | 36.25 | 0.88 | 0.284 |
| Platelet count (cell × 104/mm3) | 60.65 | 55.88 | 44.89 | 3.49 | 0.170 |
| MCV (fL) | 52.50 b | 57.13 a | 60.50 a | 1.12 | 0.008 |
| MCH (pg) | 17.38 | 17.25 | 18.63 | 0.31 | 0.131 |
| MCHC (g/dL) | 33.00 a | 30.00 b | 30.50 b | 0.33 | <0.001 |
| RDW (%) | 16.54 b | 19.65 a | 20.13 a | 0.55 | 0.009 |
| Neutrophil (%) | 35.75 | 30.88 | 35.75 | 2.69 | 0.712 |
| Lymphocyte (%) | 54.13 | 59.50 | 57.88 | 2.83 | 0.746 |
| Monocyte (%) | 6.38 | 7.63 | 6.13 | 0.40 | 0.265 |
| Eosinophil (%) | 3.75 a | 2.00 b | 0.25 c | 0.44 | 0.002 |
| Biochemical tests | |||||
| Total protein (g/dL) | 4.00 b | 5.16 a | 5.13 a | 0.15 | <0.001 |
| Albumin (g/dL) | 3.39 | 3.65 | 3.36 | 0.07 | 0.155 |
| Globulin (g/dL) | 0.61 b | 1.51 a | 1.76 a | 0.14 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongkeaw, A.; Tartrakoon, W.; Numthuam, S.; Incharoen, T.; Hwanhlem, N.; Loor, J.J.; Charoensook, R. Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin Improves Growth and Survival in Suckling Piglets. Animals 2025, 15, 2881. https://doi.org/10.3390/ani15192881
Kongkeaw A, Tartrakoon W, Numthuam S, Incharoen T, Hwanhlem N, Loor JJ, Charoensook R. Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin Improves Growth and Survival in Suckling Piglets. Animals. 2025; 15(19):2881. https://doi.org/10.3390/ani15192881
Chicago/Turabian StyleKongkeaw, Adisak, Wandee Tartrakoon, Sonthaya Numthuam, Tossaporn Incharoen, Noraphat Hwanhlem, Juan J. Loor, and Rangsun Charoensook. 2025. "Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin Improves Growth and Survival in Suckling Piglets" Animals 15, no. 19: 2881. https://doi.org/10.3390/ani15192881
APA StyleKongkeaw, A., Tartrakoon, W., Numthuam, S., Incharoen, T., Hwanhlem, N., Loor, J. J., & Charoensook, R. (2025). Medium-Chain Triglyceride Emulsion with Phytocannabinoids and Monolaurin Improves Growth and Survival in Suckling Piglets. Animals, 15(19), 2881. https://doi.org/10.3390/ani15192881









