Simple Summary
During the late laying period, reduced egg production causes economic losses, while the industry aims to extend laying cycles, emphasizing the need to improve the laying performance of aged hens. The decline in laying rate is mainly associated with diminished ovarian function. Nutritional strategies that support ovarian function are therefore crucial for aged hens. Flos lonicerae and Baikal skullcap Extracts (PE) have been reported to enhance laying rate of laying hens. Thus, we speculate that dietary supplemented with PE may improve ovarian function of older hens. In this study, PE supplementation improved laying performance, immunity, antioxidant capacity, and ovarian function of aged hens. Specifically, PE supplementation increased pre-hierarchical follicle numbers, ovarian index, and serum estrogen levels. 16S rRNA sequencing revealed that PE induced alterations in the composition of the cecal microbiota, with these changes primarily linked to the production of short-chain fatty acids. Ovarian metabolomics further indicated that PE regulated metabolites related to follicle growth, estrogen secretion, and antioxidant and anti-inflammatory responses. In summary, PE improved laying performance by enhancing antioxidant and immune functions, promoting follicular development and estrogen secretion, and modulating gut microbiota and ovarian metabolites. These findings offer a mechanistic foundation for the nutritional regulation of ovarian function in aged hens.
Abstract
The aim of this study was to evaluate the effects of Flos lonicerae and Baikal skullcap extracts (PE) on laying performance, antioxidant capacity, immune function, follicular development, estrogen secretion, ovarian metabolomics, and cecal microbiota in aged laying hens. The total number of 70-week-old XinYang Black-Feathered laying hens was 240. These hens were randomly divided into two groups, with each group consisting of six replicates of 20 birds. Control (CON) group was fed a basal diet, whereas the PE group received the same basal diet supplemented with 500 mg/kg of PE. The duration of the experiment was 10 weeks. The findings indicated that the supplementation of PE improved laying performance, antioxidant capacity, and immune function. This was reflected by significant increases (p < 0.05) in laying rate, feed conversion ratio, antioxidant indicators (such as glutathione peroxidase, total antioxidant capacity, and catalase), and immunoglobulin levels. Additionally, there were notable decreases (p < 0.05) in the malondialdehyde levels and pro-inflammatory markers. Moreover, the PE group exhibited a greater number of large yellow and white follicles, as well as higher serum estrogen levels, compared to the CON group (p < 0.05). 16S rRNA sequencing revealed that PE supplementation altered the composition of the cecal microbiota by increasing Ruminococcus_torques_group, Butyricoccus and Christensenellaceae_R-7_group abundances and decreasing Bacteroides, Prevotellaceae_UCG-001 and Megamonas abundances (at genus level), which are primarily associated with short-chain fatty acid production. Ovarian metabolomic analysis showed that the major metabolites altered by PE supplementation were mainly involved in follicular development, estrogen biosynthesis, anti-inflammatory and antioxidant properties. Moreover, changes in both the cecal microbiota (at genus level) and ovarian metabolites were strongly correlated with laying performance, antioxidant status, and immune function. In conclusion, PE supplementation improved laying performance in aged hens by enhancing antioxidant, immune, and ovarian functions, promoting follicular development and estrogen secretion, and modulating the gut microbiota and ovarian metabolites. These findings will offer novel insights into the mechanisms that underlie egg production in the ovaries of aged poultry.
1. Introduction
During the late laying period, the continuous decline in laying rate leads to economic losses for poultry farms [1,2]. On the other hand, in recent years, the egg industry has been actively exploring strategies to extend the laying cycle in order to maximize the utilization of housing facilities and equipment [3]. Thus, it is essential to find effective ways to improve the laying performance of aged hens.
The decline in laying rate in aged hens is mainly linked to ovarian oxidative stress [4], which in turn accelerates ovarian aging. This results in a diminished number of follicles and a decrease in estrogen levels, which consequently leads to a reduced laying rate in older hens [1]. Therefore, identifying appropriate nutritional strategies to improve ovarian function in aged laying hens is of great importance.
Flos lonicerae, cultivated in China for thousands of years. Its various products, including herbal drinks, wine, tea, and traditional Chinese medicine preparations such as honeysuckle granules, are highly valued [5,6]. China is the primary producer of Baikal skullcap for medicinal purposes, and its extract is widely applied in diverse industries, including pharmaceuticals, nutraceuticals, cosmetics, and food and beverages. Both plants hold significant medicinal, economic, and ecological value [7]. Chlorogenic acid (CGA) and baicalin are the major active components of Flos lonicerae and Baikal skullcap, respectively [8]. Both baicalin and CGA demonstrate a range of biological activities, encompassing antioxidant, anti-inflammatory, antibacterial, and antiviral effects [9]. A study by Xie et al. [10] showed that supplementing the diet of 52-week-old Lohman pink-shell hens with 0.1% Flos lonicerae and 0.1% Baikal skullcap extracts for 12 weeks significantly decreased plasma malondialdehyde levels and increased hen-day egg production. Additionally, Liu et al. [11] demonstrated that dietary supplementation with 300, 500, or 1000 mg/kg Flos lonicerae extract for 11 weeks significantly improved hen-day egg production in 83-week-old Roman Pink laying hens.
Therefore, we hypothesized that supplementation with Flos lonicerae and Baikal skullcap extracts (PE) could improve laying performance of aged hens by enhancing ovarian function. However, a comprehensive and in-depth understanding of how PE precisely regulates ovarian function is still lacking, particularly with regard to the key metabolites, metabolic pathways and gut microbiota through which this regulatory process is mediated. Consequently, a feeding trial was undertaken to evaluate the effect of PE supplementation on laying performance, antioxidant capacity, immune function, follicular development, estrogen secretion, ovarian metabolomics, and cecal microbiota of aged laying hens. The aim of this study is to identify key metabolites targeting the enhancement of ovarian function, thereby providing a theoretical basis for their application as novel additives in reproductive health regulation, and offering scientific support for the development of personalized nutritional interventions based on core ovarian metabolites.
2. Materials and Methods
2.1. Experimental Design, Birds and Diets
The experimental procedures were conducted in adherence to the Chinese Guidelines for Animal Welfare and were approved by the Animal Care and Use Committee at Zhejiang A&F University.
A total of 240 healthy, 70-week-old XinYang Black-Feathered laying hens with a comparable laying rate of 71% were randomized into two groups of six replicates, each consisting of 20 birds. Initial body weight and laying rate were standardized across all replicates after a two-week pre-test period. In the pre-test phase, all birds were given a basal diet consisting of corn-soybean meal, as outlined in Table A1. The composition of this diet met the nutritional requirements for laying hens as per the NY/T-33-2004 feeding standard (China National Standard, 2004) [12].
The two experimental groups were as follows: the control (CON) group, where the birds were fed the same corn-soybean meal basal diet as during the pre-test; and the PE group, where the birds received the basal diet supplemented with 500 mg/kg of PE. This optimal dose of PE was selected based on prior findings from Liu et al. [11]. The PE consisted of Flos lonicerae extract (10% chlorogenic acid) and Baikal skullcap extract (90% baicalin) in a 3:2 ratio, providing final concentrations of 300 mg/kg chlorogenic acid and 200 mg/kg baicalin, respectively. The supplement was provided by Beijing Centre Biology Co., Ltd. (Beijing, China).
Five-tier ladder cages, with a capacity of four birds per cage, were used to house all the hens. The photoperiod was set at 16 h of light per day, and the room temperature was maintained at 20 ± 3 °C throughout the 10-week study. The birds had unrestricted access to food and water, and eggs were collected daily at 4 pm.
2.2. Laying Performance Measurement
The total number of eggs and the egg weight per replicate were recorded on a daily basis. Weekly records were kept of residual feed consumption. At the end of week 75 and 80, laying rate, average egg weight, average daily feed intake (ADFI) and feed/egg ratio were calculated. All calculations are based on the above records.
2.3. Sample Collection and Preparation
At the conclusion of the experiment, two birds with comparable body weight were selected from each replicate. After an 8-h fasting period, the birds were weighed, and blood samples were drawn from the wing vein into vacuum tubes designed to promote coagulation. These blood samples were kept at room temperature for 4 h, after which serum was obtained by centrifuging the samples at 3000 rpm for 15 min at 4 °C. Subsequently, the hens were anesthetized using sodium pentobarbital and euthanized via exsanguination from the jugular vein. Follicles were classified according to their diameter as either pre-grade or preovulatory follicles (≥12 mm) [13]. Pre-grade follicles were further categorized into small white follicles (SWF, 1–2 mm), large white follicles (LWF, 2–6 mm), small yellow follicles (SYF, 6–8 mm), and large yellow follicles (LYF, 8–12 mm) [14]. These follicles were identified and counted. Ovaries lacking follicles larger than 1 mm in diameter were weighed to determine the ovarian index (g/kg), calculated as the ovary weight (g) divided by the bird’s body weight (kg). Aseptic collection of cecal contents was performed for each bird. Serum, ovaries (excluding follicles larger than 1 mm), and cecal samples were then stored at −80 °C for subsequent analysis.
2.4. Antioxidant Indices
The kits used for the determination of glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), catalase (CAT), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in the serum and ovary were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The procedures were carried out as previously described [15].
2.5. Immune Parameters
Immunoglobulins Y (IgY), immunoglobulins A (IgA) and immunoglobulins M (IgM) concentrations in the serum were determined using commercially available chicken specific enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The experimental approach was based on the protocol described by Wattrang E et al. [16].
Interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and interferon-γ (IFN-γ) kits were purchased from Cloud-Clone Corp. (Wuhan, China), and interleukin-1β (IL-1β) kit was purchased from Cusabio Biotech Co., Ltd. (Wuhan, China). The methodology followed that described in earlier publication [17].
2.6. Hormonal Assays
Estradiol (E2), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in the serum were measured using commercial ELISA kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing China). The procedures were carried out in the same way as they were previously described [18].
2.7. 16S rRNA Gene Sequencing of Cecal Microbiota
Microbia DNA was extracted from the cecal content using the DNAiso Reagent (TAKARA, Kyoto, Japan). The quality of extracted DNA was checked with gel electrophoresis. TheV3-V4 hypervariable regions of bacterial 16S rRNA were amplified using universal primer pairs (338F: 5′-ACTCCTACGGAGGCACAG-3′; 806R: 5′-GGACTACHVGGGTWTCTAAT-3′). Pair sequencing was performed on an Illumina HiSeq2500 PE300 platform (Illumina, San Diego, CA, USA) at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw paired-end reads were submitted to the Trimmomatic software (version 0.39) for quality filtering and then merged using FLASH 1.2.7 (https://ccb.jhu.edu/software/FLASH/index.shtml, accessed on 6 September 2024). Clustering of Amplicon Sequence Varian (ASV) with the requirement of 100% similarity by means of UPARSE (version 7.1, http://drive5.com/uparse/, accessed on 6 September 2024). The taxonomy of each 16S rRNA gene sequence was analyzed using the RDP classifier algorithm with a confidence threshold of 70%. Data analysis was performed using the Majorbio Cloud Platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The Ace, Chao and Shannon indices were calculated using Mothur-1.30.2. To estimate pairwise distances between samples and to determine β-diversity, the Principal Coordinates Analysis (PCoA) plot based on unweighted Unifrac was used. Linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe) were used to identify the microbial taxa biomarkers between groups, with a selection criterion of an LDA score greater than 2.0. Wilcoxon rank-sum test was used to evaluate the functional differences between the CON and VE groups.
2.8. Untargeted Metabolomics Analysis of Ovary
A total of 12 ovarian samples, each weighing 20 ± 1 mg, were obtained for metabolomic analysis, with 6 samples from the CON group and 6 from the PE group. Metabolites were extracted from the samples using methanol/water (70%, v/v) and 2-chlorophenylalanine (1 μg/mL). A 200 μL supernatant was collected in a liquid chromatographic bottle for LC-MS/MS analysis after centrifugation (4 °C, 12,000 rpm, 10 min). The extraction solution of all samples in the same group is mixed in equal volumes for the preparation of the quality control sample. Metabolite samples were analyzed using a UHPLC system (LC20, Shimadzu, Kyoto, Japan) coupled with an ACQUITY UPLC HSS T3 C18 column (1.8 µm, 2.1 mm × 100 mm; Waters Corp., Milford, MA, USA). The mobile phases consisted of ultrapure water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The elution gradient was as follows: 95% A and 5% B (v/v) at 0 min; 10% A and 90% B (v/v) at 11.0 min; 10% A and 90% B (v/v) at 12.0 min; 95% A and 5% B (v/v) at 12.1 min; 95% A and 5% B (v/v) at 14.0 min. The column temperature was maintained at 40 °C, with a 2 µL injection volume and a flow rate of 0.4 mL/min. Mass spectrometric detection was performed using both positive and negative ion modes on the Triple TOF-6600 mass spectrometer (AB SCIEX, Framingham, MA, USA).
Data analysis was performed using the self-built MWDB database (Metware Biotechnology Co., Ltd., Hubei, Wuhan, China). Metabolites exhibiting a variable importance in projection (VIP) greater than 1, along with a fold change (FC) exceeding 2 or less than 0.05, were utilized as criteria for the identification of potential biomarkers. The KEGG pathway analysis was used to conduct metabolite pathway enrichment analysis on the differential metabolites to determine the differential metabolic pathways between the CON and PE groups.
2.9. Statistical Analysis
Student’s t-test was used to analyze the data (expected for gut microbes and ovarian metabolites). Results were presented as “mean ± standard deviation.” A significance level of p < 0.05 was established for statistical analysis. Spearman correlation analysis was carried out to explore the intricate relationships among gut microbes (at the genus level), the significantly identified ovarian metabolites, immunoglobulins, antioxidant parameters, and performance-related parameters. GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) was used to create the figures.
3. Results
3.1. Laying Performance
As shown in Table 1, the PE group exhibited a notable (p < 0.05) improvement in laying rates (6.36%, 9.27%, 8.12%) compared to the CON group, while also showing a reduction in feed/egg ratio (7.42%, 9.43%, 8.8%) across weeks 71 to 75, 76 to 80, and 71 to 80. However, no significant differences (p > 0.05) were observed between the CON and PE groups regarding average egg weight and average daily feed intake (ADFI) during the same periods.

Table 1.
Effect of PE increment on laying performance of laying hens.
3.2. Antioxidant Parameters
The data presented in Figure 1 demonstrate that the antioxidant capacity of hens in the PE group was generally superior to that observed in the CON group. This was supported by the following findings: a significant increase (p < 0.05) in serum T-SOD activity (9.37%); elevated (p < 0.05) levels of GSH-Px (14.62% and 13.40%), T-AOC (12.14% and 12.78%), and CAT (7.52% and 13.26%) in both serum and ovarian tissues; and a notable reduction (p < 0.05) in the concentrations of MDA in both serum (8.10%) and ovary (13.96%).
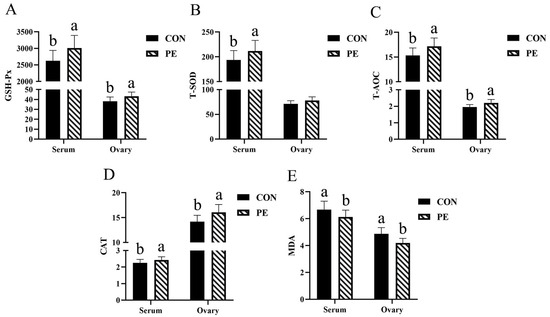
Figure 1.
Effect of PE on antioxidant parameters of laying hens. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. The data are expressed as the mean ± standard deviation (n = 12 per group). Statistical significance (p < 0.05) is indicated by different letters in the bar chart. (A) GSH-Px: glutathione peroxidase. (B) T-SOD: total superoxidedismutase. (C) T-AOC, total antioxidant capacity. (D) CAT, catalase; (E) MDA: malondialdehyde. The GSH-Px, T-SOD, T-AOC and CAT were expressed as specific activity (U/mgprot) in ovary and as (U/mL) in serum, respectively. The MDA was expressed as (nmol/mgprot) in ovary and as (nmol/mL) in serum, respectively.
3.3. Cytokine Levels
As illustrated in Figure 2, the PE group exhibited a statistically significant reduction (p < 0.05) in serum levels of IL-6, IL-1β, TNF-α and IFN-γ, with reductions of 14.34%, 10.07%, 12.72% and 16.84%, respectively, in comparison to the CON group (Figure 2A–D).
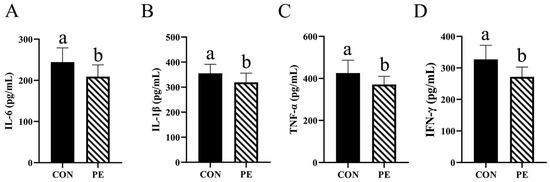
Figure 2.
Effect of PE on the level of cytokine. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. The data are expressed as the mean ± standard deviation (n = 12 per group). Statistical significance (p < 0.05) is indicated by different letters in the bar chart. (A) IL-6: Interleukin-6. (B) IL-1β: interleukin-1β. (C) TNF-α, tumor necrosis factor-alpha. (D) IFN-γ: interferon-γ. The IL-6, IL-1β, TNF-α and IFN-γ were expressed as (pg/mL) in serum.
3.4. Immune Indices
According to Figure 3, we found that PE group increased (p < 0.05) IgY, IgA and IgM levels in serum by 14.06%, 11.74% and 6.82%, respectively, compared with CON group.
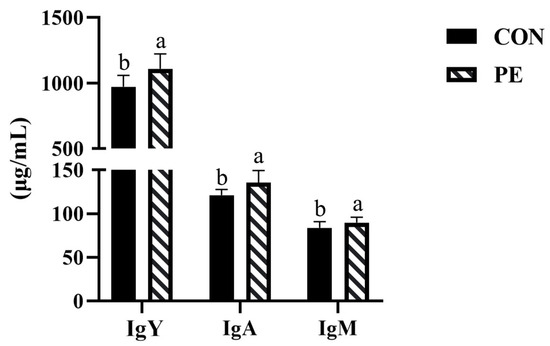
Figure 3.
Effect of PE on serum immune indexes of laying hens. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. Data were presented as mean ± standard deviation (n = 12 per group). Different superscript letters in the bar chart indicate significant differences (p < 0.05). IgA, immunoglobulin A; IgM, immunoglobulin M; IgY, immunoglobulin Y.
3.5. Ovary Index and Follicles Numbers
The results for ovary index and follicle numbers are shown in Figure 4. In comparison to the CON group, the PE group exhibited significant increases (p < 0.05) SWF (7.66%), LWY (20.52%), LYF (55.50%) and total follicles numbers (15.56%), as well as a rise in ovary index (6.28%) (Figure 4A–C,E,G). Nevertheless, the numbers of SYF and grade follicles were not significantly altered by PE supplementation (Figure 4D,F).
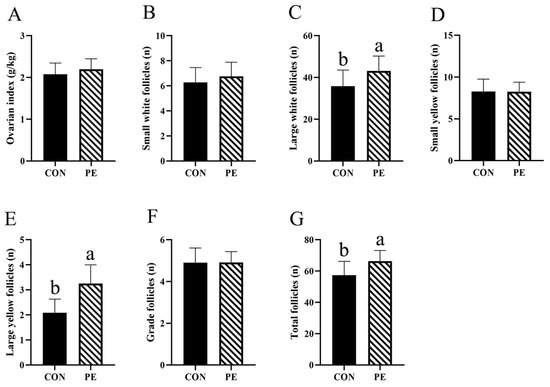
Figure 4.
Effect of PE on the number of follicles. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. The data are expressed as the mean ± standard deviation (n = 12 per group). Statistical significance (p < 0.05) is indicated by different letters in the bar chart. (A) Ovarian index; (B) The number of small white follicles; (C) The number of large white follicles; (D) The number of small yellow follicles; (E) The number of large yellow follicles; (F) The number of grade follicles; (G) The number of grade follicles total follicles.
3.6. Serum Estrogen Levels
The results presented in Figure 5 show that PE supplementation significantly (p < 0.05) increased serum FSH (38.99%), E2 (39.16%) and LH (20.92%) levels (Figure 5A–C).
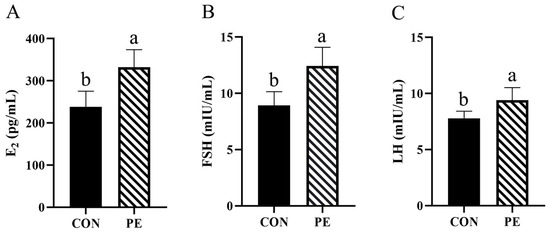
Figure 5.
Effect of PE on serum estrogen levels of laying hens. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. The data are expressed as the mean ± standard deviation (n = 12 per group). Statistical significance (p < 0.05) is indicated by different letters in the bar chart. (A) E2: estradiol; (B) FSH: follicle-stimulating hormone; (C) LH: luteinizing hormone.
3.7. Cecal Microbiota
The Ace and Chao indices are used to measure the community richness of samples, while the Shannon index serves to assess the community diversity. The results indicated that the Ace and Chao indices, which represent α-diversity, were significantly greater (p < 0.05) in the PE group compared to the CON group (Figure 6A,B). There were no apparent alterations (p > 0.05) in the Shannon index (also representative of α-diversity) between the PE and CON groups (Figure 6C). To assess the similarity in microbial community structure (β-diversity) between the PE and CON groups, we performed principal coordinate analysis (PCoA) (Figure 6D). The PCoA results demonstrated a distinct separation of microbial communities between the PE and CON groups. The microbial communities were clearly clustered, allowing for the clear division of the data into two distinct groups (Figure 6D). As demonstrated in Figure 6E, the cecal microbiotas of hens in the PE and CON groups exhibited significant overlap, with the two groups sharing a total of 666 OTUs. The birds from the CON and PE groups exhibited 493 and 490 specific OTUs, respectively (Figure 6E).

Figure 6.
An analysis of microbial diversity in the cecal contents was conducted between the PE and CON groups. The α-diversity comparison between the PE and CON groups was assessed using the Ace index (A), Chao index (B) and Shannon index (C). (D) β-diversity between PE and CON groups display as principal coordinates analysis. (E) Venn diagram of two microbial communities based on ASV. CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. Data are expressed as mean ± standard deviation (n = 4 per group).
We examined the alterations in microbiota composition at the genus level (Table 2). The Bacteroides, Phascolarctobacterium and unclassified_f__Lachnospiraceae accounted for the largest proportion of the microbiota. Compared with CON group, PE group increased (p < 0.05) Bacteroides and Butyricicoccus abundance. Marked upregulation of Phascolarctobacterium, unclassified_f__Lachnospiraceae, Ruminococcus_torques_group, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group, Lactobacillus, Christensenellaceae_R-7_group and Colidextribacter, along with a downregulation in the abundance of Prevotellaceae_UCG-001, Faecalibacterium, Desulfovibrio, Subdoligranulum, Megamonas, norank_f__Muribaculaceae, Romboutsia, Alistipes and norank_f__Prevotellaceae. However, these changes were not statistically significant (p > 0.05).

Table 2.
Effect of PE increment on relative abundance of cecal microbiota species at genus level.
The LEfSe analysis revealed that the taxonomic markers identified in the PE group included Barnesiellaceae (family level), Butyricicoccaceae (family level), Paludibacteraceae (family level), Barnesiella (genus level), Butyricicoccus (genus level), CHKCI001 (genus level), and norank_f__Paludibacteraceae (genus level). In contrast, the taxonomic markers found in the CON group encompassed Bacteroidota (phylum level), Bacteroidia (class level), Bacteroidales (order level), Bacteroidaceae (family level), Enterococcaceae (family level), Bacteroides (genus level), and Enterococcus (genus level) (Figure 7).

Figure 7.
Effect size measurements analysis of cecal microbiota. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. (A) Effect size measurements multi-level species hierarchy tree diagram. (B) Taxonomic biomarkers with linear discriminant analysis (LDA) score (log10) > 4. The LDA score is represented by the length of the histogram.
3.8. Ovary Metabolome
The metabolic patterns of the CON group were clearly separated from those of the PE group based on OPLS-DA and PCA analyses (Figure 8A,B), suggesting that PE supplementation induced notable alterations in ovarian metabolites. The OPLS-DA score plot further demonstrated strong model performance, with values of R2Y = 1 and Q2 = 0.943, confirming the robustness and reliability of the model (Figure 8C).

Figure 8.
Untargeted ovary metabolomics multivariate analysis. Note: CON, control group; PE, plant extracts of Flos lonicerae and Baikal skullcap group. (A) The principal component analysis (PCA) scatter plot (n = 6 per group) illustrates the variation in metabolites between the CON and PE groups. (B) The partial least squares discriminant analysis (PLS-DA) score plot (n = 6 per group) highlights the metabolic distinctions separating the CON and PE groups. (C) The PLS-DA validation plot presents the R2 and Q2 parameters, which serve to confirm the robustness of the model.
Furthermore, as presented in Table 3, a total of 17 metabolites (both upregulated and downregulated) were identified as potential biomarkers, based on the screening criteria of p < 0.01 and FC ≥ 20 or FC ≤ 0.01. Relative to the CON group, the levels of Leu-Enkephalin (L-ENK), palmitoyl serotonin, repaglinide, 2′-Hydroxy-4,4′,6′-trimethoxychalcone, fenaminosulf, lasalocid, 2-methoxyestradiol (2ME), amsacrine, vanillin, nicotinic acid adenine dinucleotide (NAD), carboplatin, enalapril, bosentan, rosuvastatin were markedly elevated in the PE group (p < 0.01). In contrast, the concentrations of urobilin, paxilline, and hexaconazole were significantly reduced in the PE group (p < 0.01).

Table 3.
Differential metabolites between the CON and PE groups.
To explore the potential metabolic pathways, KEGG enrichment analysis was conducted on the metabolites that differed significantly between the CON and PE groups. As illustrated in Figure 9, supplementation with PE was primarily associated with modifications in stilbenoid, diarylheptanoid, and gingerol biosynthesis (p = 0.002), followed by phenylpropanoid biosynthesis (p = 0.002), cutin, suberine and wax biosynthesis (p = 0.002), isoquinoline alkaloid biosynthesis (p = 0.004) and nicotinate and nicotinamide (NAM) metabolism (p = 0.006).

Figure 9.
Analysis of KEGG pathways.
3.9. Spearman Correlation Analysis
Spearman’s correlation analysis was conducted to investigate the potential associations among the significantly altered metabolites, specific gut microbial populations, production traits, cytokine concentrations, as well as antioxidant and immune indices.
As represented in Figure 10A. Urobilin and paxilline were negatively correlated with laying rate, immunoglobulins (particularly IgY and IgM) and ovary antioxidant parameters (particularly GSH-Px and T-SOD) (p < 0.05). Hexaconazole was negatively correlated with average egg weight (p < 0.01) and IL-6 and IFN-γ (p < 0.05). Fenaminosulf, NAD and repaglinide were positively correlated with laying rate (p < 0.01). Bosentan was positively correlated with average egg weight and ADFI (p < 0.01). L-ENK, NAD, amsacrine, repaglinide and rosuvastatin were positively correlated with immunoglobulins and ovary antioxidant parameters (particularly GSH-Px, T-SOD, T-AOC and MDA) (p < 0.05). Enalapril, 2′-Hydroxy-4,4′,6′-trimethoxychalcone and lasalocid were positively correlated with ovary antioxidant parameters (particularly CAT) (p < 0.05).
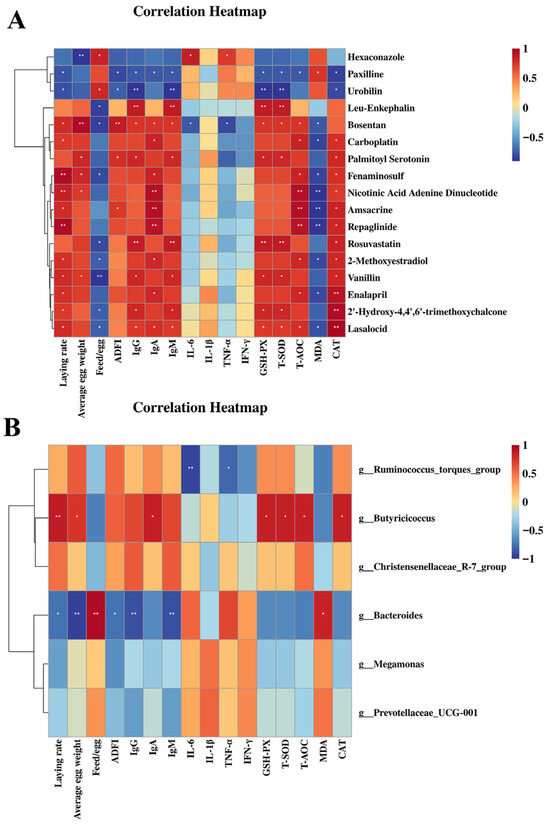
Figure 10.
Correlation analysis of ovary metabolites and gut microbes (at genus level) with immune, antioxidant and performance parameters. Note: positive and negative correlations are shown in red and blue panels (color intensity indicates Spearman’s r-value of the correlation in each panel). * p< 0.05, ** p< 0.01. (A) The correlation of significantly changed ovary metabolites with immunoglobulins, ovary antioxidant parameters and performance parameters. (B) The correlation of gut microbes (at genus level) with immunoglobulins, serum antioxidant parameters and performance parameters.
As shown in Figure 10B, Butyricicoccus (at genus level) showed a significant positive correlation with laying rate (p < 0.01), average egg weight (p < 0.05), IgA (p < 0.05) and serum GSH-Px, T-SOD, T-AOC and CAT (p < 0.05). Bacteroides (at genus level) was negatively correlated with laying rate, ADFI (p < 0.05), average egg weight (p < 0.01), IgY and IgM (p < 0.05), and positively correlated with the feed/egg ratio (p < 0.01) and serum MDA (p < 0.05). Ruminococcus_torques_group showed a significant positive correlation with IL-1β (p < 0.01) and IFN-γ (p < 0.05).
4. Discussion
The results of this experiment indicate that the addition of 500 mg/kg PE to the diet increased the laying rate and feed conversion efficiency of laying hens from 71 to 75 weeks, 76 to 80 weeks and 71 to 80 weeks, which were basically consistent with previous report [8]. Wang et al. [8] reported that dietary supplementation with 1000 mg/kg of PE enhanced egg production in 41-week-old Jinghong laying hens. While the improvement in laying rate has often been attributed to the antioxidant, antibacterial, anti-inflammatory, and antiviral properties of CGA and baicalin [19,20], our metabolomic results provide more specific insights. PE supplementation significantly altered stilbenoid, diarylheptanoid, gingerol, and phenylpropanoid biosynthesis, which generate bioactive metabolites with estrogen-like and anti-inflammatory activities. These metabolites could enhance ovarian sensitivity to estrogen and reduce follicular atresia, thereby supporting sustained egg production in aged hens. Additionally, the enrichment of nicotinate and nicotinamide metabolism may regulate mitochondrial redox balance and energy metabolism, further contributing to improved feed efficiency.
The main active components of Flos lonicerae and Baikal skullcap extract are CGA and baicalin, both possessing phenolic hydroxyl groups that scavenge free radicals and exert strong antioxidant effects [21,22]. Study of Chen et al. [23] revealed that diets supplemented with 0.2%, 0.4% and 0.8% Flos lonicerae increased the GSH-Px activities and decreased the MDA contents in the hepatopancreas of Penaeus monodon. Analogously, Liao et al. [24] confirmed that dietary supplementation with 60, 120, 180 or 240 mg/kg Baikal skullcap increased liver T-SOD and GSH-Px activities in 42 d Arbor Acres male broilers. Consistently, our study found that 500 mg/kg PE supplementation increased serum and ovarian CAT, T-AOC, T-SOD, and GSH-Px levels, while reducing MDA concentrations in the serum and ovary, indicating that PE addition may improve ovarian antioxidant status of aged laying hens.
Immunoglobulins (IgM, IgA, IgY) are key indicators of humoral immunity [25]. In this study, dietary supplementation with 500 mg/kg PE significantly increased serum IgM, IgA, and IgY levels in late-phase laying hens, suggesting enhanced immune function. These results are consistent with Wang et al. [8], who observed a significant increase in serum IgM levels in 41-week-old Jinghong laying hens that were challenged with Salmonella pullorum, following dietary supplementation of 1000 mg/kg PE. Although the improved immunity has been previously attributed to protection of immune cells from oxidative stress [23], our data suggest additional mechanisms. For instance, nicotinate and nicotinamide metabolism may enhance NAD+-dependent signaling pathways that regulate immune cell activation. Moreover, PE supplementation increased the abundance of Butyricicoccus, which was positively correlated with IgA levels and antioxidant enzyme activities. This genus is known to produce short-chain fatty acids (SCFAs) that modulate gut-immune interactions, thereby providing a plausible explanation for the enhanced immune response observed in PE-treated hens.
During the late laying period, excessive fat deposition can induce oxidative stress and stimulate pro-inflammatory cytokine release [26,27,28]. PE supplementation enhanced the antioxidant capacity of laying hens, suggesting more effective ROS scavenging and a potential reduction in pro-inflammatory factors. This effect is consistent with the reported anti-inflammatory properties of CGA and baicalin, which can inhibit the secretion of pro-inflammatory factors, particularly TNF-α, IL-1β and IL-6 [29,30], further supporting the potential immunomodulatory role of PE. Importantly, specific metabolites elevated by PE, such as 2-methoxyestradiol and vanillin, are known to suppress NF-κB–mediated transcription of pro-inflammatory cytokines. Additionally, the enrichment of gingerol biosynthesis, which produces compounds that inhibit IL-1, TNF-α, and IL-8 synthesis [31]. As expected, the levels of TNF-α, IFN-γ, IL-1β and IL-6inthe serum were higher in the CON group than those in the PE group in this study, which were in line with previous study of Ishfaq et al. [32].
The ovary plays a vital role in reproduction, with ovarian index and follicle number positively correlated with laying rate [33,34]. In our study, 500 mg/kg PE supplementation increased ovarian index and follicle numbers (SWF, LWF, LYF). High egg production is linked to reduced follicular atresia [35], whereas oxidative stress accelerates granulosa cell apoptosis and corpus luteum degeneration, leading to follicular loss [36]. In this study, PE supplementation improved antioxidant and immune functions in laying hens, thereby alleviating oxidative stress-induced follicular atresia and increasing the number of different follicular patterns. Estrogen promotes the development of ovarian follicles by helping the granulosa cells to proliferate [37]. During the late laying period of hens, estrogen secretion decreases as ovarian function declines, resulting in an increase in follicular atresia and a decrease in the number of developing follicles [38]. Our findings revealed that PE supplementation significantly enhanced serum E2, FSH and LH levels. This improvement may be attributed to the potential role of PE in enhancing ovarian antioxidant capacity and attenuating the progression of ovarian aging. In this study, the capacity of PE to elevate estrogen levels could potentially account for the enhanced number of different follicular patterns (SWF, LWF and LYF) in the PE group.
The gut microbiota strongly influences nutrient utilization and host immunity [39]. In the current study, dietary supplementation with 500 mg/kg PE increased the α-diversity of the cecal microbial community, as reflected by higher Ace and Chao indices. The possible reason for this phenomenon is that PE may enable certain indigenous bacteria to become dominant bacteria, which in turn optimizes the original microbial community structure, leading to an increase in microbial community diversity. However, the specific mechanism still needs to be investigated in more detail. Notably, the relative abundance of Ruminococcus_torques_group, Butyricicoccus, and Christensenellaceae_R-7_group at the genus level was elevated in the PE group. Both Ruminococcus_torques_group and Butyricicoccus are reported butyrate producers [40,41]. Butyrate contributes to intestinal health by promoting villus growth [42], supporting the balance of gut microbiota through stimulation of beneficial bacteria and inhibition of harmful bacteria [43], providing the majority of energy required for colonic epithelial metabolism, and enhancing intestinal mucin protein secretion [44]. Therefore, an increase in Ruminococcus_torques_group and Butyricicoccus abundances in the PE group meant that PE may play a crucial role in maintaining the normal physiological functions of the intestine. However, it should be noted that the present study did not directly measure butyrate levels in the small intestine, and most butyrate produced in the cecum is utilized locally by colonocytes. Therefore, additional analyses of butyrate levels in the small intestine and intestinal transport would be required to confirm whether cecal butyrate reaches the small intestine and exert systemic or extra-cecal effects. Christensenellaceae_R-7_group, which possess cellulase and hemicellulase genes, enhance ruminants’ ability to degrade polysaccharides and extract energy [45], and are positively correlated with the average daily gain of Yak [46]. Hence, our results suggest that PE may improve feed conversion efficiency and production performance of laying hens, which is consistent with the results of laying rate and feed/egg ratio in this experiment. In this study, correlation analysis showing that Butyricicoccus was positively associated with serum antioxidant and immune parameters as well as laying rate, while Ruminococcus_torques_group and Christensenellaceae_R-7_group displayed positive, though not statistically significant, correlations with these traits. In addition, Ruminococcus_torques_group and Butyricicoccus showed a negative correlation with serum pro-inflammatory factors. Therefore, we propose that the microbiota modified by PE supplementation played a crucial role in enhancing antioxidant and immune functions, maintaining intestinal health, and alleviating inflammatory responses, which ultimately leads to an improvement in the laying rate and feed conversion efficiency of laying hens. LEfSe analysis showed Butyricicoccus (at genus level) dominated in the PE group, consistent with overall microbial abundance results.
Furthermore, PE supplementation reduced the abundance of Bacteroides, Prevotellaceae_UCG-001 and Megamonas at the genus level. Bacteroides, Prevotellaceae_UCG-001 and Megamonas are generally involved in SCFAs production [47,48,49]. SCFAs, serving as an energy source for colonocytes, play a crucial role in modulating immune responses, maintaining intestinal barrier integrity, and regulating systemic metabolic pathways [50]. However, our study showed that Bacteroides, Prevotellaceae_UCG-001 and Megamonas were negatively correlated with serum antioxidant and immune parameters as well as laying rate. Bacteroides, Prevotellaceae_UCG-001 and Megamonas are generally important for host health; however, certain species within these genera can contribute to disease. Bacteroides fragilis is a recognized opportunistic pathogen, promoting chronic inflammation and producing enterotoxins that induce secretory diarrhea and colitis [51,52]. Similarly, Megamonas hypermegale and Megamonas funiformis have been linked to elevated inflammatory biomarkers in individuals with metabolic syndrome [53]. Prevotella is associated with intestinal inflammation and mucosal dysfunction and tends to accumulate in the mucosal tissue of ulcerative colitis patients [54,55]. In our study, PE supplementation reduced the abundance of Bacteroides, Megamonas and Prevotellaceae_UCG-001, suggesting a potential mitigation of inflammatory responses in laying hens. This interpretation was supported by Spearman correlation analysis, which showed positive associations between these genera and serum pro-inflammatory factors.
Metabolomics is a powerful tool for profiling metabolites in biological samples [56]. In this study, ovarian metabolomic analysis showed that multiple metabolites, including L-ENK, Palmitoyl Serotonin, Repaglinide, 2′-Hydroxy-4,4′,6′-trimethoxychalcone, Fenaminosulf, Lasalocid, 2ME, Amsacrine, Vanillin, NAD, Carboplatin, Enalapril, Bosentan and Rosuvastatin were elevated in the PE group compared with the CON group.
Hexaconazole has been reported to disrupt estrogen synthesis by interfering with steroidogenic enzymes and aromatase activity [57,58,59], whereas L-ENK acts as a regulator of female reproduction via the hypothalamic-pituitary-ovarian axis (HPOA) [60]. Ovarian index and oocyte diameter were significantly increased in shrimp (Penaeus indicus) after injection of L-ENK [61]. In addition, by regulating HPOA, L-ENK was observed to stimulate oogonia proliferation in Oreochromis mossambicus [62]. In this study, L-ENK was positively correlated with laying rate, while hexaconazole showed the opposite trend, suggesting that PE may enhance egg production by increasing ovarian L-ENK and reducing ovarian hexaconazole levels. This aligns with previous evidence that baicalin promotes steroid hormone production in granulosa cells of CD-1 mice [63].
2ME exhibits both anti-inflammatory and antioxidant activities, suppressing pro-inflammatory cytokines production and ROS-induced proliferation and migration of vascular smooth muscle cells [64,65]. Similarly, vanillin can neutralize ROS via self-polymerization and reduce inflammatory cytokine expression in mice [66,67]. Enalapril has been shown to eliminate oxidative stress in rodent models [68,69]. Age-related factors such as oxidative stress and mitochondrial activity are profoundly affected by NAD [70]. Many models have suggested that a decline in NAD levels is a hallmark of aging [71]. Our results showed that 2ME, vanillin, enalapril and NAD were positively associated with immune and ovarian antioxidant parameters as well as laying rate, suggesting that PE may improve the laying rate by improving immune and anti-inflammatory functions as well as ovarian antioxidant capacity.
Fenaminosulf, a common fungicide [72], and lasalocid, used against coccidiosis [73], were positively correlated with immune and ovarian antioxidant parameters. Therefore, the increase in fenaminosulf and lasalocid in the PE group suggests PE may exert antibiotic-like effects.
Vanillin is an important flavor ingredient used worldwide [67]. Our results suggest that PE may have a role in improving the aroma of egg yolks. Rosuvastatin lowers cholesterol synthesis in the liver [74]. Our findings suggest that PE may reduce cholesterol levels in egg yolks and plasma, consistent with CGA’s cholesterol-lowering effects in rats [75]. Moreover, as reduced urobilin levels are linked to better liver health [76], our findings suggest that PE might exert hepatoprotective effects, which may ultimately enhance the laying rate.
KEGG analysis showed that PE supplementation enhanced metabolic pathways, primarily stilbenoid, diarylheptanoid and gingerol biosynthesis as well as phenylpropanoid biosynthesis, and secondarily cutin, suberine and wax biosynthesis, isoquinoline alkaloid biosynthesis as well as nicotinate and NAM metabolism.
Stilbenoid, diarylheptanoid and gingerol possess significant medicinal value due to their anticancer, antioxidant and anti-inflammatory properties [31,77,78]. Representative stilbenoid compounds such as resveratrol, pinosylvin, and pinosylvin monomethyl ether exhibit strong antioxidant and anti-inflammatory effects [79], and resveratrol has been shown to alleviate aflatoxin B2-induced oxidative stress in mice [80]. Diarylheptanoids display estrogen-like activity and can activate estrogen receptors in vivo [78], while gingerol, a major active component of ginger, possesses antioxidant capacity and inhibits the synthesis of pro-inflammatory cytokines such as IL-1, TNF-α and IL-8 [81]. In addition, phenylpropanoids are widely applied as antioxidants and anti-inflammatory or antimicrobial agents [82]. In our study, the enrichment of stilbenoid, diarylheptanoid and gingerol biosynthesis as well as phenylpropanoid biosynthesis in the PE group indicated that PE can not only enhance ovarian anti-inflammatory and antioxidant capacity, but also improve estrogen sensitivity, thereby delaying ovarian aging.
Isoquinoline alkaloids are an important class of alkaloids with diverse pharmacological activities such as antibacterial, antiviral, anti-parasitic, anti-inflammatory, antioxidant and anti-ulcer [83]. Ni et al. [84] reported that isoquinoline alkaloid can improve gut health and digestive function in swine and poultry. In our experiment, the isoquinoline alkaloid biosynthesis was enriched by PE supplementation, suggesting that PE may improve intestinal antioxidant, anti-inflammatory, digestive and absorptive functions, thus increasing laying rate and reducing feed/egg ratio in old laying hens. This aligns with Vieira et al. [85], who found that isoquinoline alkaloids supplementation improved growth performance and feed conversion rate in broiler chicks.
NAM is a key regulator of mitochondrial metabolism and redox balance, acting to inhibit protein oxidation and lipid peroxidation [86,87]. Kwak et al. [87] reported that NAM can significantly reduce ROS levels in both senescent cells and cells undergoing senescence, while external NAM supplementation may mitigate ROS-induced cellular damage [88]. Niacin contributes to mitochondrial health by forming NAD and NADP, maintaining mitochondrial integrity and promoting mitochondrial biogenesis. Study by Adebowale et al. [89] demonstrated that niacin supplementation improved the heterophil/lymphocyte ratio and leukocytes in Aviagen turkeys. The enrichment of nicotinate and NAM metabolism due to the PE increment revealed that PE may enhance ovarian antioxidant capacity and have a positive modulation of the immune system.
5. Conclusions
This study provides novel evidence that PE supplementation improves laying performance and ovarian function of aged hens by modulating antioxidant status, inflammatory responses, ovarian metabolites, and cecal microbiota. These findings highlight PE as a promising natural green feed additive with potential to extend the laying cycle of laying hens and enhance sustainability in the poultry industry. Future studies should explore how the identified key metabolites, which target the enhancement of ovarian function, influence the production performance of laying hens during the late laying period, thereby enabling precise modulation of ovarian aging.
Author Contributions
Conceptualization, Y.W. (Yongxia Wang); Methodology, J.L.; Software, J.L.; Validation, J.L.; Formal analysis, X.Y.; Investigation, Y.W. (Yongxia Wang), X.Y. and J.L.; Resources, Y.W. (Yongxia Wang), X.Z., W.Y., Y.L. and Y.W. (Yufang Wang); Data curation, X.Y.; Writing—original draft preparation, X.Y. and J.L.; Writing—Review and Editing, Y.W. (Yongxia Wang), X.Y. and J.L.; Visualization, R.P.; Supervision: Y.W. (Yongxia Wang), X.Z., W.Y., Y.L. and Y.W. (Yufang Wang); Project administration, Y.W. (Yongxia Wang), X.Z. and W.Y. Funding Acquisition, Y.W. (Yongxia Wang) All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Zhejiang Xinmiao Talents Program (2025R412A035); the fourth round of the Team Science and Technology Commissioner Project of Zhejiang Province in 2025.
Institutional Review Board Statement
All animal experiments were conducted in accordance with the Chinese Guidelines for Animal Welfare and approved by the Animal Care and Use Committee of Zhejiang A&F University, identification number: ZAFUAC202490, approval date: 8 March 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PE | Flos lonicerae and Baikal skullcap extracts |
| CGA | Chlorogenic acid |
| CON | Control group |
| ADFI | Average daily feed intake |
| SWF | Small white follicles |
| LWF | Large white follicles |
| SYF | Small yellow follicles |
| LYF | Large white follicles |
| GSH-Px | Glutathione peroxidase |
| T-SOD | Superoxide dismutase |
| CAT | Catalase |
| T-AOC | Total antioxidant capacity |
| MDA | malondialdehyde |
| IgY | Immunoglobulins Y |
| IgA | Immunoglobulins A |
| IgM | Immunoglobulins M |
| ELISA | Enzyme-linked immunosorbent assay |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| E2 | Estradiol |
| FSH | Follicle-stimulating hormone |
| LH | Luteinizing hormone |
| ASV | Amplicon Sequence Varian |
| PCoA | Principal Coordinates Analysis |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| VIP | variable importance in projection |
| FC | Fold change |
| L-ENK | Leu-enkephalin |
| 2ME | 2-methoxyestradiol |
| NAD | Nicotinic acid adenine dinucleotide |
| NAM | Nicotinate and nicotinamide |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| HPOA | Hypothalamic-pituitary-ovarian axis |
Appendix A

Table A1.
Composition and nutrient levels of the control diet (% dry matter).
Table A1.
Composition and nutrient levels of the control diet (% dry matter).
| Items | Content |
|---|---|
| Ingredients | |
| Corn | 60.30 |
| Soybean meal | 24.70 |
| Soybean oil | 1.00 |
| Limestone | 9.50 |
| Dicalcium phosphate | 2.00 |
| Sodium chloride | 0.30 |
| DL-Methionine | 0.20 |
| Premix 1 | 2.00 |
| Total | 100.00 |
| Nutrient levels 2 | |
| Metabolic energy (ME)/(MJ/kg) | 10.88 |
| Crude protein | 15.70 |
| Total phosphorus | 0.66 |
| Available phosphorus | 0.48 |
| Calcium | 4.00 |
| Methionine 3 | 0.42 |
| Methionine + cysteine 3 | 0.72 |
| Lysine 3 | 0.83 |
1 Provided per kg of diet: Vitamin A, 11,000 IU; Vitamin D3, 3000 IU; Vitamin E, 20 mg; Vitamin K3, 3 mg; Vitamin B1, 2.5 mg; Vitamin B2, 8.5 mg; Vitamin B6, 4 mg; Vitamin B12, 0.025 mg; nicotinic acid, 40 mg; D-pantothenate calcium, 14 mg; folic acid, 0.8 mg; D-biotin, 0.125 mg; choline chloride, 500 mg; copper, 8 mg; manganese, 100 mg; iron, 80 mg; zinc, 85 mg; iodine, 0.8 mg; selenium, 0.3 mg. 2 ME was a calculated value, while the others were measured values. 3 The amino acid values were expressed as total.
References
- Guo, Y.; Zhao, Z.H.; Pan, Z.Y.; An, L.L.; Balasubramanian, B.; Liu, W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020, 99, 2100–2107. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Zhang, Y.; Wang, S.; Shao, Y.; Xiong, X.; Hu, X.; Yu, R.; Lan, W.; Cui, Y.; et al. Peppermint extract improves egg production and quality, increases antioxidant capacity, and alters cecal microbiota in late-phase laying hens. Front. Microbiol. 2023, 14, 1252785. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, Y.; Chen, J.; Wu, H.; Huang, L.; Hu, J.; Zhang, Z.; Lu, Y.; Liu, X. Dietary naringin supplementation on laying performance and antioxidant capacity of Three-Yellow breeder hens during the late laying period. Poult. Sci. 2022, 101, 102023. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, H.; Tang, C.; Zhao, Q.; Zhang, J. Dietary supplementation with astaxanthin alleviates ovarian aging in aged laying hens by enhancing antioxidant capacity and increasing reproductive hormones. Poult. Sci. 2023, 102, 102258. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yu, Y.; Wang, X.; Xu, J.; Wang, X.; Feng, Z.; Zhou, Y.; Xiao, H.; Sun, L. Structural Characterization and Anti-Oxidation Activity Evaluation of Pectin from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 998462. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, R. Compound-honeysuckle-induced Drug Eruption with Special Manifestations: A Case Report. World J. Clin. Cases 2022, 10, 8018–8024. [Google Scholar] [CrossRef]
- Chanchal, D.K.; Singh, K.; Bhushan, B.; Chaudhary, J.S.; Kumar, S.; Varma, A.K.; Agnihotri, N.; Garg, A. An updated review of Chinese skullcap (Scutellaria baicalensis): Emphasis on phytochemical constituents and pharmacological attributes. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100326. [Google Scholar] [CrossRef]
- Wang, W.W.; Jia, H.J.; Zhang, H.J.; Wang, J.; Lv, H.Y.; Wu, S.G.; Qi, G.H. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by salmonella pullorum. Front. Microbiol. 2019, 10, 1681. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; Xu, P.; Yin, G. Effects of dietary baicalin supplementation on growth performance, antioxidative status and protection against oxidative stress-induced liver injury in GIFT tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108919. [Google Scholar] [CrossRef]
- Xie, T.; Bai, S.P.; Zhang, K.Y.; Ding, X.M.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Lu, H.Y.; Bai, J.; Xuan, Y.; et al. Effects of Lonicera confusa and Astragali radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019, 98, 4838–4847. [Google Scholar] [CrossRef]
- Liu, Z.P.; Chao, J.R.; Xu, P.T.; Lv, H.Y.; Ding, B.Y.; Zhang, Z.F.; Li, L.L.; Guo, S.S. Lonicera flos and Cnicus japonicus extracts improved egg quality partly by modulating antioxidant status, inflammatory-related cytokines and shell matrix protein expression of oviduct in laying hens. Poult. Sci. 2023, 102, 102561. [Google Scholar] [CrossRef]
- NY/T-33-2004; Feeding Standard of Chicken. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004.
- Hao, E.Y.; Wang, D.H.; Chen, Y.F.; Zhou, R.Y.; Chen, H.; Huang, R.L. The relationship between the mTOR signaling pathway and ovarian aging in peak-phase and late-phase laying hens. Poult. Sci. 2021, 100, 334–347. [Google Scholar] [CrossRef]
- Sun, X.; Chen, X.; Zhao, J.; Ma, C.; Yan, C.; Liswaniso, S.; Xu, R.; Qin, N. Transcriptome comparative analysis of ovarian follicles reveals the key genes and signaling pathways implicated in hen egg production. BMC Genom. 2021, 22, 899. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Fu, Z.; Ma, Z.; Bai, Z. The Photoperiod Significantly Influences the Growth Rate, Digestive Efficiency, Immune Response, and Antioxidant Activities in the Juvenile Scalloped Spiny Lobster (Panulirus homarus). J. Mar. Sci. Eng. 2024, 12, 389. [Google Scholar] [CrossRef]
- Wattrang, E.; Erksson, H.; Albihn, A.; Dalgaard, T.S. Quantification of IgY to Erysipelothrix rhusiopathiae in serum from Swedish laying hens. BMC Vet. Res. 2021, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Krzysica, P.; Verhoog, L.; de Vries, S.; Smits, C.; Savelkoul, H.F.J.; Tijhaar, E. Optimization of Capture ELISAs for Chicken Cytokines Using Commercially Available Antibodies. Animals 2022, 12, 3040. [Google Scholar] [CrossRef] [PubMed]
- Peclaris, G.M.; Pappa, A.; Deligiannis, K.; Koutsotolis, K. Enzyme immunoassays for the determination of ovine LH and FSH. Reprod. Domest. Anim. 2003, 38, 367–372. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Miao, M.S.; Xiang, L.L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–78. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M.A. comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.Z.; Jiang, S.G.; Wu, K.C.; Liu, Y.J.; Tian, L.X.; Zhang, Y.Q.; Niu, J. Dietary supplementation of honeysuckle improves the growth, survival and immunity of Penaeus monodon. Fish Shellfish Immunol. 2013, 35, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.D.; Wen, Q.; Zhang, L.Y.; Lu, L.; Zhang, L.Y.; Luo, X.G. Effect of dietary supplementation with flavonoid from Scutellaria baicalensis Georgi on growth performance, meat quality and antioxidative ability of broilers. J. Integr. Agric. 2018, 17, 1165–1170. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Nankam, P.N.A.; Nguelefack, T.B.; Goedecke, J.H.; Blüher, M. Contribution of adipose tissue oxidative stress to obesity-associated diabetes risk and ethnic differences: Focus on women of African ancestry. Antioxidants 2021, 10, 622. [Google Scholar] [CrossRef]
- Miao, S.S.; Li, Y.; Mu, T.M.; Wang, X.M.; Zhao, W.Y.; Li, R.; Dong, X.; Zou, X. Dietary coated sodium butyrate ameliorates hepatic lipid accumulation and inflammation via enhancing antioxidative function in post-peaking laying hens. Metabolites 2023, 13, 650. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Yan, Y.W.; Li, Q.; Shen, L.; Guo, K.X.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, Q.; Zou, Y.; Liu, Y.; Lan, J. Transcriptome Analysis Reveals the Genetic Basis Underlying the Biosynthesis of Volatile Oil, Gingerols, and Diarylheptanoids in Ginger (Zingiber officinale Rosc.). Bot. Stud. 2017, 58, 41. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhang, W.; Liu, Y.; Wang, J.; Wu, Z.; Shah, S.W.; Li, R.; Miao, Y.; Chen, C.; Li, J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in chicken bursa of fabricius through modulation of autophagy and inhibited inflammation and apoptosis. J. Sci. Food Agric. 2021, 101, 880–890. [Google Scholar] [CrossRef]
- Xu, W.; Ayu, Y.; Wang, J.; Zeng, Q.; Bai, S.; Ding, X.; Lv, L.; Peng, H.; Xuan, Y.; Zhang, K. Effects of dietary theabrownins on production performance, egg quality, and ovarian function of laying hens with different ages. Poult. Sci. 2023, 102, 102545. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Geng, S.; Liu, M.; Zhao, L.; Zhang, J.; Huang, S.; Ma, Q. Effects of different methionine levels in low protein diets on production performance, reproductive system, metabolism, and gut microbiota in laying hens. Front. Nutr. 2021, 8, 739676. [Google Scholar] [CrossRef]
- Brady, K.; Liu, H.C.; Hicks, J.A.; Long, J.A.; Porter, T.E. Transcriptome analysis during follicle development in turkey hens with low and high egg production. Front. Genet. 2021, 12, 619196. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Uyanga, V.A.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Li, H.; Zhou, Y.; Lin, H. Insulin-like growth factor-1 is involved in the deteriorated performance of aged laying hens. J. Anim. Sci. 2022, 100, skac286. [Google Scholar] [CrossRef]
- Colella, M.; Cuomo, D.; Peluso, T.; Falanga, I.; Mallardo, M.; De Felice, M.; Ambrosino, C. Ovarian aging: Role of pituitary-ovarian axis hormones and ncRNAs in regulating ovarian mitochondrial activity. Front. Endocrinol. 2021, 12, 791071. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.F.; Mesalam, N.M.; Elsadek, M.F.; Abdel-Moneim, A.M.E. The impact of dietary supplementation of polysaccharide derived from Polygonatum sibiricum on growth, antioxidant capacity, meat quality, digestive physiology, and gut microbiota in broiler chickens. Poult. Sci. 2024, 103, 103675. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, B.; Lan, F.; Zhong, C.; Jin, J.; Li, X.; Zhou, Q.; Li, J.; Yang, N.; Wen, C.; et al. Host genetics and gut microbiota jointly regulate blood biochemical indicators in chickens. Appl. Microbiol. Biotechnol. 2023, 107, 7601–7620. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Gerunova, L.K.; Gerunov, T.V.; P’yanova, L.G.; Lavrenov, A.V.; Sedanova, A.V.; Delyagina, M.S.; Fedorov, Y.N.; Kornienko, N.V.; Kryuchek, Y.O.; Tarasenko, A.A. Butyric acid and prospects for creation of new medicines based on its derivatives: A literature review. J. Vet. Sci. 2024, 25, e23. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Q.; Li, Y.; Wei, L.; Zhang, Z.; Khan, A.; Khan, M.Z.; Wang, C. Butyrate supplementation improves intestinal health and growth performance in livestock: A Review. Biomolecules 2025, 15, 85. [Google Scholar] [CrossRef]
- Price, C.E.; Valls, R.A.; Ramsey, A.R.; Loeven, N.A.; Jones, J.T.; Barrack, K.E.; Schwartzman, J.D.; Royce, D.B.; Cramer, R.A.; Madan, J.C.; et al. Intestinal Bacteroides modulates inflammation, systemic cytokines, and microbial ecology via propionate in a mouse model of cystic fibrosis. mBio 2024, 15, e03144-23. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Tian, Y.; Li, J.; Luo, Y.; Liu, D.; Zheng, H.; Wang, J.; Dong, Z.; Hu, S.; Huang, L. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, B.; Zuo, Z.; Qi, Y.; Zhao, S.; Zhang, X.; Lan, L.; Shi, Y.; Liu, X.; Li, S.; et al. Effects of two different straw pellets on yak growth performance and ruminal microbiota during cold season. Animals 2023, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Comparative genomics of transport proteins in seven Bacteroides species. PLoS ONE 2018, 13, e0208151. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, D.; Liu, Y.; Yang, X.; Zhang, M.; Wei, F.; Li, D.; Hu, Y.; Guo, Y. Host-genotype-dependent cecal microbes are linked to breast muscle metabolites in Chinese chickens. iScience 2022, 25, 104469. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, X.; Li, D.; Yang, H.; Lin, X.; Zhao, H.; Li, P.; Wang, Y.; Wang, X.; Ge, J. Leonurine regulates hippocampal nerve regeneration in rats with chronic and unpredictable mild stress by activating SHH/GLI signaling pathway and restoring gut microbiota and microbial metabolic homeostasis. Neural Plast. 2023, 2023, 1455634. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: From gut development to neuroprotection. Front. Microbiol. 2024, 15, 1456793. [Google Scholar] [CrossRef]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef]
- Jean, S.; Wallace, M.J.; Dantas, G.; Burnham, C.D. Time for some group therapy: Update on identification, antimicrobial resistance, taxonomy, and clinical significance of the Bacteroides fragilis group. J. Clin. Microbiol. 2022, 60, e0236120. [Google Scholar] [CrossRef]
- Sheng, S.; Yan, S.; Chen, J.; Zhang, Y.; Wang, Y.; Qin, Q.; Li, W.; Li, T.; Huang, M.; Ding, S.; et al. Gut microbiome is associated with metabolic syndrome accompanied by elevated gamma-glutamyl transpeptidase in men. Front. Cell. Infect. Microbiol. 2022, 12, 946757. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Chen, K.Y.; Lee, H.H.; Huang, L.K.; Yang, Y.S.H.; Liu, Y.R.; Hu, C.J. Rifaximin modifies gut microbiota and attenuates inflammation in Parkinson’s disease: Preclinical and clinical studies. Cells 2022, 11, 3468. [Google Scholar] [CrossRef]
- Lucke, K.; Miehlke, S.; Jacobs, E.; Schuppler, M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J. Med. Microbiol. 2006, 55, 617–624. [Google Scholar] [CrossRef]
- Resurreccion, E.P.; Fong, K.W. The Integration of Metabolomics with Other Omics: Insights into Understanding Prostate Cancer. Metabolites 2022, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Alquraini, A. Potency of Hexaconazole to Disrupt Endocrine Function with Sex Hormone-Binding Globulin. Int. J. Mol. Sci. 2023, 24, 3882. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.A.H.; Alzahrani, A.; Alghamdi, S.; Alquraini, A.; Alghamdi, A. Hexaconazole exposure ravages biosynthesis pathway of steroid hormones: Revealed by molecular dynamics and interaction. Toxicol. Res. 2021, 11, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Pang, J.; Sun, D.; Zhang, Q. Stereoselective toxicokinetic and distribution study on the hexaconazole enantiomers in mice. Toxics 2023, 11, 145. [Google Scholar] [CrossRef]
- Ganeyan, A.; Ganesh, C.B. The opioid peptide leucine-enkephalin disrupts seasonal and gonadotropin-induced ovarian recrudescence in the gecko Hemidactylus frenatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 283, 111454. [Google Scholar] [CrossRef]
- Reddy, P.S. Involvement of opioid peptides in the regulation of reproduction in the prawn Penaeus indicus. Naturwissenschaften 2000, 87, 535–538. [Google Scholar] [CrossRef]
- Vijayalaxmi; Ganesh, C.B. Influence of leucine-enkephalin on pituitary-ovary axis of the cichlid fish Oreochromis mossambicus. Fish Physiol. Biochem. 2017, 43, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; He, J.; Bai, Y.; He, Q.; Zhang, T.; Zhang, J.; Yang, G.; Xu, Z.; Hu, J.; Yao, G. Baicalin improves the functions of granulosa cells and the ovary in aged mice through the mTOR signaling pathway. J. Ovarian Res. 2022, 15, 34. [Google Scholar] [CrossRef]
- Liao, W.I.; Wu, S.Y.; Tsai, S.H.; Pao, H.P.; Huang, K.L.; Chu, S.J. 2-Methoxyestradiol protects against lung ischemia/reperfusion injury by upregulating annexin A1 protein expression. Front. Immunol. 2021, 12, 596376. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.; Mueck, A.O.; Lippert, T.H. Effect of estradiol metabolites on the susceptibility of low density lipoprotein to oxidation. Life Sci. 1997, 61, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.; de Sousa, D.P. Overview of the role of vanillin on redox status and cancer development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef]
- Tian, N.; Thrasher, K.D.; Gundy, P.D.; Hughson, M.D.; Manning, R.D., Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension 2005, 45, 934–939. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ali, T.M.; Abdel Gaid, M.A.; Elberry, A.A. Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, p53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. PLoS ONE 2019, 14, e0214349. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Zhong, H.; Chen, J. Germicide fenaminosulf promotes gall formation of Zizania latifolia without directly affecting the growth of endophytic fungus Ustilago esculenta. BMC Plant Biol. 2022, 22, 418. [Google Scholar] [CrossRef]
- Mahtal, N.; Wu, Y.; Cintrat, J.C.; Barbier, J.; Lemichez, E.; Gillet, D. Revisiting old ionophore lasalocid as a novel inhibitor of multiple toxins. Toxins 2020, 12, 26. [Google Scholar] [CrossRef]
- Hanke, N.; Gómez-Mantilla, J.D.; Ishiguro, N.; Stopfer, P.; Nock, V. Physiologically based pharmacokinetic modeling of rosuvastatin to predict transporter-mediated drug-drug interactions. Pharm. Res. 2021, 38, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Bates, E.A.; Kipp, Z.A.; Martinez, G.J.; Badmus, O.O.; Soundarapandian, M.M.; Foster, D.; Xu, M.; Creeden, J.F.; Greer, J.R.; Morris, A.J.; et al. Suppressing hepatic UGT1A1 increases plasma bilirubin, lowers plasma urobilin, reorganizes kinase signaling pathways and lipid species and improves fatty liver disease. Biomolecules 2023, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, G.; Hasan, R.; Medina-Bolivar, F. Antioxidant Assessment of Prenylated Stilbenoid-Rich Extracts from Elicited Hairy Root Cultures of Three Cultivars of Peanut (Arachis hypogaea). Molecules 2021, 26, 6778. [Google Scholar] [CrossRef] [PubMed]
- Winuthayanon, W.; Piyachaturawat, P.; Suksamrarn, A.; Ponglikitmongkol, M.; Arao, Y.; Hewitt, S.C.; Korach, K.S. Diarylheptanoid Phytoestrogens Isolated from the Medicinal Plant Curcuma comosa: Biologic Actions in Vitro and in Vivo Indicate Estrogen Receptor-Dependent Mechanisms. Environ. Health Perspect. 2009, 117, 1155–1161. [Google Scholar] [CrossRef]
- Aalto, A.L.; Saadabadi, A.; Lindholm, F.; Kietz, C.; Himmelroos, E.; Marimuthu, P.; Salo-Ahen, O.M.H.; Eklund, P.; Meinander, A. Stilbenoid Compounds Inhibit NF-κB-Mediated Inflammatory Responses in the Drosophila Intestine. Front. Immunol. 2023, 14, 1253805. [Google Scholar] [CrossRef]
- Gündüz, A.; Yalçın, E.; Çavuşoğlu, K. Combined toxic effects of aflatoxin B2 and the protective role of resveratrol in Swiss albino mice. Sci. Rep. 2021, 11, 18081. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013, 4 (Suppl. S1), S36–S42. [Google Scholar]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef]
- Shang, X.F.; Yang, C.J.; Morris-Natschke, S.L.; Li, J.C.; Yin, X.D.; Liu, Y.Q.; Guo, X.; Peng, J.W.; Goto, M.; Zhang, J.Y.; et al. Biologically active isoquinoline alkaloids covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef]
- Ni, H.; Martínez, Y.; Guan, G.; Rodríguez, R.; Más, D.; Peng, H.; Valdivié Navarro, M.; Liu, G. Analysis of the Impact of Isoquinoline Alkaloids, Derived from Macleaya cordata Extract, on the Development and Innate Immune Response in Swine and Poultry. Biomed. Res. Int. 2016, 2016, 1352146. [Google Scholar] [CrossRef]
- Vieira, S.L.; Oyarzabal, O.A.; Freitas, D.M.; Berres, J.; Peña, J.E.M.; Torres, C.A. Performance of Broilers Fed Diets Supplemented with Sanguinarine-Like Alkaloids and Organic Acids. J. Appl. Poult. Res. 2008, 17, 128–133. [Google Scholar] [CrossRef]
- Jung, M.; Lee, K.M.; Im, Y.; Seok, S.H.; Chung, H.; Kim, D.Y.; Han, D.; Lee, C.H.; Hwang, E.H.; Park, S.Y.; et al. Nicotinamide (niacin) supplement increases lipid metabolism and ROS-induced energy disruption in triple-negative breast cancer: Potential for drug repositioning as an anti-tumor agent. Mol. Oncol. 2022, 16, 1795–1815. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Ham, H.J.; Kim, C.M.; Hwang, E.S. Nicotinamide exerts antioxidative effects on senescent cells. Mol. Cells 2015, 38, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Byun, K.A.; Oh, S.; Kim, H.M.; Chung, M.S.; Son, K.H.; Byun, K. The Combination of Niacinamide, Vitamin C, and PDRN Mitigates Melanogenesis by Modulating Nicotinamide Nucleotide Transhydrogenase. Molecules 2022, 27, 4923. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, T.; Oso, A.; Liu, H.; Tossou, M.; Chen, J.; Li, H.; Kang, B.; Yao, K. Effect of Dietary Niacin Supplementation on Growth Performance, Nutrient Digestibility, Hematology, and Lipoprotein Concentrations of Young Turkeys, Meleagris gallopavo. J. Poult. Sci. 2019, 56, 112–119. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).