Revealing Phenotypic Differentiation in Ochetobius elongatus from the Middle Yangtze River Through Geometric Morphometrics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
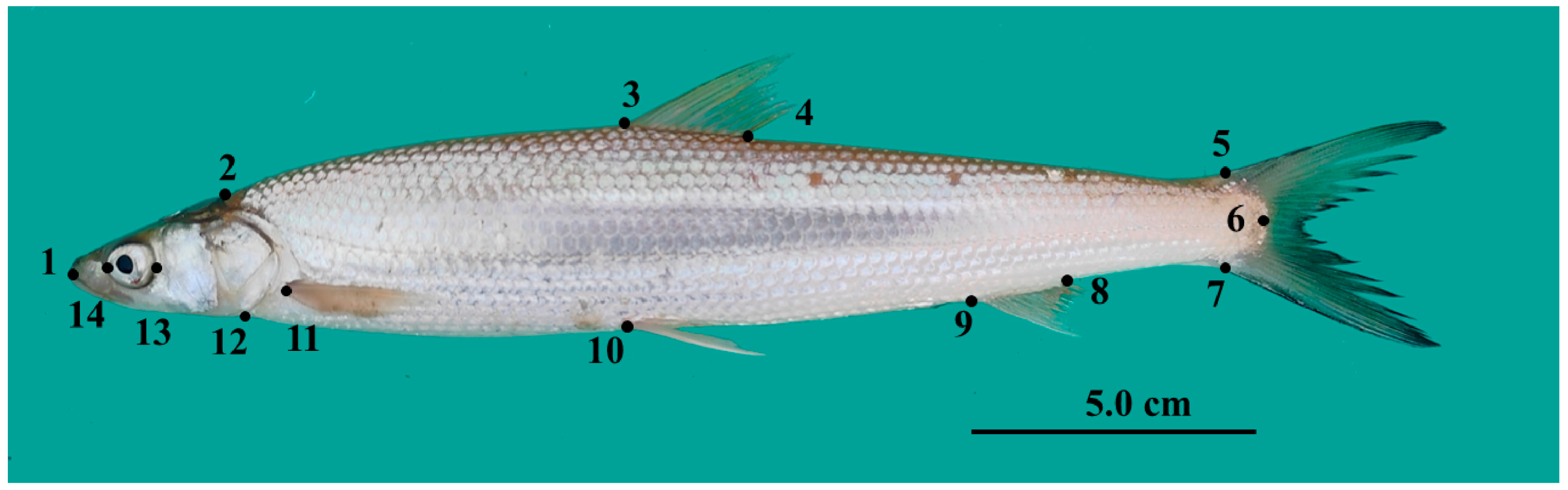
2.2. Data Collecting and Processing
2.3. Geometric Morphometric Analysis
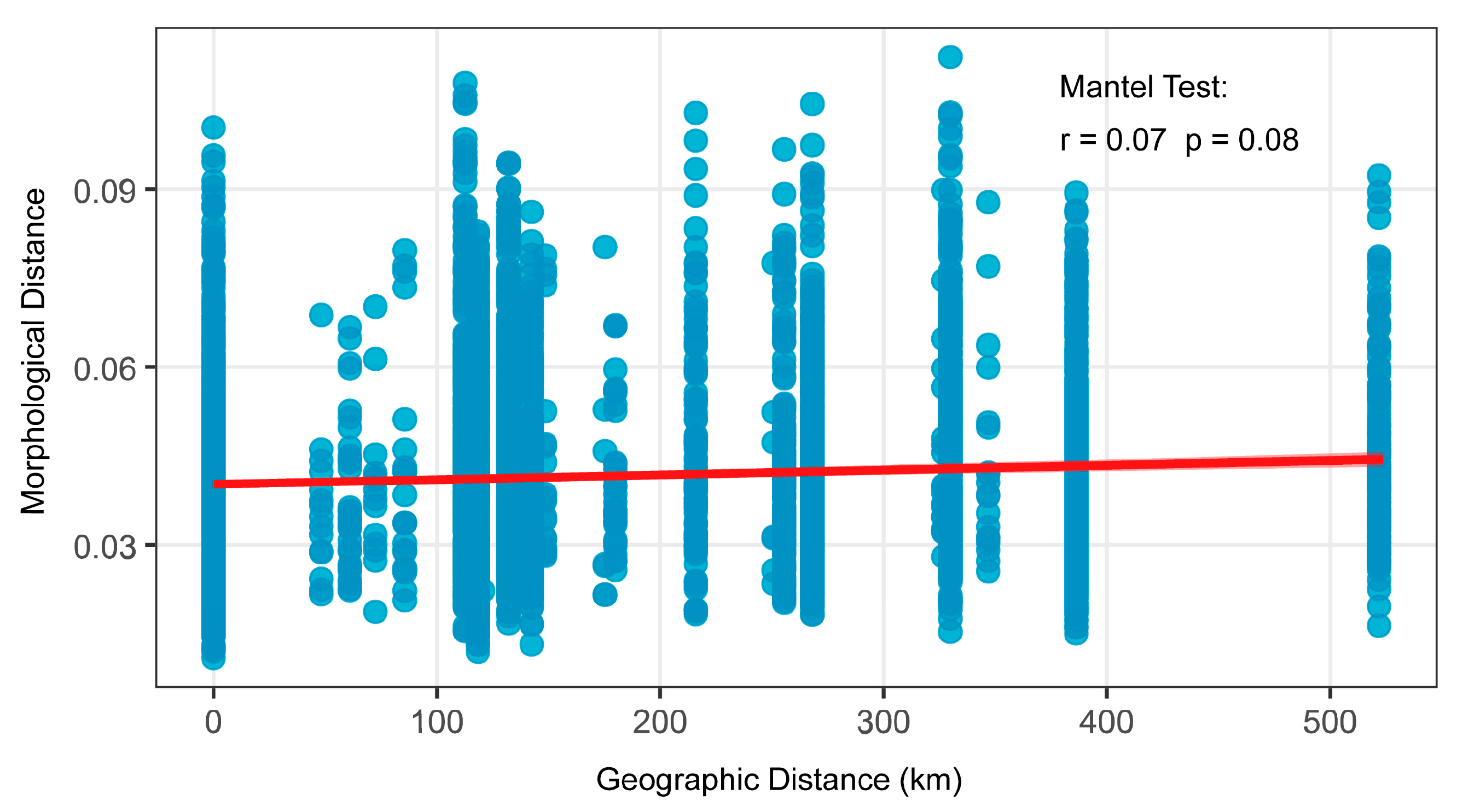
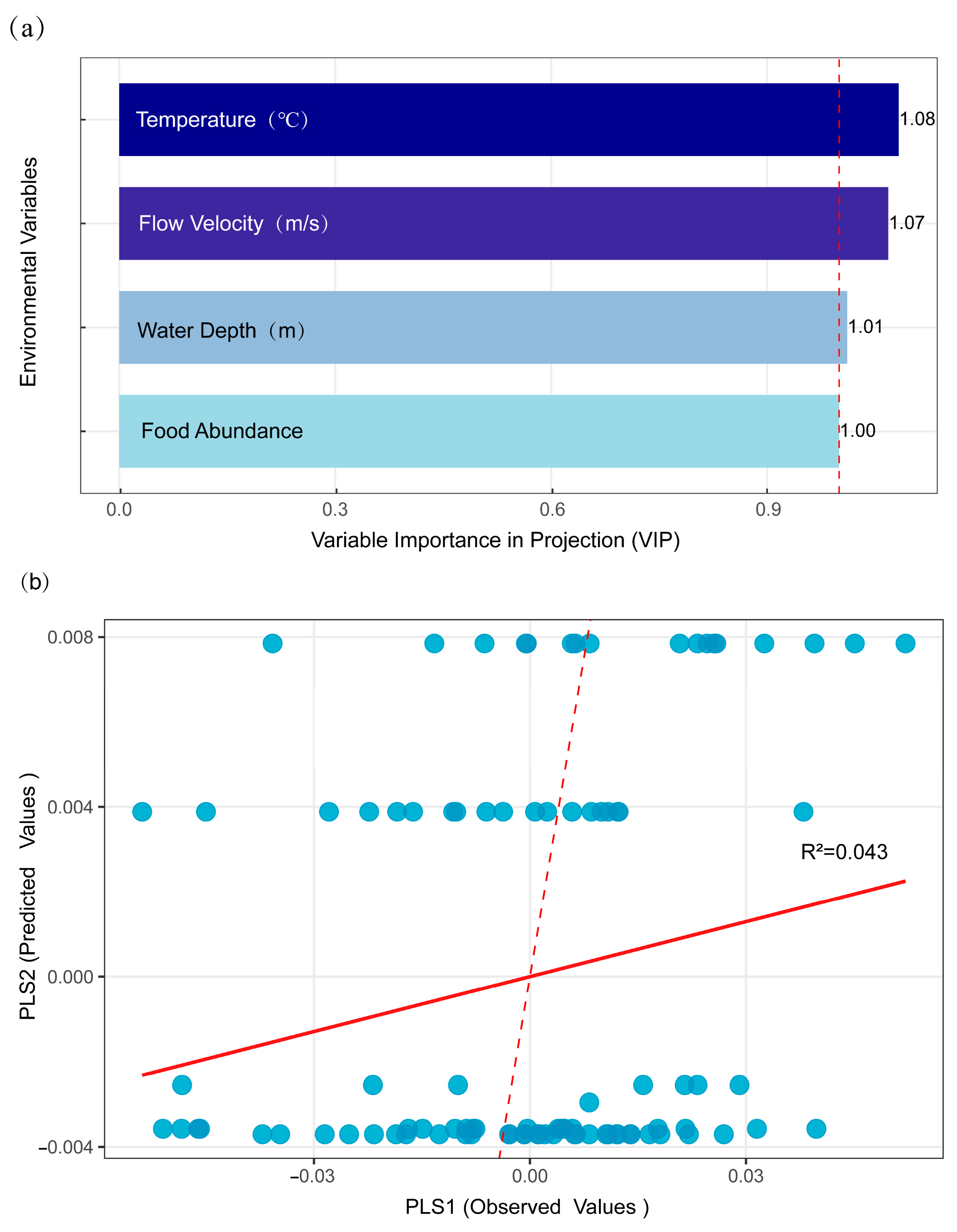
2.4. Correlation of Morphology with Geographical Distance and Environmental Factors
3. Results
3.1. Data Reliability Validation
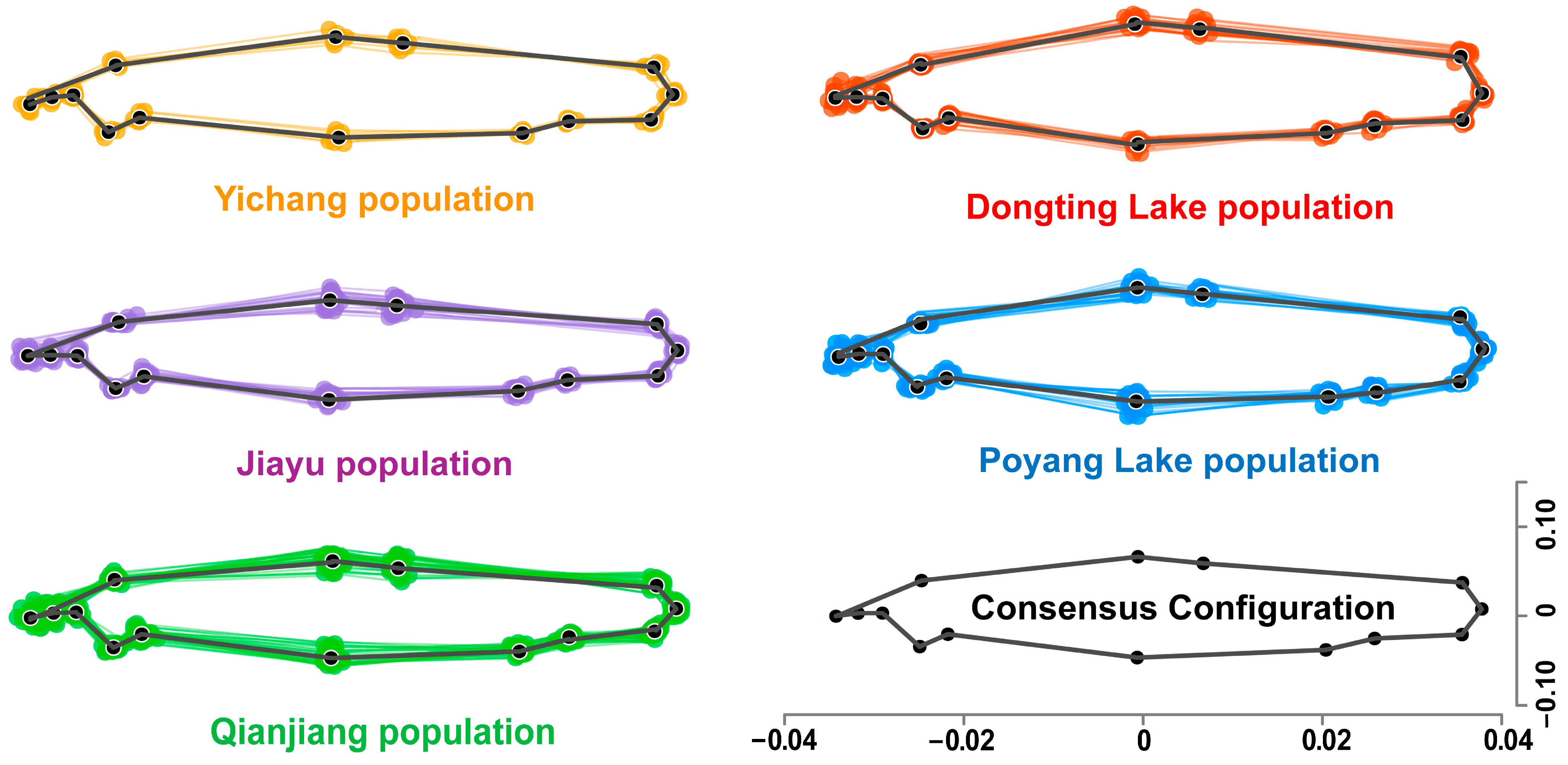
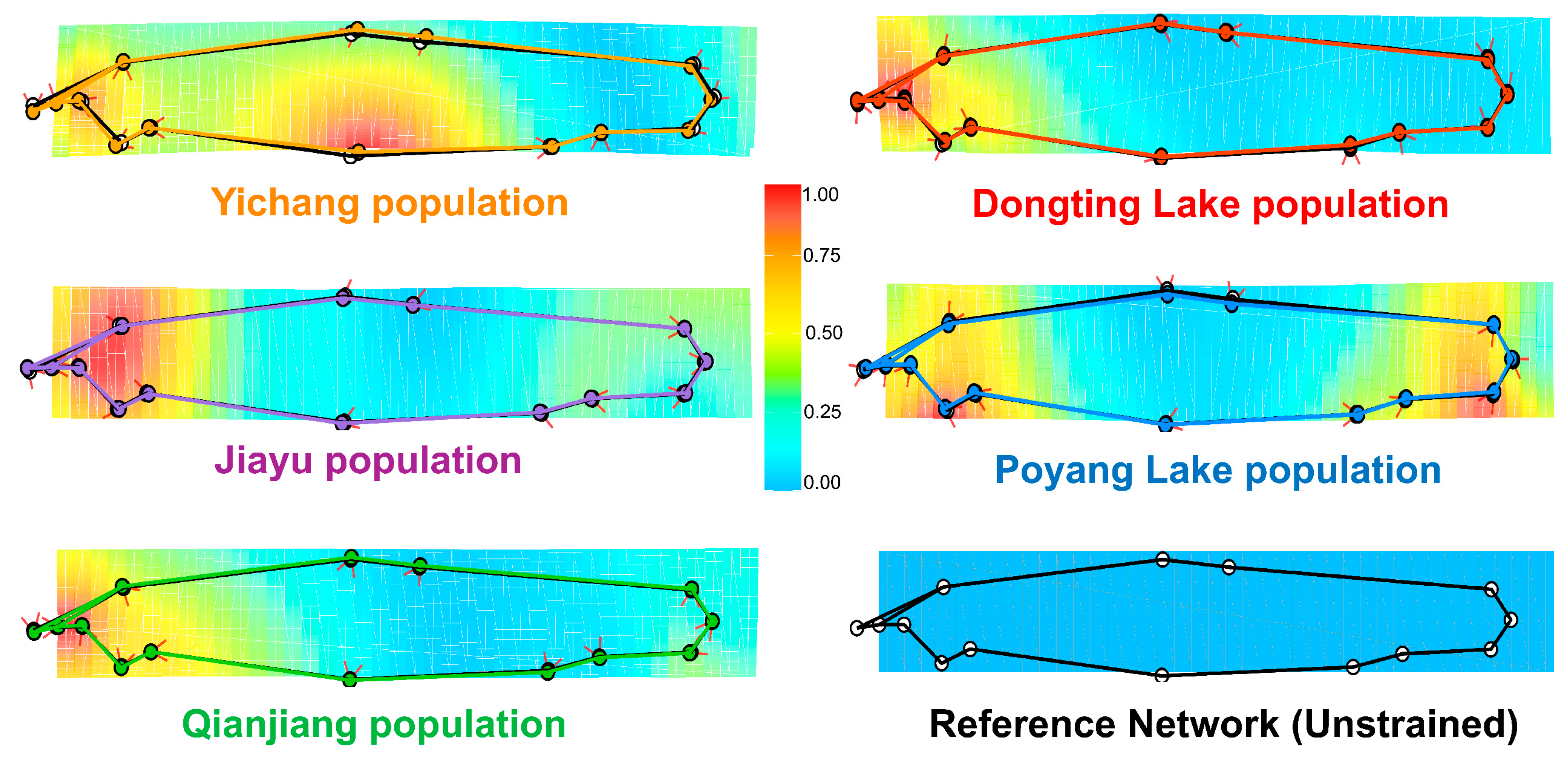
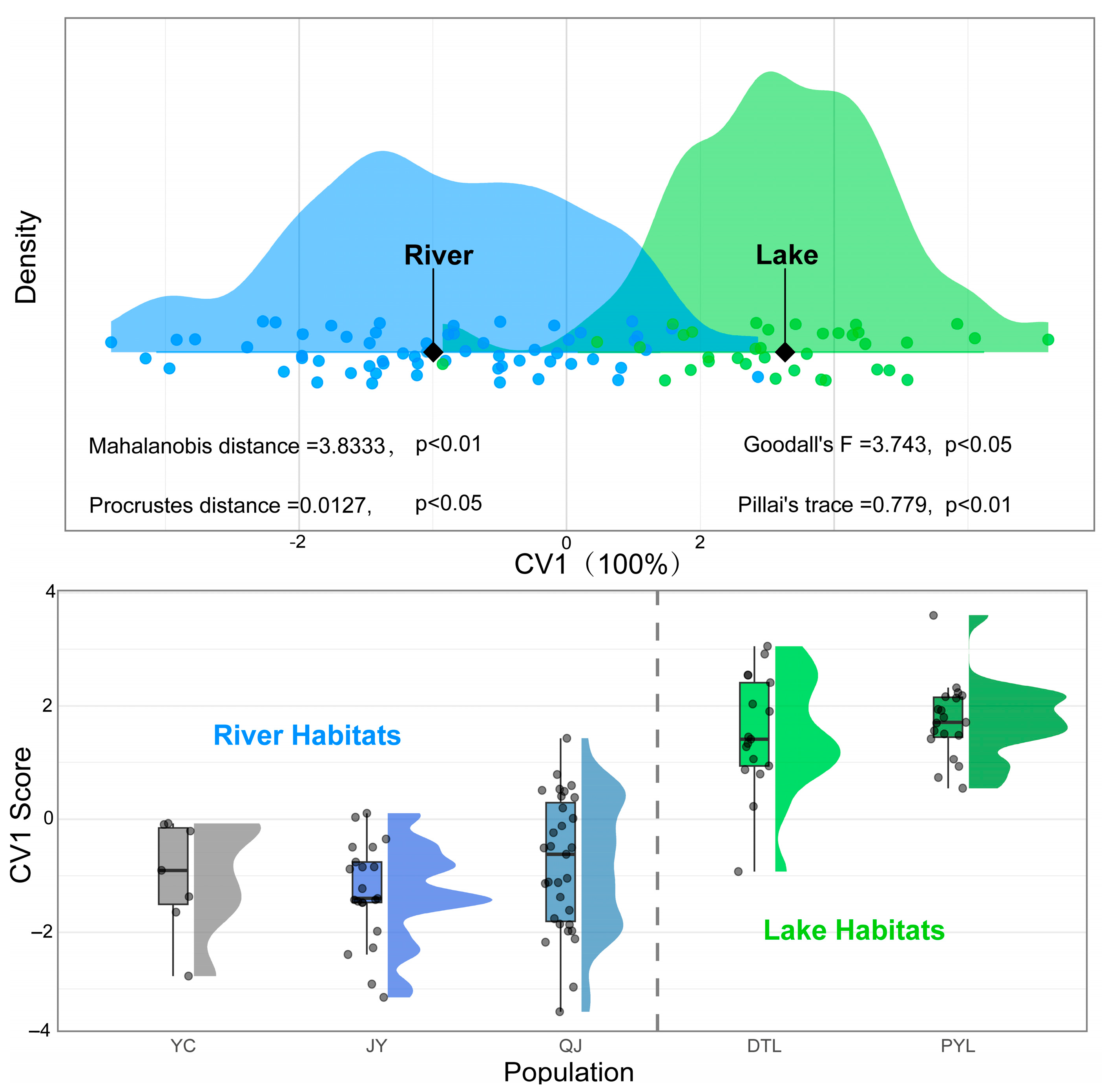
3.2. Geometric Morphometric Differentiation Among Populations
3.3. Influence of Geographic and Environmental Variables on Morphological Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langerhans, R.B. Predictability of phenotypic differentiation across flow regimes in fishes. Integr. Comp. Biol. 2008, 48, 750–768. [Google Scholar] [CrossRef]
- Gómez, J.M.; González-Megías, A.; Armas, C.; Narbona, E.; Navarro, L.; Perfectti, F. The role of phenotypic plasticity in shaping ecological networks. Ecol. Lett. 2023, 26, S47–S61. [Google Scholar] [CrossRef]
- Shuai, F.; Yu, S.; Lek, S.; Li, X. Habitat effects on intra-species variation in functional morphology: Evidence from freshwater fish. Ecol. Evol. 2018, 8, 10902–10913. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; Johns Hopkins University Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Imre, I.; McLaughlin, R.L.; Noakes, D.L.G. Phenotypic plasticity in brook charr: Changes in caudal fin induced by water flow. J. Fish Biol. 2005, 61, 1171–1181. [Google Scholar] [CrossRef]
- Rovito, S.M. Lineage Divergence and Species Formation in Plethodontid Salamanders. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2009. [Google Scholar]
- Langerhans, R.B.; Layman, C.A.; Langerhans, A.K.; DeWitt, T.J. Habitat-associated morphological divergence in two Neotropical fish species. Biol. J. Linn. Soc. 2003, 80, 689–698. [Google Scholar] [CrossRef]
- Franssen, N.R.; Stewart, L.K.; Schaefer, J.F. Morphological divergence and flow-induced phenotypic plasticity in a native fish from anthropogenically altered stream habitats. Ecol. Evol. 2013, 3, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Hincapié-Cruz, J.; Márquez, E. Variación fenotípica de los peces Curimata mivartii (Characiformes: Curimatidae) y Pimelodus grosskopfii (Siluriformes: Pimelodidae) en hábitats lóticos y lénticos. Rev. Biol. Trop. 2021, 69, 434–444. [Google Scholar] [CrossRef]
- Kristjánsson, B.K.; Leblanc, C.A.-L.; Skúlason, S.; Snorrason, S.S.; Noakes, D.L.G. Phenotypic plasticity in the morphology of small benthic Icelandic Arctic charr (Salvelinus alpinus). Ecol. Freshw. Fish 2018, 27, 636–645. [Google Scholar] [CrossRef]
- Hernandez, J.; Villalobos-Leiva, A.; Bermúdez, A.; Ahumada, C.D.; Suazo, M.J.; Correa, M.; Díaz, A.; Benítez, H.A. Ecomorphology and Morphological Disparity of Caquetaia kraussii (Perciformes: Cichlidae) in Colombia. Animals 2022, 12, 3438. [Google Scholar] [CrossRef]
- Wang, Z.T.; Duan, P.X.; Akamatsu, T.; Chen, Y.W.; An, X.; Yuan, J.; Lei, P.Y.; Li, J.; Zhou, L.; Liu, M.C.; et al. Riverside underwater noise pollution threaten porpoises and fish along the middle and lower reaches of the Yangtze River, China. Ecotoxicol. Environ. Saf. 2021, 226, 112860. [Google Scholar] [CrossRef]
- Du, J.; Tian, H.W.; Xiang, Z.Y.; Zhao, K.S.; Yu, L.Y.; Duan, X.B.; Chen, D.Q.; Xu, J.; Liu, M.D. Impact of the fishing ban on fish diversity and population structure in the middle reaches of the Yangtze River, China. Front. Environ. Sci. 2025, 12, 1530716. [Google Scholar] [CrossRef]
- Cui, L.J.; Gao, C.J.; Zhao, X.S.; Ma, Q.F.; Zhang, M.Y.; Li, W.; Song, H.T.; Wang, Y.F.; Li, S.G.; Zhang, Y. Dynamics of the lakes in the middle and lower reaches of the Yangtze River basin, China, since late nineteenth century. Environ. Monit. Assess. 2013, 185, 4005–4018. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Hydrological Characteristics of the Yangtze River. In Evolution and Water Resources Utilization of the Yangtze River; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Chen, Y.Y. Fauna Sinica, Osteichthyes Cypriniformes II; Science Press: Beijing, China, 1998; pp. 106–107. [Google Scholar]
- Chen, W.T.; Duan, X.B.; Gao, L.; Li, X.H.; Yang, J.P.; Wang, D.Q. Genetic structure analysis of Ochetobius elongatus between Yangtze River and Pearl River using multiple loci. South China Fish. Sci. 2022, 18, 19–25. [Google Scholar] [CrossRef]
- Yang, J.P.; Li, C.; Chen, W.T.; Li, Y.F.; Li, X.H. Genetic diversity and population demographic history of Ochetobius elongatus in the middle and lower reaches of the Xijiang River. Biodiv. Sci. 2018, 26, 1289–1295. [Google Scholar] [CrossRef]
- Yang, J.P.; Li, Y.F.; Zhu, S.L.; Chen, W.T.; Li, J.; Xue, H.M.; Li, X.H. Development and characterization of 26 SNP markers in Ochetobius elongatus based on restriction site-associated DNA sequencing (RAD-seq). Conserv. Genet. Resour. 2020, 12, 53–55. [Google Scholar] [CrossRef]
- Li, L.K.; Liang, X.G.; Zhang, J.T.; Xu, Q.; Wang, L.; Gao, X.P.; Song, X.C.; Zhang, B.; Huang, D.; Wang, H.; et al. A chromosome-level genome assembly of critically endangered Ochetobius elongatus. Sci. Data. 2024, 11, 1399. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Jiang, J.P.; Wang, Y.Z.; Zhang, E.; Zhang, Y.Y.; Li, L.L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.M.; et al. Red List of China’s vertebrates. Biodiv. Sci. 2016, 24, 500–551. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment; Chinese Academy of Sciences. Announcement on Releasing the China Biodiversity Red List: Volume of Vertebrates (2020) and China Biodiversity Red List: Volume of Higher Plants (2020); Ministry of Ecology and Environment; Chinese Academy of Sciences: Beijing, China, 2023. Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202305/t20230522_1030745.html (accessed on 20 June 2025).
- Zhai, D.D.; Wang, D.; He, T.Q.; Cai, F.T.; Duan, X.B.; Hu, X.K.; Zhu, B.; Xie, Z.G.; Xia, M.; Liu, H.Y.; et al. Genetic diversity and population historical dynamics of Ochetobius elongatus in the middle reaches of the Yangtze River. Acta Hydrobiol. Sin. 2024, 48, 1736–1744. [Google Scholar] [CrossRef]
- Baken, E.K.; Collyer, M.L.; Kaliontzopoulou, A.; Adams, D.C. geomorph v4.0 and gmShiny: Enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol. Evol. 2021, 12, 2355–2363. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar] [CrossRef]
- Adams, D.C.; Otárola-Castillo, E. Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J. Ecological character displacement in Plethodon: Biomechanical differences found from a geometric morphometric study. Proc. Natl. Acad. Sci. USA 2000, 97, 4106–4111. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.W. Body form, locomotion and foraging in aquatic vertebrates. Amer. Zool. 1984, 24, 107–120. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Schaefer, K. Thirty years of geometric morphometrics: Achievements, challenges, and the ongoing quest for biological meaningfulness. Am. J. Biol. Anthropol. 2022, 178, 181–210. [Google Scholar] [CrossRef]
- Bookstein, F.L. Thin-plate splines and the atlas problem for biomedical images. In Information Processing in Medical Imaging; Colchester, A.C.F., Hawkes, D.J., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 1991; Volume 511, pp. 326–342. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, F.Q. Geometric morphometrics and its application in fish ecology: A review. J. Shanghai Ocean Univ. 2022, 31, 1180–1189. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y.A. Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Luo, D. Quantitative Analysis of Fish Morphology Through Landmark and Outline-based Geometric Morphometrics with Free Software. Bio. Protoc. 2024, 14, e5087. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Amoutchi, A.I.; Ugbor, O.N.; Kouamelan, E.P.; Mehner, T. Morphological variation of African snakehead (Parachanna obscura) populations along climate and habitat gradients in Côte d’Ivoire, West Africa. Environ. Biol. Fishes. 2023, 106, 1233–1246. [Google Scholar] [CrossRef]
- Andersson, J. Effects of diet- induced resource polymorphism on performance in arctic charr (Salvelinus alpinus). Evol. Ecol. Res. 2003, 5, 213–228. [Google Scholar]
- Andersson, J.; Bystrom, P.; Persson, L.; De Roos, A.M. Plastic resource polymorphism: Effects of resource availability on Arctic char (Salvelinus alpinus) morphology. Biol. J. Linn. Soc. 2005, 85, 341–351. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.Q.; Gu, Z.M.; Peng, S.M.; Li, Y.K. Analysis of Morphological Variations among Seven Populations of Erythroculter ilishaeformis. Fresh. Fish. 2007, 37, 40–44. [Google Scholar]
- Winans, G.A. Multivariate Morphometric Variability in Pacific Salmon: Technical Demonstration. Can. J. Fish. Aquat. Sci. 1984, 41, 1150–1159. [Google Scholar] [CrossRef]
- Hjelm, J.; Svanbäck, R.; Byström, P.; Persson, L.; Wahlström, E. Diet-dependent body morphology and ontogenetic reaction norms in Eurasian perch. Oikos 2001, 95, 311–323. [Google Scholar] [CrossRef]
- Li, Y.; Feng, G.P.; Zhang, T.; Zhuang, P.; Song, C.; Wang, H.H.; Zhang, Y.P.; Chen, J.H. Morphological Characteristics and Cluster Analysis of Coilia nasus Populations from Six Different Waters. J. Hydroecol. 2022, 43, 116–123. [Google Scholar] [CrossRef]
- Wang, H.K.Y.; Tang, Y.T.; Li, G.G.; Zhang, R.Y.; Feng, C.G.; Tong, C.; Liu, S.J.; Zhang, C.F.; Tian, F.; Zhao, K. Geometric Morphometrics of the Cephalic Contour and Its Morphological Variation in Schizopygopsis stoliczkanii (Teleostei: Cyprinidae). Acta Hydrobiol. Sin. 2017, 41, 182–193. [Google Scholar] [CrossRef]
- Sultan, S.E.; Spencer, H.G. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 2002, 160, 271–283. [Google Scholar] [CrossRef]
- Hetzel, C.; Forsythe, P. Phenotypic plasticity of a generalist fish species resident to lotic environments: Insights from the Great Lakes region. Ecol. Evol. 2023, 13, e10715. [Google Scholar] [CrossRef]


| Centroid Size: | |||||||
|---|---|---|---|---|---|---|---|
| Effect | SS | MS | df | F | p (param.) | ||
| Individual | 866,562.758 | 216,640.690 | 4 | 27.43 | <0.01 | ||
| Residual | 710,864.676 | 7898.496 | 90 | ||||
| Shape, Procrustes ANOVA: | |||||||
| Effect | SS | MS | df | F | p (param.) | Pillai tr. | p (param.) |
| Individual | 0.012400 | 0.000129 | 96 | 3.45 | <0.01 | 2.36 | <0.01 |
| Residual | 0.080980 | 0.000038 | 2160 | ||||
| Classification Method | Group | Correct Size | Misclassified Size | Accuracy | Overall Accuracy |
|---|---|---|---|---|---|
| Original Classification | River | 57 | 2 | 96.6% | 95.8% |
| Lake | 34 | 2 | 94.4% | ||
| Cross-Validation | River | 54 | 5 | 91.5% | 91.6% |
| Lake | 33 | 3 | 91.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Qi, Z.; Hu, Z.; Zhai, D.; Chen, Y.; Xiong, F.; Liu, H. Revealing Phenotypic Differentiation in Ochetobius elongatus from the Middle Yangtze River Through Geometric Morphometrics. Animals 2025, 15, 2870. https://doi.org/10.3390/ani15192870
Cai F, Qi Z, Hu Z, Zhai D, Chen Y, Xiong F, Liu H. Revealing Phenotypic Differentiation in Ochetobius elongatus from the Middle Yangtze River Through Geometric Morphometrics. Animals. 2025; 15(19):2870. https://doi.org/10.3390/ani15192870
Chicago/Turabian StyleCai, Fangtao, Zhiyuan Qi, Ziheng Hu, Dongdong Zhai, Yuanyuan Chen, Fei Xiong, and Hongyan Liu. 2025. "Revealing Phenotypic Differentiation in Ochetobius elongatus from the Middle Yangtze River Through Geometric Morphometrics" Animals 15, no. 19: 2870. https://doi.org/10.3390/ani15192870
APA StyleCai, F., Qi, Z., Hu, Z., Zhai, D., Chen, Y., Xiong, F., & Liu, H. (2025). Revealing Phenotypic Differentiation in Ochetobius elongatus from the Middle Yangtze River Through Geometric Morphometrics. Animals, 15(19), 2870. https://doi.org/10.3390/ani15192870





