Molecular Detection of Pythium insidiosum in Cutaneous Lesions of Horses from Northeastern Brazil
Simple Summary
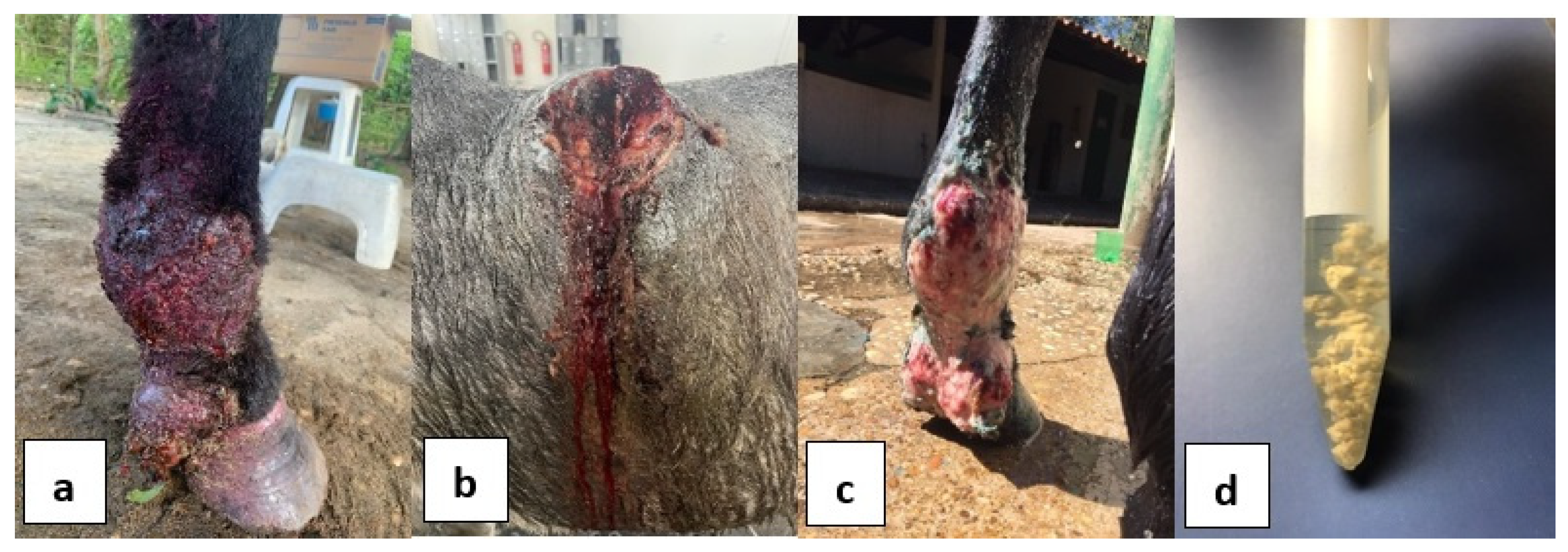
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Huang, X.; Yee, N.H.; Meng, H.; Jiang, L.; Liang, L.; Chen, X. Pythium insidiosum: An emerging pathogen that is easily misdiagnosed and given treatment as a fungus. Front. Cell. Infect. Microbiol. 2024, 14, 1430032. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Global distribution and clinical features of pythiosis in humans and animals. J. Fungi 2022, 8, 182. [Google Scholar] [CrossRef]
- Sae-Chew, P.; Rujirawat, T.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Yurayart, N.; Yurayart, C.; Krajaejun, T. Generation of protoplasts provides a powerful experimental research tool for biological and pathogenicity studies of Pythium insidiosum. J. Mycol. Med. 2023, 33, 101430. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Worasilchai, N.; Chindamporn, A. Human pythiosis: Emergence of fungal-like organism. Mycopathologia 2020, 185, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Nutthapan, N.; Sutichaiworapong, W.; Wu, Y.H. Human cutaneous pythiosis: A case report. J. Cutan. Pathol. 2024, 51, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, W.; Lipman, L.J.A.; De Cock, A.W.A.M.; Mendoza, L.; Grooters, A.M. Pythium insidiosum: An overview. Vet. Microbiol. 2010, 146, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.B.; Botton, S.A.; Ianiski, L.B.; Braga, C.Q.; Maciel, A.F.; Melo, L.G.; Zambrano, C.G.; Bruhn, F.R.P.; Santurio, J.M. Equidae pythiosis in Brazil and the world: A systematic review of the last 63 years (1960–2023). Braz. J. Microbiol. 2024, 55, 2969–2981. [Google Scholar] [CrossRef]
- Dos Santos, C.E.P.; Loreto, E.S.; Zanette, R.A.; Santurio, J.M.; Marques, L.C. Epidemiology of horse pythiosis in the Pantanal of Mato Grosso: Exploring the host-parasite-vector relationship. J. Equine Vet. Sci. 2024, 132, 104976. [Google Scholar] [CrossRef]
- Leal, A.B.M.; Santos, R.L.; Rodrigues, A.M.; Menezes, R.C.; Ogliari, D.; Castro, M. Pitiose equina no pantanal brasileiro: Aspectos clínico-patológicos de casos típicos e atípicos. Pesq. Vet. Bras. 2001, 21, 151–156. [Google Scholar] [CrossRef]
- Botton, S.A.; Pereira, D.I.; Costa, M.M.; Azevedo, M.I.; Argenta, J.S.; Jesus, F.P.; Alves, S.H.; Santurio, J.M. Identification of Pythium insidiosum by nested PCR in cutaneous lesions of Brazilian horses and rabbits. Curr. Microbiol. 2011, 62, 1225–1229. [Google Scholar] [CrossRef]
- Chagoya-Fuentes, J.L.; Gaona-López, J.; Hernández-Carbajal, G.R.; Torres-Guerrero, H.; Lammoglia-Villagómez, M.A.; Huerta-Peña, J.; Pérez-Brígido, C.D.; Jácome-Sosa, E.; Nieto-Rosaliano, S.O.; Rojas-Ronquillo, R.; et al. First confirmed case of equine pythiosis in Northern Veracruz, Mexico. Acta Trop. 2024, 254, 107195. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.P.F.; Maia, L.A.; Miranda Neto, E.G.; Kommers, G.D.; Garino Junior, F.; Riet-Correa, F.; Galiza, G.J.N.; Dantas, A.F.M. Pythiosis in equidae in Northeastern Brazil: 1985–2020. J. Equine Vet. Sci. 2021, 105, 103726. [Google Scholar] [CrossRef] [PubMed]
- Offer, K.S.; Dixon, C.E.; Sutton, D.G.M. Treatment of equine sarcoids: A systematic review. Equine Vet. J. 2024, 56, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; El-Gameel, S.M.; Kamel, M.S.; Elsamman, E.M.; Ramadan, R.M. Innovative diagnostic strategies for equine habronemiasis: Exploring molecular identification, gene expression, and oxidative stress markers. Parasit. Vectors 2025, 18, 325. [Google Scholar] [CrossRef]
- Grooters, A.M.; Gee, M.K. Development of a nested polymerase chain reaction assay for the detection and identification of Pythium insidiosum. J. Vet. Intern. Med. 2002, 16, 147–152. [Google Scholar] [CrossRef]
- Kosrirukvongs, P.; Chaiprasert, A.; Canyuk, C.; Wanachiwanawin, W. Comparison of nested PCR and culture identification of Pythium insidiosum in patients with Pythium keratitis. J. Med. Assoc. Thai. 2016, 99, 1033–1038. [Google Scholar]
- Krajaejun, T.; Rujirawat, T.; Lowhnoo, T.; Yingyong, W.; Lerksuthirat, T.; Suriyaphol, P.; Smittipat, N.; Juthayothin, T.; Kittichotirat, W.; Reamtong, O.; et al. Pythium insidiosum and human pythiosis, Thailand. Emerg. Infect. Dis. 2019, 25, 2295–2305. [Google Scholar]
- Htun, Z.M.; Laikul, A.; Pathomsakulwong, W.; Yurayart, C.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Sae-Chew, P.; Rujirawat, T.; et al. An initial survey of 150 horses from Thailand for anti-Pythium insidiosum antibodies. J. Mycol. Med. 2021, 31, 101085. [Google Scholar] [CrossRef]
- Tonpitak, W.; Pathomsakulwong, W.; Sornklien, C.; Krajaejun, T.; Wutthiwithayaphong, S. First confirmed case of nasal pythiosis in a horse in Thailand. JMM Case Rep. 2018, 5, e005136. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K.; Agarwal, S.; Lalgudi, V.G.; Shekhawat, N.S.; Venugopal, A.; Tripathy, K.; Srinivasan, B.; Iyer, G.; Gubert, J. Pythium insidiosum keratitis: Past, present, and future. Ophthalmol. Ther. 2022, 11, 1629–1653. [Google Scholar] [CrossRef]
- Vilela, R.; Grady, S.C.; Vilela, P.R.; Mendoza, L. Geographical distribution of human pythiosis in the USA. Microbiol. Spectr. 2025, 13, e0210324. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Hamad, M.H.; Abouzeid, N.Z.; El-Belkemy, F.A. Equine pythiosis in Egypt: Clinicopathological findings, detection, identification and genotyping of Pythium insidiosum. Vet. Dermatol. 2020, 31, 298-e73. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Alfaro, A.A. Equine pythiosis in Costa Rica: Report of 39 cases. Mycopathologia 1986, 94, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Canelón, J.; Perazzo, Y.; Colmenárez, V.; López, J.A. Equine pythiosis: Report in crossed bred (Criole Venezuelan) horses. Mycopathologia 2012, 174, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, M.K.; Schumacher, J.; McMullan, W.C. Cutaneous pythiosis in the horse. Vet. Clin. North. Am. Equine Pract. 1995, 11, 91–103. [Google Scholar] [CrossRef]
- Reis, J.L., Jr.; de Carvalho, E.C.; Nogueira, R.H.; Lemos, L.S.; Mendoza, L. Disseminated pythiosis in three horses. Vet. Microbiol. 2003, 96, 289–295. [Google Scholar] [CrossRef]
- Paz, G.S.D.; Camargo, G.G.; Cury, J.E.; Apolonio, E.V.P.; Garces, H.G.; Prado, A.C.D.; Chechi, J.L.; Oliveira, A.L.; Watanabe, M.J.; Bagagli, E.; et al. Outbreak of equine pythiosis in a southeastern region of Brazil: Environmental isolation and phylogeny. Transbound. Emerg. Dis. 2022, 69, 1617–1624. [Google Scholar] [CrossRef]
- Schurko, A.M.; Mendoza, L.; de Cock, A.W.A.M.; Shahin, E.; de Hoog, G.S. Evidência para agrupamentos geográficos: Diferenças genéticas moleculares entre cepas de Pythium insidiosum da Ásia, Austrália e Américas são exploradas. Mycologia 2003, 95, 200–208. [Google Scholar] [CrossRef]
- Krajaejun, T.; Chongtrakool, P.; Angkananukul, K.; Brandhorst, T.T. Effect of temperature on growth of the pathogenic oomycete Pythium insidiosum. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1462–1466. [Google Scholar]
- Chitasombat, M.N.; Jongkhajornpong, P.; Lekhanont, K.; Krajaejun, T. Recent update in diagnosis and treatment of human pythiosis. PeerJ 2020, 8, e8555. [Google Scholar] [CrossRef]
- Sridapan, T.; Krajaejun, T. Nucleic acid-based detection of Pythium insidiosum: A systematic review. J. Fungi 2022, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Garma-Aviña, A.; Valli, V.E.; Lumsden, J.H. Equine congenital cutaneous papillomatosis: A report of 5 cases. Equine Vet. J. 1981, 13, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Thongsri, Y.; Wonglakorn, L.; Chaiprasert, A.; Svobodova, L.; Hamal, P.; Pakarasang, M.; Prariyachatigul, C. Evaluation for the Clinical Diagnosis of Pythium insidiosum Using a Single-Tube Nested PCR. Mycopathologia 2013, 176, 369–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krajaejun, T.; Sathapatayavongs, B.; Pracharktam, R.; Nitiyanant, P.; Leelachaikul, P.; Wanachiwanawin, W.; Chaiprasert, A.; Assanasen, P.; Saipetch, M.; Mootsikapun, P.; et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 2006, 43, 569–576. [Google Scholar] [CrossRef]
- Ud-Naen, S.; Tansit, T.; Kanistanon, D.; Chaiprasert, A.; Wanachiwanawin, W.; Srinoulprasert, Y. Defective cytokine production from monocytes/macrophages of E-beta thalassemia patients in response to Pythium insidiosum infection. Immunobiology 2019, 224, 427–432. [Google Scholar] [CrossRef]
- Weiblen, C.; Leite, F.P.; Kommers, G.D.; Mattos, C.L.; Barros, C.S.; Piazer, J.V. Seroprevalence of Pythium insidiosum infection in equine in Rio Grande do Sul, Brazil. Cienc. Rural. 2015, 46, 126–131. [Google Scholar] [CrossRef]
- França, D.A.; Langoni, H. Prevalent zoonoses in Sao Paulo State, Brazil: The role of bats and molecular diagnosis. Rev. Inst. Med. Trop. Sao Paulo 2025, 67, e17. [Google Scholar] [CrossRef]
- França, D.A.; Mioni, M.S.R.; Fernandes, J.; Lemos, E.R.S.; Duré, A.Í.L.; Silva, M.V.F.; Langoni, H.; Megid, J. Overview of Q fever in Brazil: An underestimated zoonosis. Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e39. [Google Scholar] [CrossRef]
- Brazilian Institute of Geography and Statistics (IBGE). Cities and States. Available online: https://cidades.ibge.gov.br/ (accessed on 4 September 2025).
- National Institute of Meteorology (INMET). Climatological Normals of Brazil (1991–2020); INMET: Brasília, Brazil, 2020. Available online: https://portal.inmet.gov.br/ (accessed on 4 September 2025).
- Leksuwankun, S.; Plongla, R.; Eamrurksiri, N.; Torvorapanit, P.; Phongkhun, K.; Langsiri, N.; Meejun, T.; Srisurapanont, K.; Thanakitcharu, J.; Lerttiendamrong, B.; et al. Needs assessment of a pythiosis continuing professional development program. PLoS Negl. Trop. Dis. 2024, 18, e0012004. [Google Scholar] [CrossRef]
- Vasconcelos, A.B.; França, D.A.; do Prado, A.C.; Yamauchi, D.H.; Garces, H.G.; Oliveira, A.L.; Carnaúba, R.T.M.D.S.; Tertuliano Dos Santos, C.B.; Lucheis, S.B.; Gimenes Bosco, S.M. Development of a multiplex-PCR assay for differentiation of Cryptococcus species using the PRP8 gene region. Braz. J. Microbiol. 2025. ahead of print. [Google Scholar] [CrossRef]
| Animal | Breed | Age | Sex | Source | Lesion Location |
|---|---|---|---|---|---|
| 1 | Mixed-breed | 2 years | Male | Mossoró, Rio Grande do Norte | Abdominal Region |
| 2 | Mixed-breed | 2 years | Female | Presidente Dutra, Maranhão | Left thoracic limb |
| 3 | Mixed-breed | 3 years | Male | Paulista, Pernambuco | Abdominal Region |
| 4 | Mixed-breed | 3 years | Male | Palmeira dos Índios, Alagoas | Left pelvic limb |
| 5 | Mixed-breed | 3 years | Female | João Pessoa, Paraíba | Dorsal region |
| Animal | Material | Culture | PCR Endogenous Gene | Nested-PCR P. insidiusum |
|---|---|---|---|---|
| 1 | tissue | − | + | + |
| 2 | tissue | + | + | + |
| 3 | tissue and kunkers | − | + | + |
| 4 | tissue and kunkers | − | + | − |
| 5 | tissue | − | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, A.B.d.; França, D.A.d.; Prado, A.C.d.; Yamauchi, D.H.; Silva, A.C.A.d.; Barros, I.d.O.; Valença, S.R.F.d.A.; Lucheis, S.B.; Bosco, S.d.M.G. Molecular Detection of Pythium insidiosum in Cutaneous Lesions of Horses from Northeastern Brazil. Animals 2025, 15, 2863. https://doi.org/10.3390/ani15192863
Vasconcelos ABd, França DAd, Prado ACd, Yamauchi DH, Silva ACAd, Barros IdO, Valença SRFdA, Lucheis SB, Bosco SdMG. Molecular Detection of Pythium insidiosum in Cutaneous Lesions of Horses from Northeastern Brazil. Animals. 2025; 15(19):2863. https://doi.org/10.3390/ani15192863
Chicago/Turabian StyleVasconcelos, Artur Bibiano de, Danilo Alves de França, Ana Carolina do Prado, Danielle Hamae Yamauchi, Andrezza Caroline Aragão da Silva, Isabella de Oliveira Barros, Sandra Regina Fonseca de Araújo Valença, Simone Baldini Lucheis, and Sandra de Moraes Gimenes Bosco. 2025. "Molecular Detection of Pythium insidiosum in Cutaneous Lesions of Horses from Northeastern Brazil" Animals 15, no. 19: 2863. https://doi.org/10.3390/ani15192863
APA StyleVasconcelos, A. B. d., França, D. A. d., Prado, A. C. d., Yamauchi, D. H., Silva, A. C. A. d., Barros, I. d. O., Valença, S. R. F. d. A., Lucheis, S. B., & Bosco, S. d. M. G. (2025). Molecular Detection of Pythium insidiosum in Cutaneous Lesions of Horses from Northeastern Brazil. Animals, 15(19), 2863. https://doi.org/10.3390/ani15192863





