Measurements and Visibility of the Pancreatic Ducts on Computed Tomography in 78 Cats Without Clinical Evidence of Pancreatitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Patients were included if:
- There was contemporaneous standard bloodwork that included normal DGGR lipase, ALT (alanine transferase) or ALKP (alkaline phosphatase).
- There was no clinical evidence of pancreatic disease nor indication in the history of previous pancreatic disease.
- There was no final diagnosis of pancreatic disease identified in the records.
- Patients were excluded if:
- An ultrasound was performed near the time of CT, and pancreatic abnormalities were identified
- fPLi was performed and was abnormal.
- Images were of inadequate quality or did not include the entire pancreas and pancreatic ducts for evaluation.
2.1. The Following Measurements Were Taken
2.1.1. Measurements and Visibility of the Pancreatic Ducts
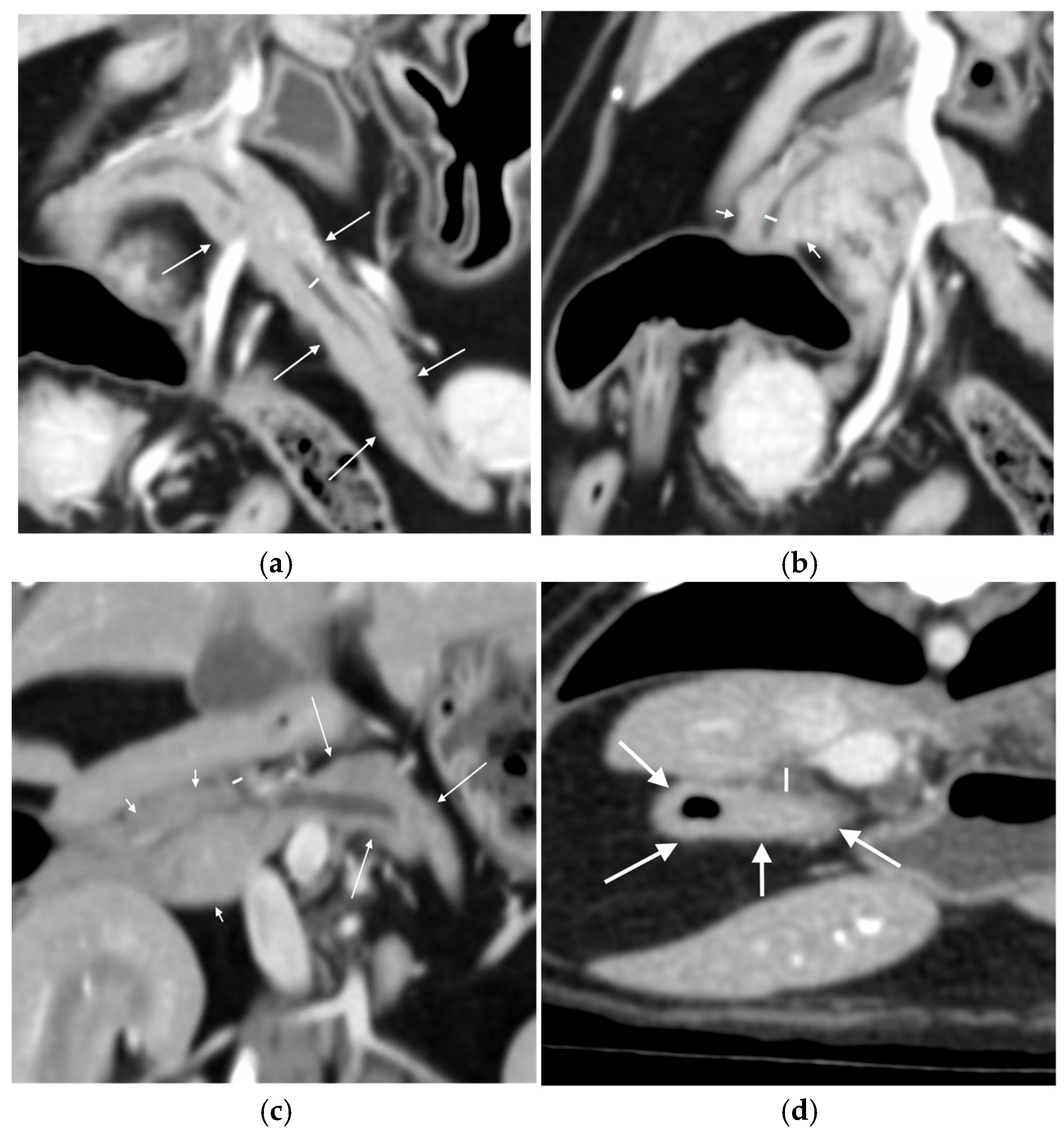
- Width from outer layer to outer layer, and Likert visibility scale pre and post-contrast, of the right pancreatic duct at its widest point on a dorsal plane reconstruction (Figure 1b).
- Width from outer layer to outer layer, and Likert visibility scale pre and post-contrast, of the common pancreatic duct as it exits the pancreatic parenchyma on a dorsal plane reconstruction (Figure 1c).
- Evaluate each segment for any subjective thickening or irregularity of the walls and for the presence of any mineral attenuation within the duct (subjectively assessed as being distinctly visible on bone window, WL 300, WW 1500).
2.1.2. Measurements of the Duodenal Papilla
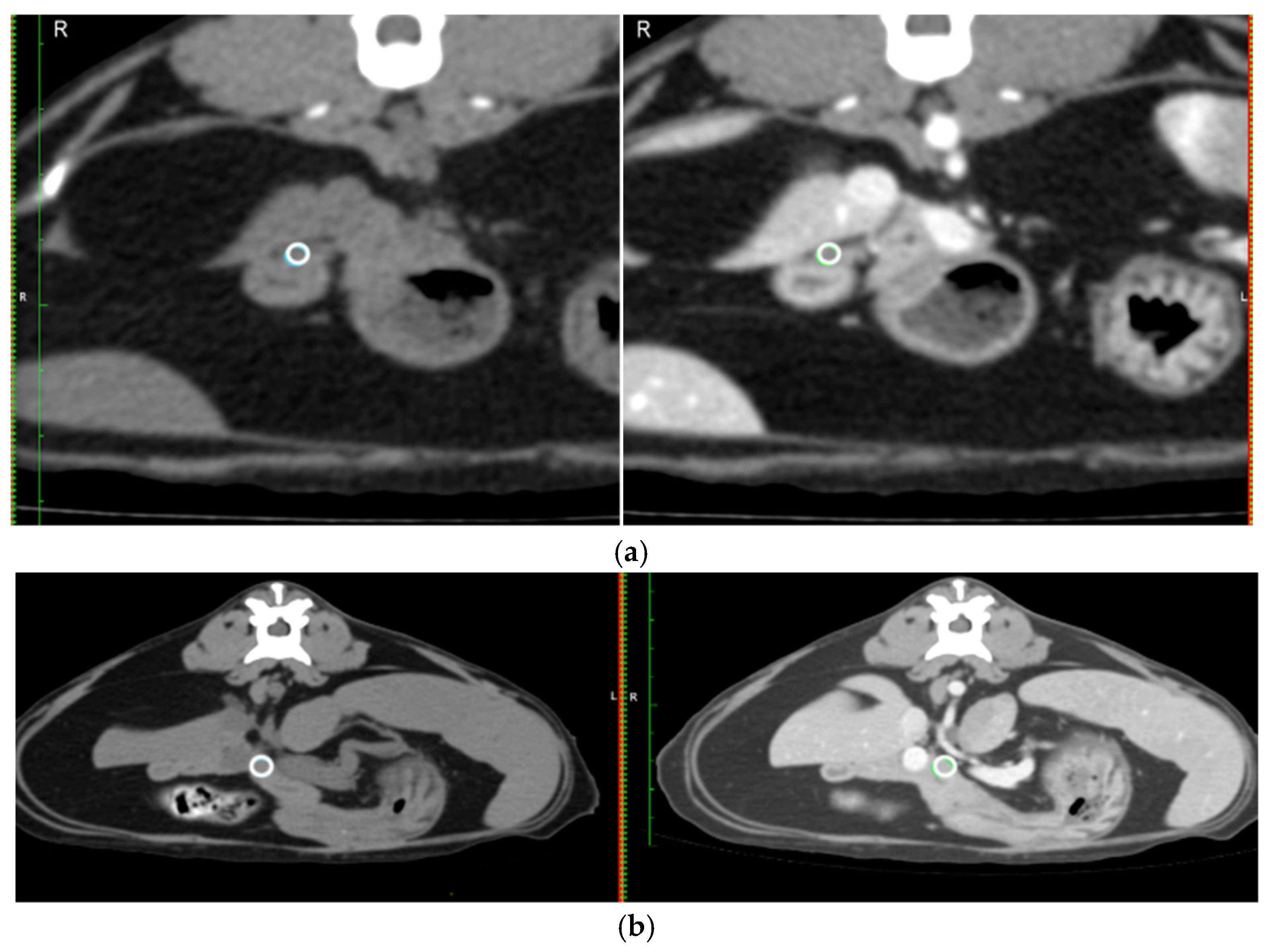
- Mean Hounsfield unit (HU) on a circular region of interest (ROI) placed over the duodenal papilla pre- and post-contrast on the transverse image (Figure 2a).
- Likert visibility scale of the duodenal papilla pre- and post-contrast.
- Diameter of the duodenal papilla post-contrast taken perpendicular to the duodenal wall on the transverse image (Figure 1d).
2.1.3. Pancreatic Parenchyma
- The width of the pancreas was measured at 90° to the long axis of the pancreas in the left and right limb at the widest point of the respective limb.
- The mean HU for the same circular ROI, pre- and post-contrast in a section of the pancreas at the level of the pancreatic body (Figure 2b).
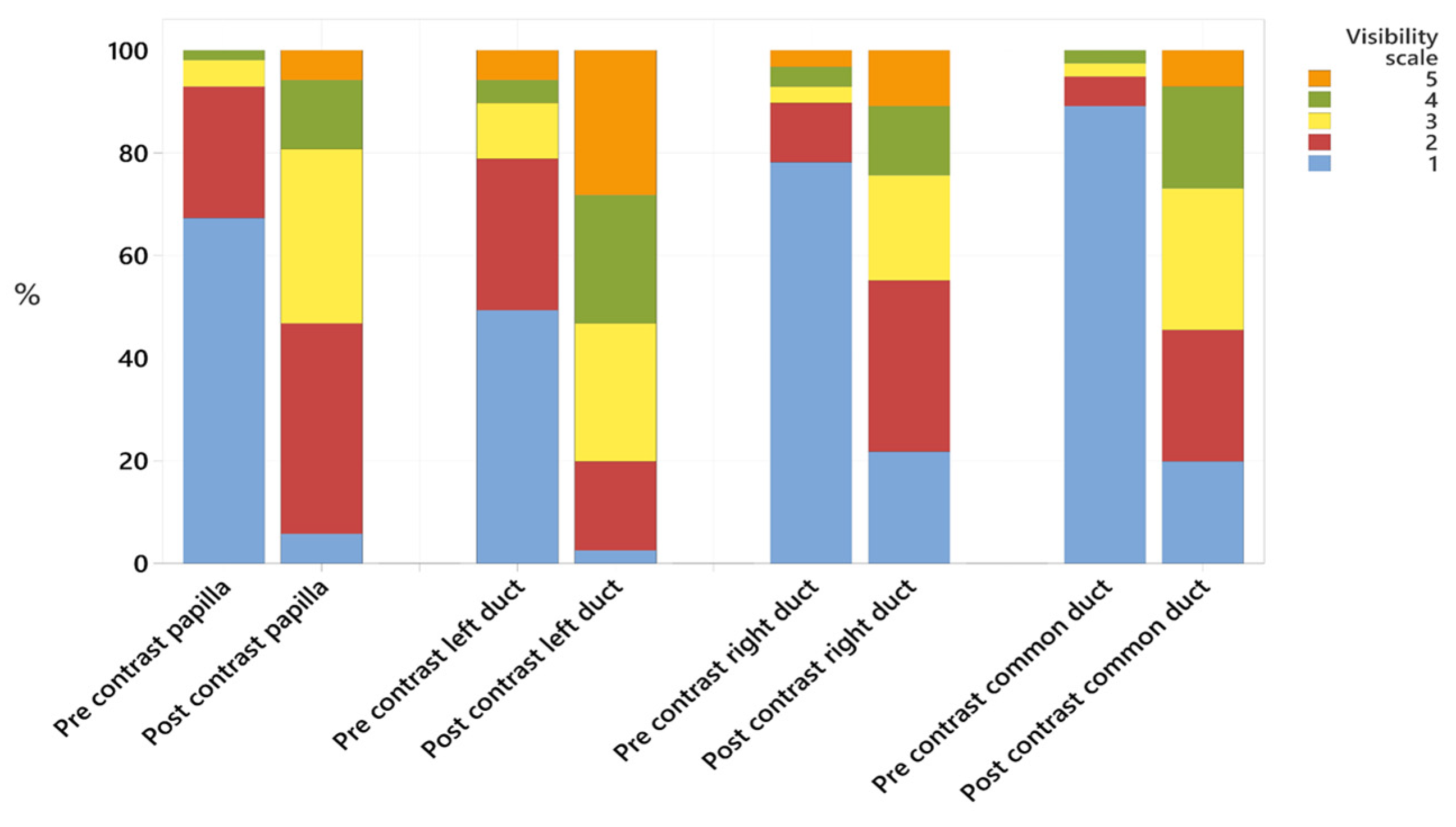
- Visibility was recorded using a 5-point Likert scale [29], defined as:
2.2. Statistical Analysis
3. Results
3.1. Measurements Obtained from Imaging
3.1.1. Pancreatic Ducts
3.1.2. Duodenal Papilla Appearance
3.1.3. Pancreatic Parenchyma
3.2. Statistical Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HU | Hounsfield unit |
| CT | Computed tomography |
| ROI | Region of interest |
| ALT | Alanine Transaminase |
| ALKP | Alkaline phosphatase |
| DGGR | (1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester) |
| fPLI | Serum feline pancreatic lipase immunoreactivity |
| RCVS | Royal college of veterinary surgeons |
Appendix A
| Broad Category | Diagnosis | Number |
|---|---|---|
| Neoplasia | Soft tissue sarcoma, including interscapular | 12 |
| Nasal tumour including lymphoma | 10 | |
| Pulmonary neoplasia | 7 | |
| Mammary tumour | 6 | |
| Lymphoma (not nasal) | 4 | |
| Thymoma | 3 | |
| Osteosarcoma/chondrosarcoma | 2 | |
| Iris melanoma | 2 | |
| Renal carcinoma | 2 | |
| Other—one each of: adrenal tumour, anal sac carcinoma, diffuse carcinoma (thorax), haemangioma, parietal bone mass, retrobulbar, salivary carcinoma, undifferentiated gastric neoplasia, undifferentiated pleural neoplasia | 9 | |
| Inflammatory/infectious | Respiratory disease including asthma | 4 |
| Pyothorax | 2 | |
| Rhinitis (including fungal) | 2 | |
| Other—one each of: Aspiration pneumonia, FIP, inflammatory lesion on soft palate, mycobacterial infection from a nasal mass. | 4 | |
| Autoimmune | Immune mediated polyarthritis | 1 |
| Other | One each of: ARDS, Chylothorax, collapsing trachea, Cruciate disease and non-healing wound, Fractured femur, idiopathic chylothorax, pleuroperitoneal hernia, renal disease | 8 |
Appendix B
| Institution | Scanner | Slices | KVP | Tube Current | Slice Thickness |
|---|---|---|---|---|---|
| A | Toshiba Aquillon | 16 | 100 | 85 | 0.5 mm |
| B | GE Revolution Evo | 64 | 120 | 190 | 1.25 |
| B | PNMS MX16 | 16 | 120 | 190 | 1 mm |
References
- De Cock, H.E.V.; Forman, M.A.; Farver, T.B.; Marks, S.L. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet. Pathol. 2007, 44, 39–49. [Google Scholar] [CrossRef]
- Bazelle, J.; Watson, P. Pancreatitis in cats: Is it acute, is it chronic, is it significant? J. Feline Med. Surg. 2014, 16, 395–406. [Google Scholar] [CrossRef]
- Forman, M.A.; Steiner, J.M.; Armstrong, P.J.; Camus, M.S.; Gaschen, L.; Hill, S.L.; Mansfield, C.S.; Steiger, K. ACVIM consensus statement on pancreatitis in cats. J. Vet. Intern. Med. 2021, 35, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A. Diagnosis and management of pancreatitis. J. Small Anim. Pract. 2008, 35, 445–454. [Google Scholar] [CrossRef]
- Ferreri, J.A.; Hardam, E.; Kimmel, S.E.; Saunders, H.M.; Van Winkle, T.J.; Drobatz, K.J.; Washabau, R.J. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996–2001). J. Am. Vet. Med. Assoc. 2003, 223, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Hittmair, K.M.; Suchodolski, J.S.; Steiner, J.M.; Tichy, A.; Dupré, G. Serum feline-specific pancreatic lipase immunoreactivity concentrations and abdominal ultrasonographic findings in cats with trauma resulting from high-rise syndrome. J. Am. Vet. Med. Assoc. 2013, 242, 1238–1243. [Google Scholar] [CrossRef]
- Bayliss, D.B.; Steiner, J.M.; Sucholdolski, J.S.; Radecki, S.V.; Brewer, M.M.; Morris, A.K.; Lappin, M.R. Serum feline pancreatic lipase immunoreactivity concentration and seroprevalences of antibodies against Toxoplasma gondii and Bartonella species in client-owned cats. J. Feline Med. Surg. 2009, 11, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Steiner, J.M. Current concepts in feline pancreatitis. Top. Companion Anim. Med. 2008, 23, 185–192. [Google Scholar] [CrossRef]
- Pratschke, K.M.; Ryan, J.; McAlinden, A.; McLauchlan, G. Pancreatic surgical biopsy in 24 dogs and 19 cats: Postoperative complications and clinical relevance of histological findings. J. Small Anim. Pract. 2015, 56, 60–66. [Google Scholar] [CrossRef]
- Schreeg, M.E.; Cullen, J.M.; Robertson, J.; Gookin, J.L. Histologic characterization of the major duodenal papilla and association with concurrent biliary, pancreatic, and intestinal pathology in cats. Vet. Pathol. 2024, 61, 207–220. [Google Scholar] [CrossRef]
- Cerna, P.; Kilpatrick, S.; Gunn-Moore, D.A. Feline comorbidities: What do we really know about feline triaditis? J. Feline Med. Surg. 2020, 22, 1047–1067. [Google Scholar] [CrossRef]
- Mansfield, C.S.; Jones, B.R. Review of feline pancreatitis part two: Clinical signs, diagnosis and treatment. J. Feline Med. Surg. 2001, 3, 125–132. [Google Scholar] [CrossRef]
- Oppliger, S.; Hartnack, S.; Riond, B.; Reusch, C.E.; Kook, P.H. Agreement of the serum Spec fPL and 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J. Vet. Intern. Med. 2013, 27, 1077–1082. [Google Scholar] [CrossRef]
- Steiner, J.M.; Wilson, B.; Williams, D.A. Development and analytical validation of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum. Can. J. Vet. Res. 2004, 68, 309–314. [Google Scholar] [PubMed]
- Oppliger, S.; Hartnack, S.; Reusch, C.E.; Kook, P.H. Agreement of serum feline pancreas–specific lipase and colorimetric lipase assays with pancreatic ultrasonographic findings in cats with suspicion of pancreatitis: 161 cases (2008–2012). J. Am. Vet. Med. Assoc. 2014, 244, 1060–1065. [Google Scholar] [CrossRef]
- Forman, M.A.; Marks, S.L.; De Cock, H.E.; Hergesell, E.J.; Wisner, E.R.; Baker, T.W.; Kass, P.H.; Steiner, J.M.; Williams, D.A. Evaluation of Serum Feline Pancreatic Lipase Immunoreactivity and Helical Computed Tomography versus Conventional Testing for the Diagnosis of Feline Pancreatitis. J. Vet. Intern. Med. 2008, 18, 807–815. [Google Scholar] [CrossRef]
- Griffin, S. Feline abdominal ultrasonography: What’s normal? What’s abnormal? The pancreas. J. Feline Med. Surg. 2020, 22, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, A.; Steiner, J.M.; Williams, D.A.; Kramer, S.; Fuchs, C.; Janthur, M.; Hewicker-Trautwein, M.; Nolte, I. Comparison of the Sensitivity of Different Diagnostic Tests for Pancreatitis in Cats. J. Vet. Intern. Med. 2008, 15, 329–333. [Google Scholar] [CrossRef]
- Williams, J.; Panciera, D.; Larson, M.; Werre, S. Ultrasonographic findings of the pancreas in cats with elevated serum pancreatic lipase immunoreactivity. J. Vet. Intern. Med. 2013, 27, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.; Mitze, S.; Teske, E.; Stockhaus, C. Evaluation of sonographic parameters as prognostic risk factors in cats with pancreatitis—A retrospective study in 42 cats. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2018, 46, 386–392. [Google Scholar] [CrossRef]
- Orrison, W.W.; Gentry, L.R.; Stimac, G.K.; Tarrel, R.M.; Espinosa, M.C.; Cobb, L.C. Blinded comparison of cranial CT and MR in closed head injury. Am. J. Neuroradiol. 1994, 15, 351–356. [Google Scholar]
- Etue, S.M.; Penninck, D.G.; Labato, M.A.; Pearson, S.; Tidwell, A. Ultrasonography of the normal feline pancreas and associated anatomic landmarks: A prospective study of 20 cats. Vet. Radiol. Ultrasound 2001, 42, 330–336. [Google Scholar] [CrossRef]
- Larson, M.M.; Panciera, D.L.; Ward, D.L.; Steiner, J.M.; Williams, D.A. Age-related changes in the ultrasound appearance of the normal feline pancreas. Vet. Radiol. Ultrasound 2005, 46, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Penninck, D.G.; Mahony, O.M.; King, R.; Rand, W.M. Relationship of pancreatic duct dilation to age and clinical findings in cats. Vet. Radiol. Ultrasound 2006, 47, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Coeuriot, C.; Pontarrasse, J.; Bouhsina, N.; Lazard, M.; Bertrand, A.; Fusellier, M. Ultrasound appearance of the duodenal papilla in clinically healthy cats. J. Feline Med. Surg. 2022, 24, 1267–1273. [Google Scholar] [CrossRef]
- Türkvatan, A.; Erden, A.; Türkoğlu, M.; Seçil, M.; Yener, Ö. Imaging of acute pancreatitis and its complications. Part 1: Acute pancreatitis. Diagn. Interv. Imaging 2015, 96, 151–160. [Google Scholar] [CrossRef]
- Marolf, A.J. Computed Tomography and MRI of the Hepatobiliary System and Pancreas. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Bugbee, A.; Sharma, A.; Secrest, S. Feline pancreatic ducts are consistently identified on CT and more likely to be dilated in the body of pancreas in cats with elevated feline pancreatic lipase immunoreactivity. Vet. Radiol. Ultrasound 2020, 61, 255–260. [Google Scholar] [CrossRef]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 22, 55. [Google Scholar]
- Parizel, P.; Makkat, S.; Van Miert, E.; Van Goethem, J.; Van den Hauwe, L.; De Schepper, A. Intracranial hemorrhage: Principles of CT and MRI interpretation. Eur. Radiol. 2001, 11, 1770–1783. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson/Prentice Hall: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sun, C.-H.; Li, X.; Chan, T.; Peng, Z.; Dong, Z.; Luo, Y.; Li, Z.-P.; Feng, S.-T. Multidetector computed tomography (MDCT) manifestations of the normal duodenal papilla. Eur. J. Radiol. 2013, 82, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Secrest, S.; Sharma, A.; Bugbee, A. Triple phase computed tomography of the pancreas in healthy cats. Vet. Radiol. Ultrasound 2018, 59, 163–168. [Google Scholar] [CrossRef] [PubMed]
| Reviewer 1 | Reviewer 2 | Both Combined | |
|---|---|---|---|
| Measurements | |||
| Papilla | 2.4 ± 0.6 (1.3–4.0) | 3.1 ± 0.7 (1.9–5.3) | 2.8 ± 0.7 (1.3–5.3) |
| Left duct | 1.3 ± 0.8 (0.6–7.6) | 1.5 ± 0.7 (0.6–4.6) | 1.4 ± 0.8 (0.6–7.6) |
| Right duct | 1.2 ± 0.5 (0.5–3.2) | 1.1 ± 0.5 (0.3–2.9) | 1.1 ± 0.5 (0.3–3.2) |
| Common duct | 1.7 ± 0.8 (0.7–4.6) | 1.3 ± 0.5 (0.6–3.7) | 1.6 ± 0.8 (0.6–4.6) |
| Likert scale | |||
| Papilla pre | 1.2 ± 0.4 (1–3) | 1.6 ± 0.8 (1–4) | 1.4 ± 0.7 (1–4) |
| Papilla post | 2.5 ± 0.7 (2–5) | 2.9 ± 1.2 (1–5) | 2.7 ± 1.0 (1–5) |
| Left pre | 1.7 ± 1.0 (1–5) | 2.0 ± 1.2 (1–5) | 1.9 ± 1.1 (1–5) |
| Left post | 3.4 ± 1.0 (2–5) | 3.8 ± 1.3 (1–5) | 3.6 ± 1.1 (1–5) |
| Right pre | 1.4 ± 0.8 (1–4) | 1.4 ± 1.0 (1–5) | 1.4 ± 1.0 (1–5) |
| Right post | 2.8 ± 0.9 (1–5) | 2.3 ± 1.5 (1–5) | 2.6 ± 1.3 (1–4) |
| Common pre | 1.3 ± 0.7 (1–4) | 1.1 ± 0.4 (1–4) | 1.2 ± 0.6 (1–4) |
| Common post | 2.9 ± 0.9 (1–5) | 2.5 ± 1.4 (1–5) | 2.7 ± 1.2 (1–5) |
| Duct | Reviewer | 1–9 Years | 10+ Years | p-Value |
|---|---|---|---|---|
| Left | 1 | 1.1 ± 0.4 (0.6–2.3) | 1.4 ± 1.0 (0.6–7.6) | 0.125 |
| 2 | 1.3 ± 0.6 (0.6–2.8) | 1.6 ± 0.7 (0.6–4.6) | 0.113 | |
| Combined | 1.2 ± 0.5 (0.6–2.8) | 1.5 ± 0.9 (0.6–7.6) | - | |
| Right | 1 | 1.1 ± 0.4 (0.6–2.6) | 1.2 ± 0.6 (0.5–3.2) | 0.124 |
| 2 | 1.1 ± 0.5 (0.3–2.0) | 1.1 ± 0.5 (0.3–2.9) | 0.984 | |
| Combined | 1.1 ± 0.4 (0.3–2.6) | 1.2 ± 0.5 (0.3–3.2) | - | |
| Common | 1 | 1.7 ± 0.8 (0.7–4.0) | 1.7 ± 0.9 (0.7–4.6) | 0.984 |
| 2 | 1.3 ± 0.7 (0.6–3.7) | 1.3 ± 0.4 (0.7–2.2) | 0.652 | |
| Combined | 1.6 ± 0.8 (0.6–4.0) | 1.5 ± 0.7 (0.7–4.6) | - |
| Reviewer 1 | Reviewer 2 | R1 vs. R2 | |
|---|---|---|---|
| ICC | ICC | ICC | |
| Measurements | |||
| Duodenal papilla diameter | 0.66 | 0.37 | 0.23 |
| Left pancreatic duct width | 0.67 | 0.93 | 0.73 |
| Right pancreatic duct width | 0.40 | 0.47 | 0.76 |
| Common pancreatic duct width | 0.24 | 0.65 | 0.22 |
| Likert visibility scales | |||
| Duodenal papilla (pre) | −0.13 | 0.40 | 0.06 |
| Duodenal papilla (post) | 0.41 | 0.62 | 0.24 |
| Left pancreatic duct (pre) | 0.41 | 0.62 | 0.59 |
| Left pancreatic duct (post) | 0.85 | 0.88 | 0.66 |
| Right pancreatic duct (pre) | 0.43 | 0.68 | 0.59 |
| Right pancreatic duct (post) | 0.74 | 0.81 | 0.47 |
| Common pancreatic duct (pre) | 0.31 | −0.04 | 0.24 |
| Common pancreatic duct (post) | 0.67 | 0.86 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caine, A.; Ma, M.-H.; Herrtage, M.; Sparks, T.; Genain, M.A. Measurements and Visibility of the Pancreatic Ducts on Computed Tomography in 78 Cats Without Clinical Evidence of Pancreatitis. Animals 2025, 15, 2857. https://doi.org/10.3390/ani15192857
Caine A, Ma M-H, Herrtage M, Sparks T, Genain MA. Measurements and Visibility of the Pancreatic Ducts on Computed Tomography in 78 Cats Without Clinical Evidence of Pancreatitis. Animals. 2025; 15(19):2857. https://doi.org/10.3390/ani15192857
Chicago/Turabian StyleCaine, Abby, Man-Hei Ma, Mike Herrtage, Tim Sparks, and Marie Aude Genain. 2025. "Measurements and Visibility of the Pancreatic Ducts on Computed Tomography in 78 Cats Without Clinical Evidence of Pancreatitis" Animals 15, no. 19: 2857. https://doi.org/10.3390/ani15192857
APA StyleCaine, A., Ma, M.-H., Herrtage, M., Sparks, T., & Genain, M. A. (2025). Measurements and Visibility of the Pancreatic Ducts on Computed Tomography in 78 Cats Without Clinical Evidence of Pancreatitis. Animals, 15(19), 2857. https://doi.org/10.3390/ani15192857






