Chimeric Fimbrial Multiepitope Antigen Fused to Double-Mutant LT (dmLT) Induces Antibodies That Inhibit Enterotoxigenic E. coli Adhesion in Porcine IPEC-J2 Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cell Line Culture Conditions
2.2. Construction of dmLT MEFA
2.3. Expression and Purification of dmLT MEFA Protein
2.4. Subcutaneous Immunization of Rabbit with dmLT MEFA
2.5. Anti-Fimbriae Specific IgG Antibody Titration
2.6. Rabbit IgG Antibody Neutralization Against Cholera Toxin Enterotoxicity
2.7. Rabbit IgG-Mediated Inhibition of K88+, F18+, K99+, 987P+, and F41+ ETEC Strain Adhesion to IPEC-J2
2.8. Statistical Analysis
3. Results
3.1. dmLT MEFA Carried the Epitopes of the Five Fimbriae Subunits of ETEC
3.2. The Fimbriae Epitopes Displayed on dmLT MEFA
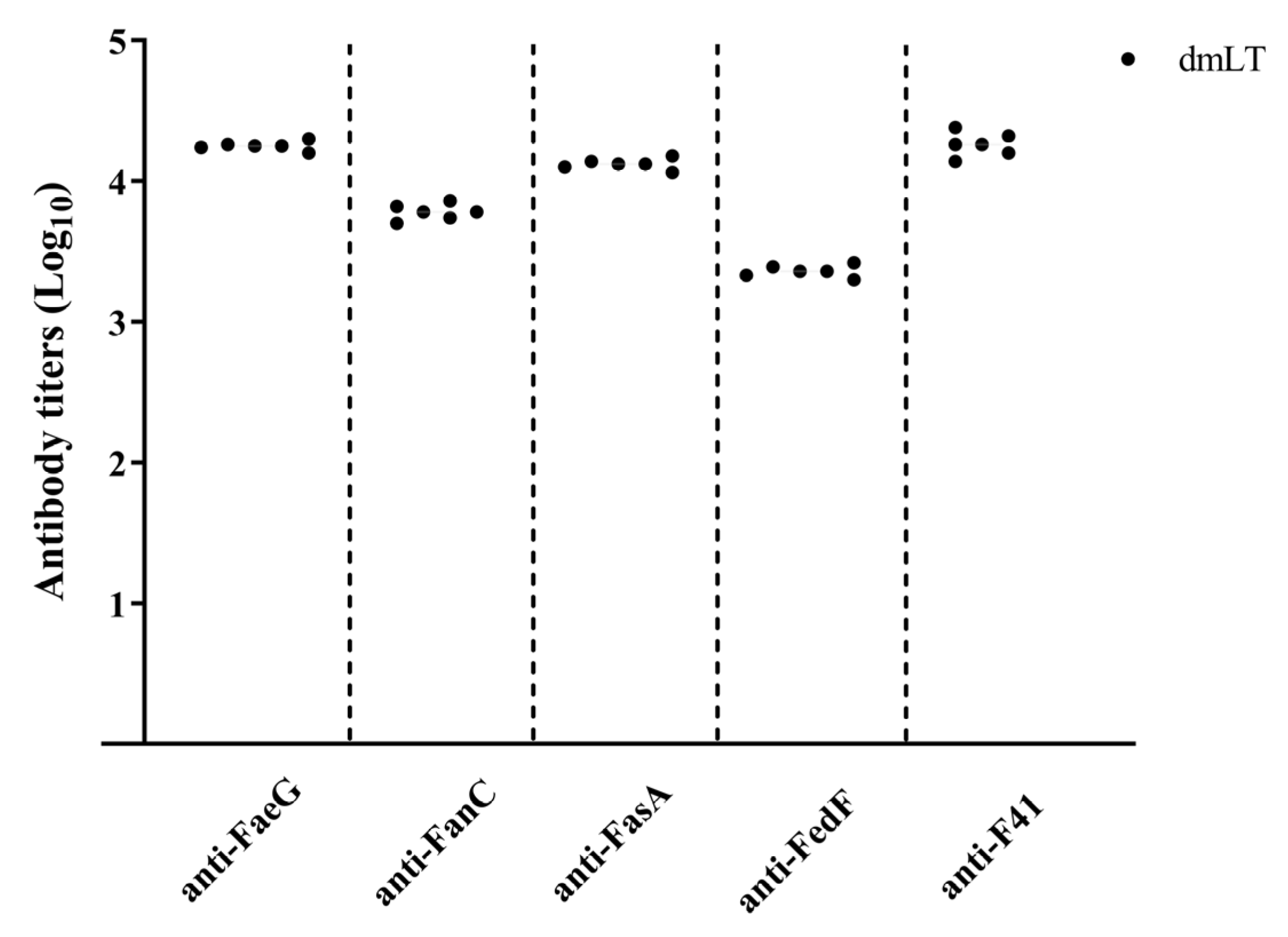
3.3. Rabbit IgG Response to All Five Fimbriae Adhesive Subunits
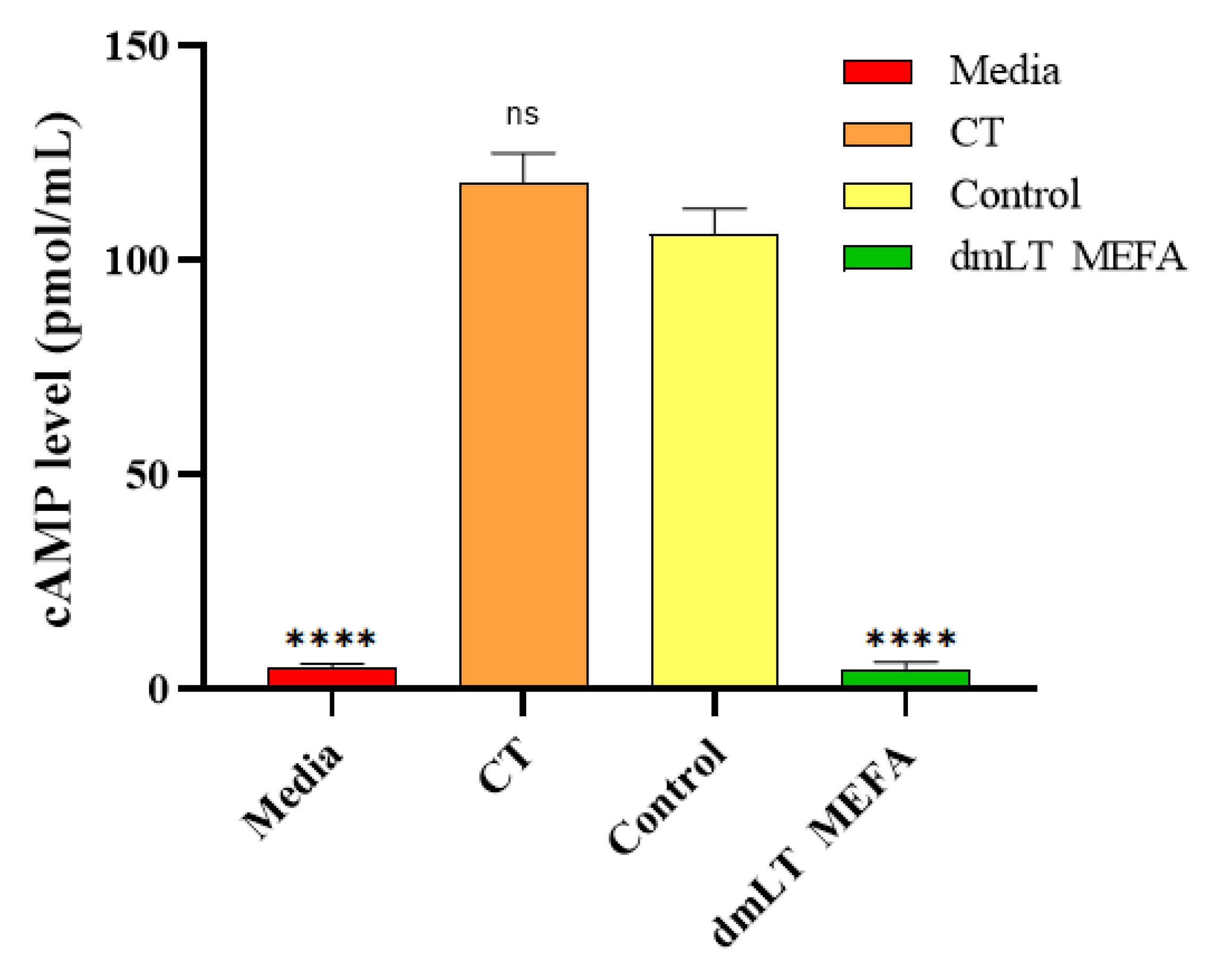
3.4. Rabbit IgG Antibody Neutralized CT In Vitro
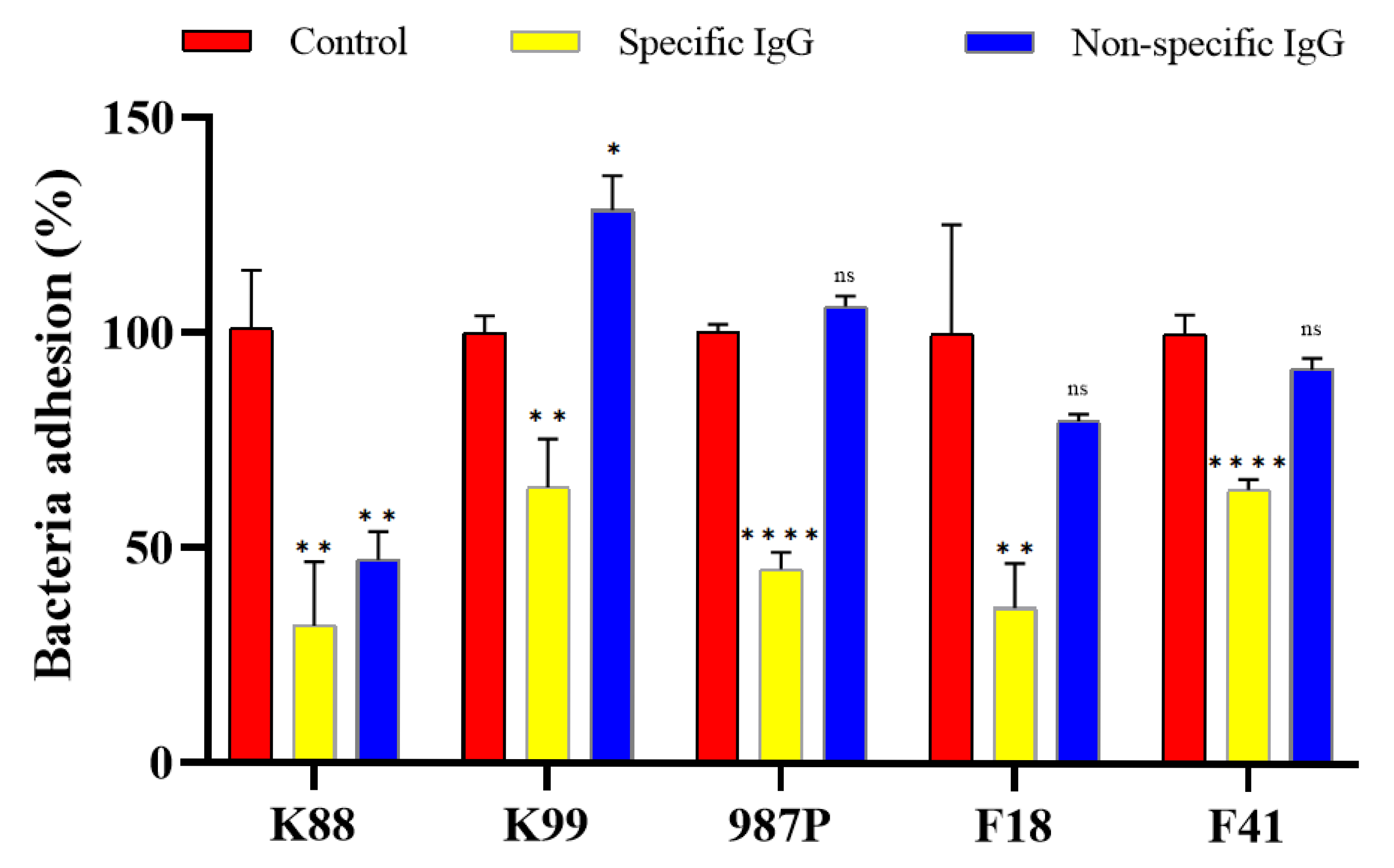
3.5. Serum Samples from Immunized Rabbits Inhibited Adherence to IPEC-J2 of ETEC Bacteria Expressing the Corresponding Fimbriae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETEC | Enterotoxigenic Escherichia coli |
| dmLT | Double-mutant heat-labile toxin |
| MEFA | Multivalent epitope fusion antigens |
| LT | Heat-labile toxin |
| ST | Heat-stable toxins |
| CFA | Colonization factor antigen |
| IPTG | Isopropyl-β-D-1-thiogalactoside |
| ELISA | Enzyme-linked immunosorbent assay |
| CFU | Colony-forming units |
References
- Seo, H.; Zhang, W. Development of effective vaccines for enterotoxigenic Escherichia coli. Lancet Infect. Dis. 2020, 20, 150–152. [Google Scholar] [CrossRef]
- Gaastra, W.; de Graaf, F.K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982, 46, 129–161. [Google Scholar] [CrossRef] [PubMed]
- Guiral, E.; Gonçalves Quiles, M.; Muñoz, L.; Moreno-Morales, J.; Alejo-Cancho, I.; Salvador, P.; Alvarez-Martinez, M.J.; Marco, F.; Vila, J. Emergence of resistance to quinolones and β-lactam antibiotics in enteroaggregative and enterotoxigenic Escherichia coli causing traveler’s diarrhea. Antimicrob. Agents Chemother. 2019, 63, e01745-18. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- von Mentzer, A.; Svennerholm, A.M. Colonization factors of human and animal-specific enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 2024, 32, 448–464. [Google Scholar] [CrossRef]
- Zhang, W.; Sack, D.A. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines 2012, 11, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Garcia, C.; Ruan, X.; Duan, Q.; Sack, D.A.; Zhang, W. Preclinical characterization of immunogenicity and efficacy against diarrhea from MecVax, a multivalent enterotoxigenic E. coli vaccine candidate. Infect. Immun. 2021, 89, e0010621. [Google Scholar] [CrossRef]
- Zhang, W.; Sack, D.A. Current progress in developing subunit vaccines against enterotoxigenic Escherichia coli (ETEC) associated diarrhea. Clin. Vaccine Immunol. 2015, 22, 983–991. [Google Scholar] [CrossRef]
- Walker, R.; Dull, P. Combination vaccine strategies to prevent enteric infections. Vaccine 2017, 35 (49 Pt A), 6790–6792. [Google Scholar] [CrossRef]
- Fleckenstein, J.M. Confronting challenges to enterotoxigenic Escherichia coli vaccine development. Front. Trop. Dis. 2021, 2, 709907. [Google Scholar] [CrossRef]
- Upadhyay, I.; Lauder, K.L.; Li, S.; Ptacek, G.; Zhang, W. Intramuscularly administered enterotoxigenic Escherichia coli (ETEC) vaccine candidate MecVax prevented H10407 intestinal colonization in an adult rabbit colonization model. Microbiol. Spectr. 2022, 10, e0147322. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I.; Bourgeois, A.L. Oral inactivated whole cell vaccine for mucosal immunization: ETVAX case study. Front. Immunol. 2023, 14, 1125102. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Svennerholm, A.M. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates. Appl. Microbiol. Biotechnol. 2012, 93, 2291–2300. [Google Scholar] [CrossRef]
- Borde, A.; Ekman, A.; Larsson, A.; Carlin, N.; Holmgren, J.; Tobias, J. Preparation and preclinical evaluation of a freeze-dried formulation of a novel combined multivalent whole-cell/B-subunit oral vaccine against enterotoxigenic Escherichia coli diarrhea. Eur. J. Pharm. Biopharm. 2016, 108, 18–24. [Google Scholar] [CrossRef]
- Lu, T.; Moxley, R.A.; Zhang, W. Application of a novel epitope- and structure-based vaccinology-assisted fimbria-toxin multiepitope fusion antigen of enterotoxigenic Escherichia coli for development of multivalent vaccines against porcine postweaning diarrhea. Appl. Environ. Microbiol. 2020, 86, e00274-20. [Google Scholar] [CrossRef]
- Duan, Q.; Lu, T.; Garcia, C.; Yañez, C.; Nandre, R.M.; Sack, D.A.; Zhang, W. Co-administered tag-less toxoid fusion 3×STaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (multiepitope fusion antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1-CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front. Microbiol. 2018, 9, 1198. [Google Scholar] [CrossRef]
- Leach, S.; Lundgren, A.; Carlin, N.; Löfstrand, M.; Svennerholm, A.M. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine ETVAX. Vaccine 2017, 35, 3966–3973. [Google Scholar] [CrossRef]
- Duan, Q.; Pang, S.; Wu, W.; Jiang, B.; Zhang, W.; Liu, S.; Wang, X.; Pan, Z.; Zhu, G. A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 2020, 51, 93. [Google Scholar] [CrossRef]
- Duan, Q.; Wu, W.; Pang, S.; Pan, Z.; Zhang, W.; Zhu, G. Coimmunization with two enterotoxigenic Escherichia coli (ETEC) fimbrial multiepitope fusion antigens induces the production of neutralizing antibodies against five ETEC fimbriae (F4, F5, F6, F18, and F41). Appl. Environ. Microbiol. 2020, 86, e00217-20. [Google Scholar] [CrossRef]
- Li, S.; Anvari, S.; Ptacek, G.; Upadhyay, I.; Kaminski, R.W.; Sack, D.A.; Zhang, W. A broadly immunogenic polyvalent Shigella multiepitope fusion antigen protein protects against Shigella sonnei and Shigella flexneri lethal pulmonary challenges in mice. Infect. Immun. 2023, 91, e0031623. [Google Scholar] [CrossRef] [PubMed]
- Molina Estupiñan, J.L.; Aradottir Pind, A.A.; Foroutan Pajoohian, P.; Jonsdottir, I.; Bjarnarson, S.P. The adjuvants dmLT and mmCT enhance humoral immune responses to a pneumococcal conjugate vaccine after both parenteral or mucosal immunization of neonatal mice. Front. Immunol. 2023, 13, 1078904. [Google Scholar] [CrossRef]
- Stone, A.E.; Rambaran, S.; Trinh, I.V.; Estrada, M.; Jarand, C.W.; Williams, B.S.; Murrell, A.E.; Huerter, C.M.; Bai, W.; Palani, S.; et al. Route and antigen shape immunity to dmLT-adjuvanted vaccines to a greater extent than biochemical stress or formulation excipients. Vaccine 2023, 41, 1589–1601. [Google Scholar] [CrossRef]
- Nandre, R.M.; Duan, Q.; Wang, Y.; Zhang, W. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine 2017, 35, 552–565. [Google Scholar] [CrossRef]
- Nandre, R.; Ruan, X.; Lu, T.; Duan, Q.; Sack, D.; Zhang, W. Enterotoxigenic Escherichia coli adhesin-toxoid multiepitope fusion antigen CFA/I/II/IV-3×STaN12S-dmLTR192G/L211A-derived antibodies inhibit adherence of seven adhesins, neutralize enterotoxicity of LT and STa toxins, and protect piglets against diarrhea. Infect. Immun. 2018, 86, e00550-17. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Howlader, D.R.; Zheng, Q.; Ratnakaram, S.S.K.; Whittier, S.K.; Lu, T.; Keith, J.D.; Picking, W.D.; Birket, S.E.; Picking, W.L. Development of a broadly protective, self-adjuvanting subunit vaccine to prevent infections by Pseudomonas aeruginosa. Front. Immunol. 2020, 11, 583008. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Poncet, D.; Seydoux, E.; Rintala, N.D.; Macie, M., Jr.; Ruiz, S.; Orr, M.T. The TLR4 agonist adjuvant SLA-SE promotes functional mucosal antibodies against a parenterally delivered ETEC vaccine. NPJ Vaccines 2019, 4, 19. [Google Scholar] [CrossRef]
- Duan, Q.D.; Yao, F.H.; Zhu, G.Q. Major virulence factors of enterotoxigenic Escherichia coli in pigs. Ann. Microbiol. 2012, 62, 7–14. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Ruan, X.; Knudsen, D.E.; Wollenberg, K.M.; Sack, D.A.; Zhang, W. Multi-epitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin. Vaccine Immunol. 2014, 21, 243–249. [Google Scholar] [CrossRef]
- Nandre, R.M.; Ruan, X.; Duan, Q.; Sack, D.A.; Zhang, W. Antibodies derived from an enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multi-epitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21 and EtpA. Vaccine 2016, 34, 3620–3625. [Google Scholar] [CrossRef]


| ETEC Strain | Fimbrial Protein | Selective Neutralizing Peptides | Predictive Value a |
|---|---|---|---|
| K88 | FaeG | LPRGSELSAGSAAAA | 0.6044 |
| K99 | FanC | NVGNGSGGANIN | 2.3671 |
| 987P | FasA | AGNNNTGSDTKYLVPASNDTSASG | 1.1994 |
| F41 | Fim41a | WDDLSHPNYTSADKASYLSYGSGVSAG | 0.5260 |
| F18 | FedF | IPSSSGTLTCQAGT | 0.9849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Liu, H.; Li, Y.; Chang, J.; Yang, Y.; Gu, S. Chimeric Fimbrial Multiepitope Antigen Fused to Double-Mutant LT (dmLT) Induces Antibodies That Inhibit Enterotoxigenic E. coli Adhesion in Porcine IPEC-J2 Cells. Animals 2025, 15, 2858. https://doi.org/10.3390/ani15192858
He J, Liu H, Li Y, Chang J, Yang Y, Gu S. Chimeric Fimbrial Multiepitope Antigen Fused to Double-Mutant LT (dmLT) Induces Antibodies That Inhibit Enterotoxigenic E. coli Adhesion in Porcine IPEC-J2 Cells. Animals. 2025; 15(19):2858. https://doi.org/10.3390/ani15192858
Chicago/Turabian StyleHe, Jinxin, Hongrui Liu, Yuexin Li, Jiashu Chang, Yayun Yang, and Shaopeng Gu. 2025. "Chimeric Fimbrial Multiepitope Antigen Fused to Double-Mutant LT (dmLT) Induces Antibodies That Inhibit Enterotoxigenic E. coli Adhesion in Porcine IPEC-J2 Cells" Animals 15, no. 19: 2858. https://doi.org/10.3390/ani15192858
APA StyleHe, J., Liu, H., Li, Y., Chang, J., Yang, Y., & Gu, S. (2025). Chimeric Fimbrial Multiepitope Antigen Fused to Double-Mutant LT (dmLT) Induces Antibodies That Inhibit Enterotoxigenic E. coli Adhesion in Porcine IPEC-J2 Cells. Animals, 15(19), 2858. https://doi.org/10.3390/ani15192858





