Integrated Analysis of Carotenoid Metabolism, Lipid Profiles, and Gut Microbiota Reveals Associations Fundamental to Skin Pigmentation in Lingshan Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals, Experiment Design, Diets, and Management
2.3. Sample Collection
2.4. Skin Color
2.5. Serum Parameters
2.6. Pigment Concentrations
2.7. RT-qPCR Analysis
2.8. Cecal Microbiota
2.9. Statistical Analysis
3. Results
3.1. Skin Color Differences in Lingshan Chickens with Different Shank Colors
3.2. Effect of Skin Color on Serum Biochemical Parameters in Lingshan Chickens
3.3. Comparative Analysis of Pigment Deposition in the Serum, Tissues, and Organs of Lingshan Chickens with Different Skin Colors
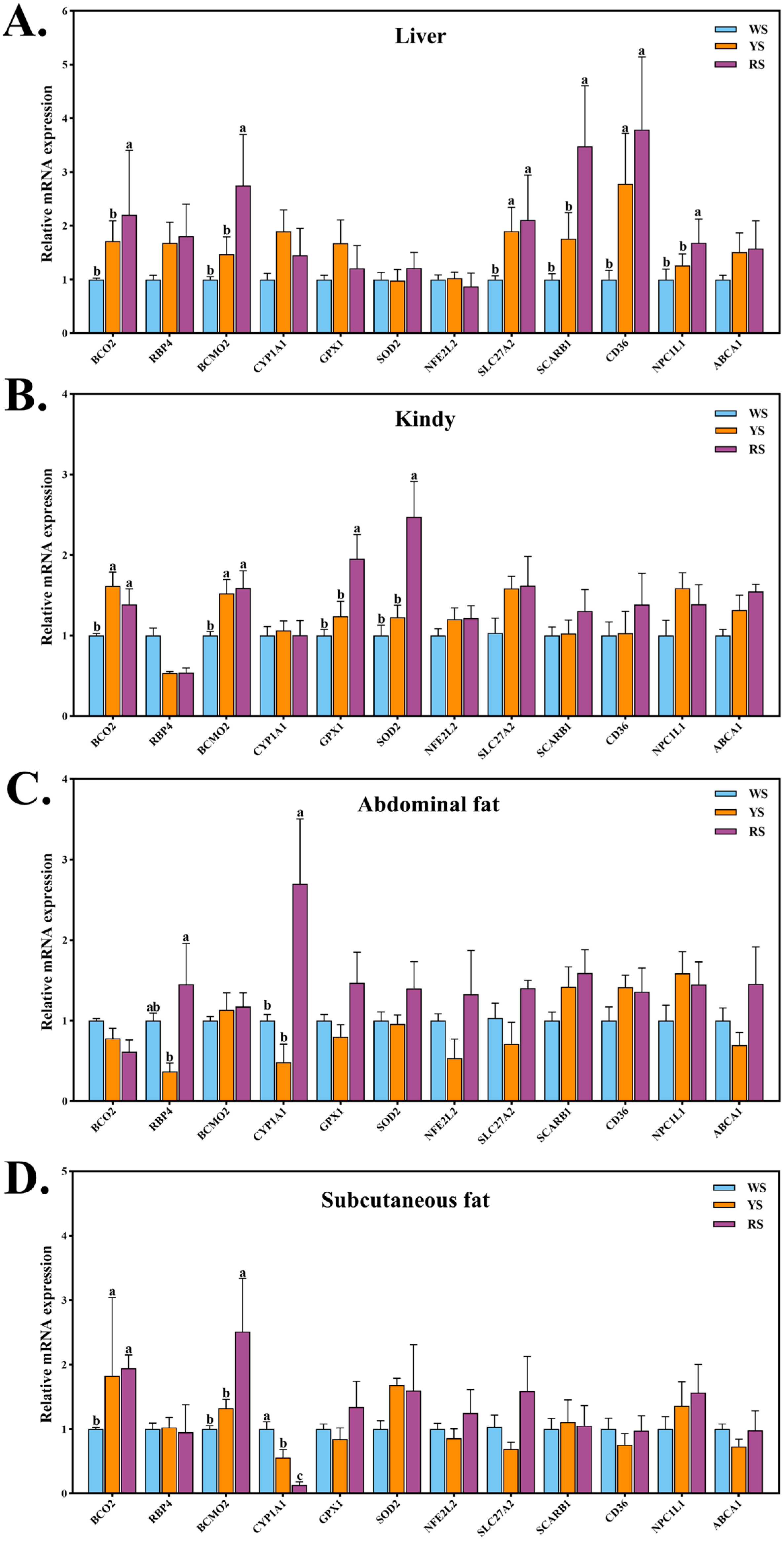
3.4. Differential Expression of Pigment Deposition–Related Genes in Lingshan Chickens with Different Skin Colors
3.5. Cecal Microbial Composition in Lingshan Chickens with Different Skin Colors
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Yu, P.; Ding, X.; Xu, M.; Guo, B.; Xu, Y. Genetic polymorphisms of the AMPD1 gene and their correlations with IMP contents in Fast Partridge and Lingshan chickens. Gene 2015, 574, 204–209. [Google Scholar] [CrossRef]
- Perini, F.; Cendron, F.; Lasagna, E.; Cassandro, M.; Penasa, M. Genomic insights into shank and eggshell color in Italian local chickens. Poult. Sci. 2024, 103, 103677. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Jiang, R.-S. Recent advances in breeding for quality chickens. World’s Poult. Sci. J. 2005, 61, 373–381. [Google Scholar] [CrossRef]
- Škrlep, M.; Poklukar, K.; Vrecl, M.; Brankovič, J.; Čandek-Potokar, M. Growth Performance, Carcass Quality, and Lipid Metabolism in Krškopolje Pigs and Modern Hybrid Pigs: Comparison of Genotypes and Evaluation of Dietary Protein Reduction. Animals 2024, 14, 3331. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Deng, X.; Wu, J.; Luo, W. Genetic and metabolic factors influencing skin yellowness in yellow-feathered broilers. Poult. Sci. 2025, 104, 104534. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Z.; Chen, G.; Luo, Q.; Nie, Q.; Zhang, X.; Luo, W. Characterization of chicken skin yellowness and exploration of genes involved in skin yellowness deposition in chicken. Front. Physiol. 2021, 12, 585089. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Li, Z.; Nong, Y.; Wang, J.; Wang, Z.; Li, Z. Whole-genome resequencing reveals melanin deposition candidate genes of Luning chicken. BMC Genomics 2024, 25, 858. [Google Scholar] [CrossRef]
- Madkour, F.A. Unique insights into morphological characterization and functional adaptation of the scaly shank skin in aquatic and terrestrial birds. Sci. Rep. 2024, 14, 28101. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Shaaban, H.A.; Abdel-Gawad, H.H.; Sleem, M.M. Composition and Health Benefits of Carotenoids in Foods and Feeds. In Carotenoids: Trends and Advances; Springer: Cham, Switzerland, 2024; pp. 227–244. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2021, 41, 1–29. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Luo, Y. Mechanisms of carotenoid intestinal absorption and the regulation of dietary lipids: Lipid transporter-mediated transintestinal epithelial pathways. Crit. Rev. Food Sci. Nutr. 2024, 64, 1791–1816. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Saadane, A.; Shen, T.; Yakovleva, D.; Banerjee, R.; Zhang, Y.; Brown, J.M.; von Lintig, J. Distinct pathways for the absorption and metabolism of β-carotene and zeaxanthin in the mouse intestine. J. Lipid Res. 2025, 66, 100758. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Papotti, B.; Escolà-Gil, J.C.; Julve, J.; Potì, F.; Zanotti, I. Impact of dietary lipids on the reverse cholesterol transport: What we learned from animal studies. Nutrients 2021, 13, 2643. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary flavonoids as modulators of lipid metabolism in poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, Q.; Zhan, Q.; Zhao, M.; Zhao, L. Oat protein isolate-Pleurotus ostreatus β-glucan conjugate nanoparticles bound to β-carotene effectively alleviate immunosuppression by regulating gut microbiota. Food Funct. 2024, 15, 1867–1883. [Google Scholar] [CrossRef]
- Kanika, N.H.; Hou, X.; Liu, H.; Dong, Y.; Wang, J.; Wang, C. Specific gut microbiome’s role in skin pigmentation: Insights from SCARB1 mutants in Oujiang colour common carp. J. Appl. Microbiol. 2024, 135, lxae226. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp. Biol. Med. 2018, 243, 613–620. [Google Scholar] [CrossRef]
- NY/T 3645-2020; Nutrient Requirements of Yellow Chickens. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- Yang, W.; Fang, C.; Liu, J.; Zhang, Q.; Lv, J.; Shang, G.; Wu, S.; Fang, R. Yeast β-glucan as a surfactant for selenium nanoparticles: Synthesis, characterization, bioavailability, and regulation of muscle Fiber type transformation. Food Res. Int. 2025, 218, 116934. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Human skin pigmentation, migration and disease susceptibility. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms of transport and delivery of vitamin A and carotenoids to the retinal pigment epithelium. Mol. Nutr. Food Res. 2019, 63, 1801046. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Díaz-Gómez, J.; Nogareda, C.; Angulo, E.; Sandmann, G.; Portero-Otin, M.; Serrano, J.C.; Twyman, R.M.; Capell, T.; Zhu, C. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci. Rep. 2016, 6, 35346. [Google Scholar] [CrossRef]
- McGraw, K.J.; Toomey, M.B. Carotenoid accumulation in the tissues of zebra finches: Predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol. Biochem. Zool. 2010, 83, 97–109. [Google Scholar] [CrossRef]
- Lopes, R.J.; Johnson, J.D.; Toomey, M.B.; Ferreira, M.S.; Araujo, P.M.; Melo-Ferreira, J.; Andersson, L.; Hill, G.E.; Corbo, J.C.; Carneiro, M. Genetic basis for red coloration in birds. Curr. Biol. 2016, 26, 1427–1434. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Ohia, S.E.; Moriyama, H.; Chakraborty, S.; Wang, C.-K.; Song, Y.S.; Goel, A.; Bagchi, M.; Bagchi, D. Current insights on the photoprotective mechanism of the macular carotenoids, lutein and zeaxanthin: Safety, efficacy and bio-delivery. J. Am. Nutr. Assoc. 2024, 43, 505–518. [Google Scholar] [CrossRef]
- McGraw, K.; Nolan, P.; Crino, O. Carotenoid accumulation strategies for becoming a colourful House Finch: Analyses of plasma and liver pigments in wild moulting birds. Funct. Ecol. 2006, 20, 678–688. [Google Scholar] [CrossRef]
- Hill, G.E.; Johnson, J.D. The vitamin A–redox hypothesis: A biochemical basis for honest signaling via carotenoid pigmentation. Am. Nat. 2012, 180, E127–E150. [Google Scholar] [CrossRef]
- Watkins, J.L.; Pogson, B.J. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020, 25, 501–512. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale delivery systems of lutein: An updated review from a pharmaceutical perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Helgeland, H.; Sandve, S.R.; Torgersen, J.S.; Halle, M.K.; Sundvold, H.; Omholt, S.; Våge, D.I. The evolution and functional divergence of the beta-carotene oxygenase gene family in teleost fish—Exemplified by Atlantic salmon. Gene 2014, 543, 268–274. [Google Scholar] [CrossRef]
- Bas, T.G. Bioactivity and bioavailability of carotenoids applied in human health: Technological advances and innovation. Int. J. Mol. Sci. 2024, 25, 7603. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018, 59, 1114. [Google Scholar] [CrossRef] [PubMed]
- Sundvold, H.; Helgeland, H.; Baranski, M.; Omholt, S.W.; Våge, D.I. Characterisation of a novel paralog of scavenger receptor class B member I (SCARB1) in Atlantic salmon (Salmo salar). BMC Genet. 2011, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Palczewski, G.; Babino, D.; Von Lintig, J. Mammalian carotenoid-oxygenases: Key players for carotenoid function and homeostasis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 78–87. [Google Scholar] [CrossRef]
- Elkin, J.; Martin, A.; Courtier-Orgogozo, V.; Santos, M.E. Analysis of the genetic loci of pigment pattern evolution in vertebrates. Biol. Rev. 2023, 98, 1250–1277. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- He, Z.; Dong, H. The roles of short-chain fatty acids derived from colonic bacteria fermentation of non-digestible carbohydrates and exogenous forms in ameliorating intestinal mucosal immunity of young ruminants. Front. Immunol. 2023, 14, 1291846. [Google Scholar] [CrossRef]
- Wassie, T.; Cheng, B.; Zhou, T.; Gao, L.; Lu, Z.; Xie, C.; Wu, X. Microbiome-metabolome analysis reveals alterations in the composition and metabolism of caecal microbiota and metabolites with dietary Enteromorpha polysaccharide and Yeast glycoprotein in chickens. Front. Immunol. 2022, 13, 996897. [Google Scholar] [CrossRef]


| Items | Content |
|---|---|
| Ingredients (%) | |
| Corn | 53.85 |
| Sorghum | 20.00 |
| Soybean meal | 16.50 |
| Fish meal | 4.20 |
| Calcium hydrogen phosphate | 1.00 |
| Stone powder | 0.90 |
| Dl-methionine | 0.15 |
| L-Lysine sulfate | 0.50 |
| L-Threonine | 0.08 |
| Salt | 0.20 |
| Choline chloride | 0.10 |
| Complex enzyme | 0.02 |
| Premix 1 | 2.50 |
| Total | 100.00 |
| Nutrition level 2 | |
| Metabolizable energy, (MJ/Kg) | 12.76 |
| Crude protein, % | 16.20 |
| Calcium, % | 1.35 |
| Total phosphorus, % | 0.42 |
| Available phosphorus, % | 0.33 |
| Gene Symbols | Accession NO. | Production Length | Primer Sequence (5′ to 3′) |
|---|---|---|---|
| SCARB1 | XM_015275627.4 | 141 | GTGAACCAGCGTGGACCGTATG CTCATCTTCAGTGCCGTTGGACAA |
| CD36 | NM_001030731.1 | 132 | GCGATTTGGTTAATGGCACTGATGG CCTTCACGGTCTTACTGGTCTGGTA |
| NPC1L1 | NM_207242.2 | 105 | CTGGCTGGCTCTCATCATCATCTTC CCTGCTGTCTTGTTCTTGTTCCTGT |
| ABCA1 | NM_204145.3 | 225 | GCTCTCCGAAGTGGCTCTGATGA ACGAGTGTGGCTGGAACGATGTA |
| BCO2 | XM_004948142.5 | 136 | GATTAACAACCAGCACAACCGCATT GCTCACATTGGCATTGTCACTTGG |
| RBP4 | NM_205238.2 | 55 | ACTGGGTAGTGGACACAGATTACGA TCACGGCAGGAATAATGAAGAGCAT |
| SLC27A2 | XM_046934089.1 | 104 | ACCAACACTACCACTGCCTCCAA GCCACCATCATCACCTTCATCTCTG |
| BCMO1 | NM_001364902.2 | 149 | CAAAGAAGAGCATCCAGAGCCCATA GCAGCAGAGCCAAGCCATCAA |
| CYP27B1 | NM_010009.2 | 403 | TCCAGAGGCAGTGAGTCGGTTC CGTTGTCCAGAGTTCCAGCATAGC |
| CYP1A1 | NM_205147.2 | 116 | TCTTCCTCTTCCTCACCACCATCC GCACTCGCACTGCTTGTACTTCA |
| GPX1 | NM_001277853.3 | 166 | GCAAAGTGCTGCTGGTGGTCAA ATCTCCTCGTTGGTGGCGTTCT |
| SOD2 | NM_204211.2 | 188 | GTCGCAAGGCAGAAGCACACT GACACCTGAGCTGTAACATCACCTT |
| NFE2L2 | NM_001396902.1 | 93 | GGACGGTGACACAGGAACAACAA CTCCACAGCGGGAAATCAGAAAGAT |
| β-actin | NM_205518.2 | 89 | CCAGCCATGTATGTAGCCATCCAG ACACCATCACCAGAGTCCATCACA |
| Items 1 | WS | YS | RS | SEM 2 | p-Value |
|---|---|---|---|---|---|
| a* | |||||
| Shank | 10.14 b | 12.53 b | 16.78 a | 0.97 | 0.009 |
| Subaxillary region | 3.83 | 4.29 | 3.39 | 0.296 | 0.506 |
| Breast | 8.83 | 7.14 | 9.76 | 0.699 | 0.319 |
| b* | |||||
| Shank | 29.70 | 31.74 | 32.74 | 0.921 | 0.413 |
| Subaxillary region | 4.14 b | 10.68 a | 11.29 a | 1.068 | 0.009 |
| Breast | 15.85 b | 20.88 a | 22.12 a | 0.994 | 0.006 |
| L* | |||||
| Shank | 66.20 | 68.66 | 61.83 | 1.311 | 0.105 |
| Subaxillary region | 71.12 | 72.23 | 73.94 | 1.054 | 0.574 |
| Breast | 65.94 | 70.81 | 67.92 | 0.922 | 0.088 |
| Roche color fan scores | |||||
| Shank | 4.17 c | 8.67 b | 11.67 a | 0.768 | <0.001 |
| Thigh | 1.00 | 1.17 | 1.33 | 0.090 | 0.342 |
| Subaxillary region | 1.00 b | 1.67 a | 2.00 a | 0.145 | 0.007 |
| Breast | 1.33 b | 3.17 a | 3.83 a | 0.298 | <0.001 |
| Abdomen | 2.33 b | 4.17 a | 4.00 a | 0.283 | 0.005 |
| Back | 1.00 | 1.33 | 1.17 | 0.090 | 0.342 |
| Items 1 | WS | YS | RS | SEM 2 | p-Value |
|---|---|---|---|---|---|
| TG (mmol/L) | 0.47 b | 0.65 b | 1.03 a | 0.075 | 0.002 |
| TC (mmol/L) | 1.83 c | 2.66 b | 3.65 a | 0.194 | <0.001 |
| HDL (mmol/L) | 3.81 b | 4.72 a | 5.07 a | 0.193 | 0.006 |
| LDL (mmol/L) | 1.51 b | 2.39 a | 2.67 a | 0.150 | <0.001 |
| VLDL (mmol/mL) | 0.54 b | 0.55 b | 0.57 a | 0.057 | 0.008 |
| Items 1 | WS | YS | RS | SEM 2 | p-Value |
|---|---|---|---|---|---|
| Xanthophyll, µg/100 mg | |||||
| Serum | 0.0030 c | 0.0125 b | 0.0202 a | 0.0028 | 0.0040 |
| Subcutaneous fat | 0.0022 b | 0.0049 a | 0.0052 a | 0.0005 | 0.0060 |
| Abdominal fat | 0.0022 b | 0.0069 a | 0.0062 a | 0.0008 | <0.001 |
| Breast muscle | 0.0000 b | 0.0010 a | 0.0013 a | 0.0002 | 0.0140 |
| Thigh muscle | 0.0015 | 0.0038 | 0.0021 | 0.0008 | 0.5690 |
| Zeaxanthin, µg/100 mg | |||||
| Serum | 0.0021 c | 0.0071 b | 0.0135 a | 0.0018 | 0.0060 |
| Subcutaneous fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Abdominal fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Breast muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| Thigh muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| β-Cryptoxanthin, µg/100 mg | |||||
| Serum | 0.0000 | 0.0010 | 0.0009 | 0.0002 | 0.0920 |
| Subcutaneous fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Abdominal fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Breast muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| Thigh muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| β-Carotene, µg/100 mg | |||||
| Serum | 0.0000 b | 0.0002 b | 0.0013 a | 0.0002 | 0.0030 |
| Subcutaneous fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Abdominal fat | 0.0000 | 0.0000 | 0.0000 | - | - |
| Breast muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| Thigh muscle | 0.0000 | 0.0000 | 0.0000 | - | - |
| Items 1 | WS | YS | RS | SEM 2 | p-Value |
|---|---|---|---|---|---|
| Xanthophyll, µg/100 mg | |||||
| Heart | 0.0030 c | 0.0125 b | 0.0202 a | 0.0027 | 0.0044 |
| Liver | 0.0090 b | 0.0167 ab | 0.0308 a | 0.0039 | 0.0398 |
| Spleen | 0.0026 b | 0.0116 b | 0.0293 a | 0.0046 | 0.0213 |
| Lung | 0.0009 b | 0.0022 b | 0.0045 a | 0.0006 | 0.0021 |
| Kidney | 0.0000 b | 0.0029 ab | 0.0037 a | 0.0008 | 0.0487 |
| α-Carotene, µg/100 mg | |||||
| Heart | 0.0000 b | 0.0000 b | 0.0038 a | 0.0007 | 0.0046 |
| Liver | 0.0000 b | 0.0011 ab | 0.0016 a | 0.0003 | 0.0460 |
| Spleen | 0.0000 | 0.0000 | 0.0000 | - | - |
| Lung | 0.0000 | 0.0000 | 0.0000 | - | - |
| Kidney | 0.0000 | 0.0000 | 0.0000 | - | - |
| β-Cryptoxanthin, µg/100 mg | |||||
| Heart | 0.0005 b | 0.0029 b | 0.0067 a | 0.0010 | 0.0081 |
| Liver | 0.0000 | 0.0000 | 0.0000 | - | - |
| Spleen | 0.0000 | 0.0000 | 0.0000 | - | - |
| Lung | 0.0000 | 0.0000 | 0.0000 | - | - |
| Kidney | 0.0000 | 0.0000 | 0.0000 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Yang, W.; Hu, S.; Li, L.; He, J.; Bian, G. Integrated Analysis of Carotenoid Metabolism, Lipid Profiles, and Gut Microbiota Reveals Associations Fundamental to Skin Pigmentation in Lingshan Chickens. Animals 2025, 15, 2832. https://doi.org/10.3390/ani15192832
Deng S, Yang W, Hu S, Li L, He J, Bian G. Integrated Analysis of Carotenoid Metabolism, Lipid Profiles, and Gut Microbiota Reveals Associations Fundamental to Skin Pigmentation in Lingshan Chickens. Animals. 2025; 15(19):2832. https://doi.org/10.3390/ani15192832
Chicago/Turabian StyleDeng, Shengting, Weiguang Yang, Shengdi Hu, Long Li, Jianhua He, and Guozhi Bian. 2025. "Integrated Analysis of Carotenoid Metabolism, Lipid Profiles, and Gut Microbiota Reveals Associations Fundamental to Skin Pigmentation in Lingshan Chickens" Animals 15, no. 19: 2832. https://doi.org/10.3390/ani15192832
APA StyleDeng, S., Yang, W., Hu, S., Li, L., He, J., & Bian, G. (2025). Integrated Analysis of Carotenoid Metabolism, Lipid Profiles, and Gut Microbiota Reveals Associations Fundamental to Skin Pigmentation in Lingshan Chickens. Animals, 15(19), 2832. https://doi.org/10.3390/ani15192832





