Impact of Thermal Manipulation of Broiler Eggs on Growth Performance, Splenic Inflammatory Cytokine Levels, and Heat Shock Protein Responses to Post-Hatch Lipopolysaccharide (LPS) Challenge
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
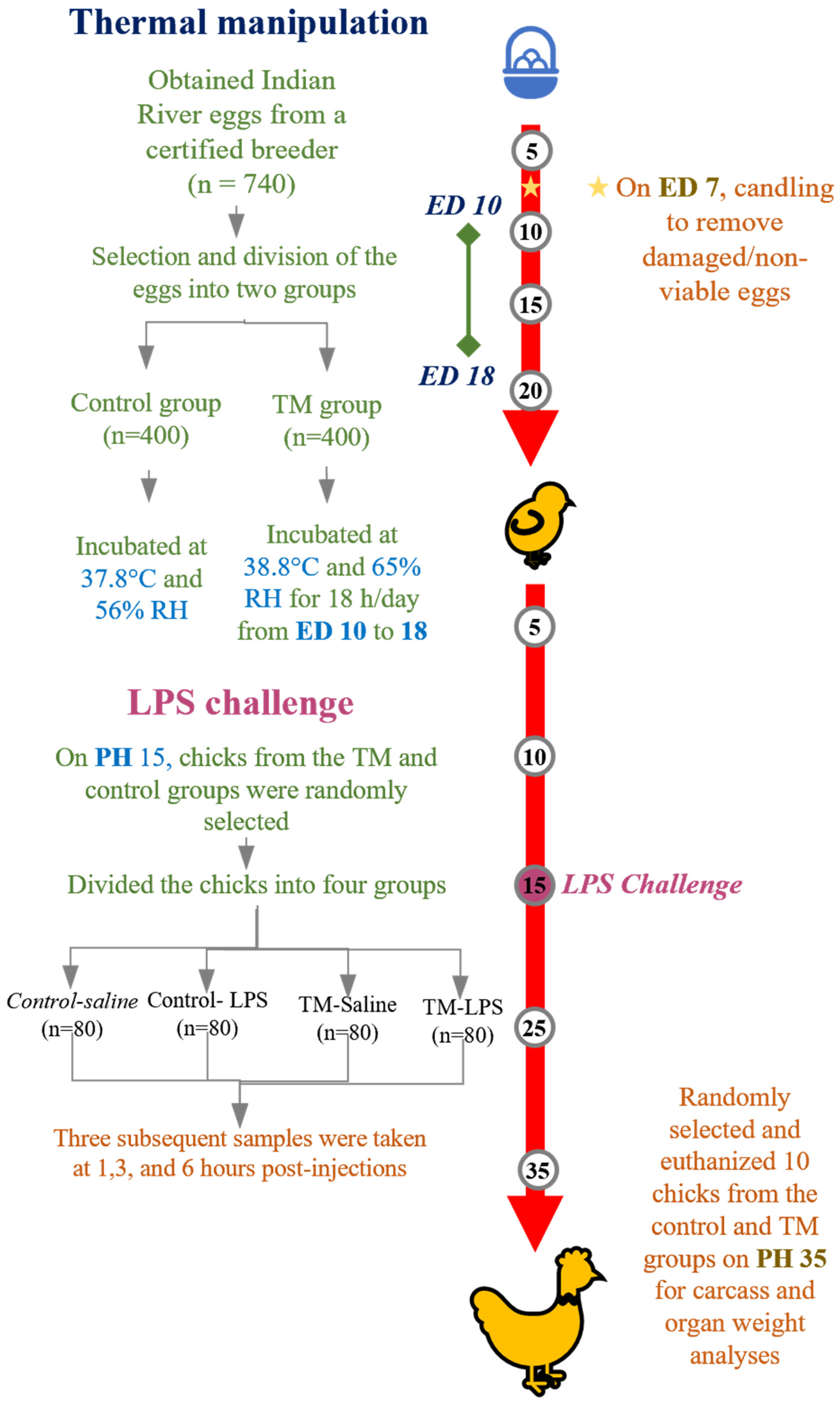
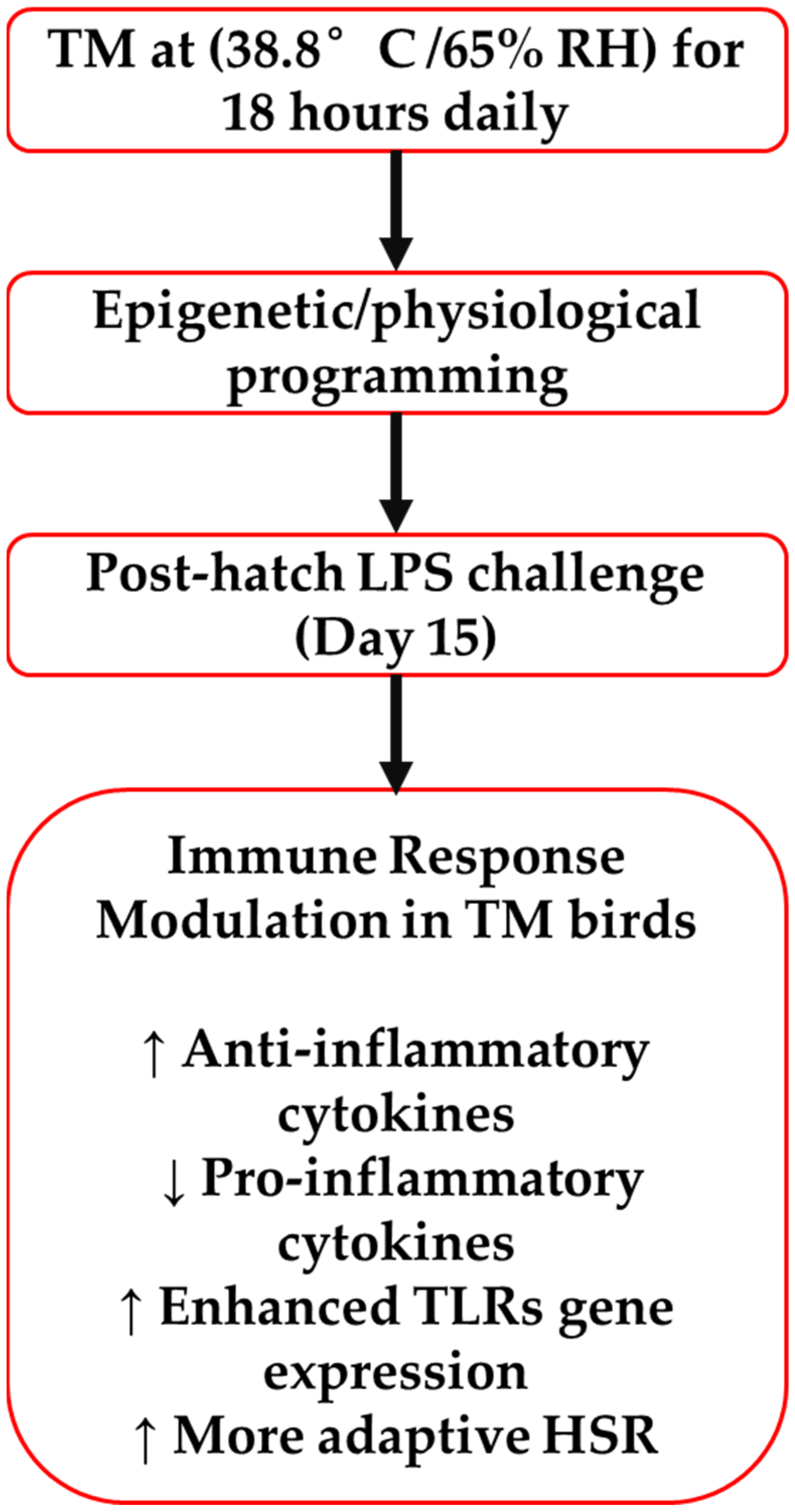
2.1. Experimental Design and Animal Management
2.2. Post-Hatch Rearing and Growth Performance Monitoring
2.3. LPS Challenge (Phase 2)
2.4. RNA Isolation and cDNA Synthesis
2.5. Relative-Quantitative Real-Time PCR (RT-qPCR)
2.6. Organ Weights
2.7. Statistical Analysis
2.8. Use and Potential Use of AI
3. Results
3.1. The Impact of TM on Hatchability Rate, Body Weight (BW), Body Temperature (Tb), Growth Performance Metrics, and Internal Organ Weight
3.2. Impact of TM and LPS Injection on Body Temperature (Tb) in Broiler Chickens
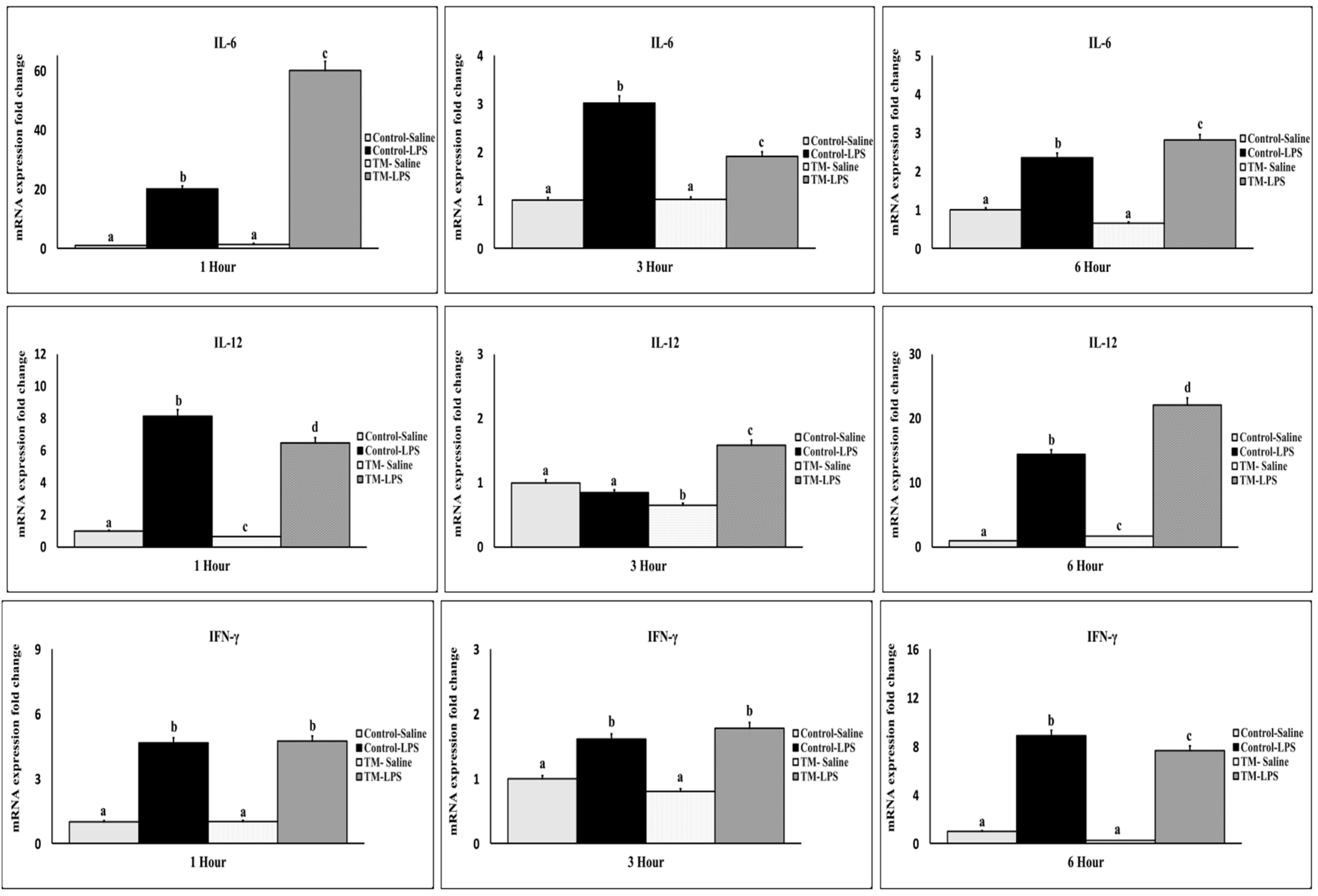
3.3. The Impact of TM and LPS Challenges on the mRNA Levels of Proinflammatory Cytokines (TNF-α, IL-1β, IL-2, IL-6, IL-12, and IFN-γ)
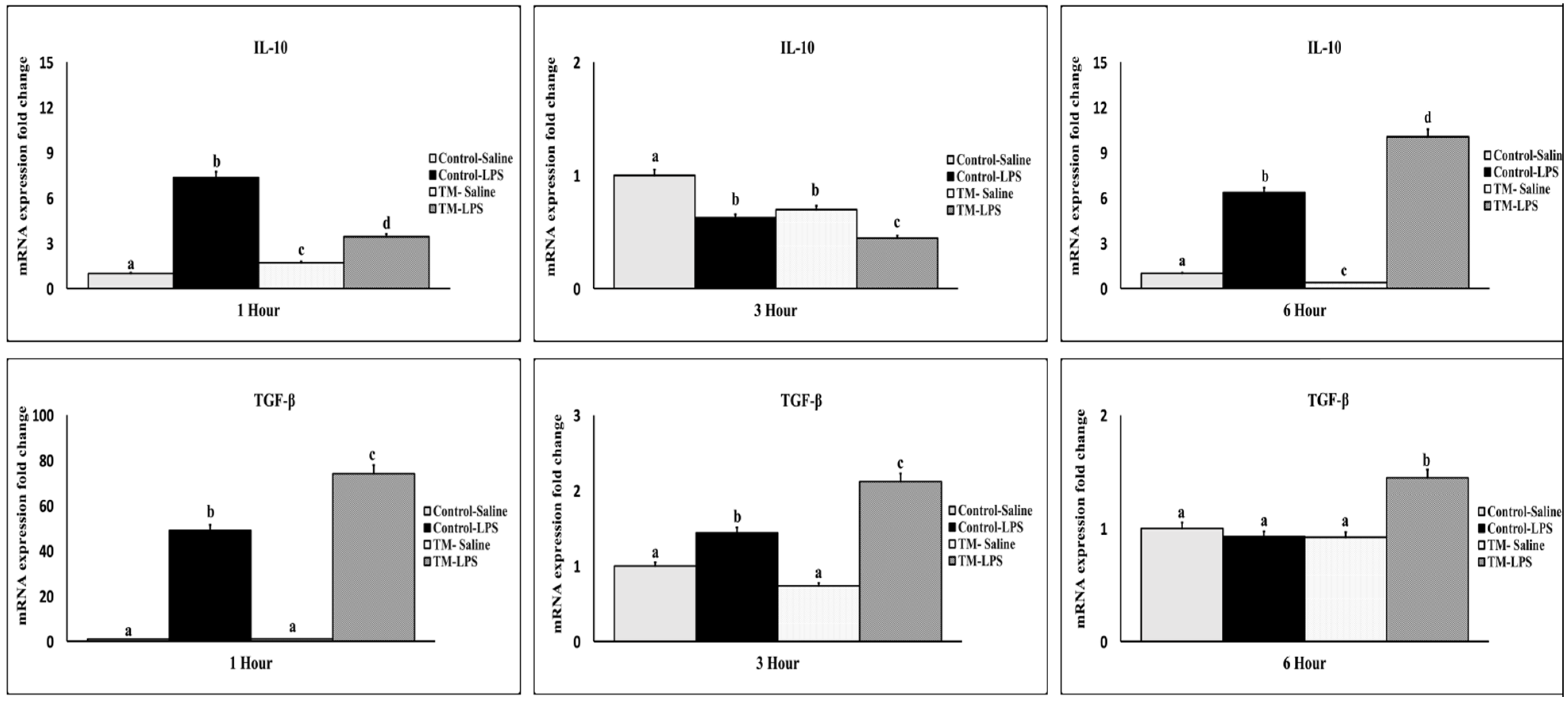
3.4. Impact of TM and LPS Challenge on mRNA Levels of Anti-Inflammatory Cytokines (IL-10 and TGF-β)
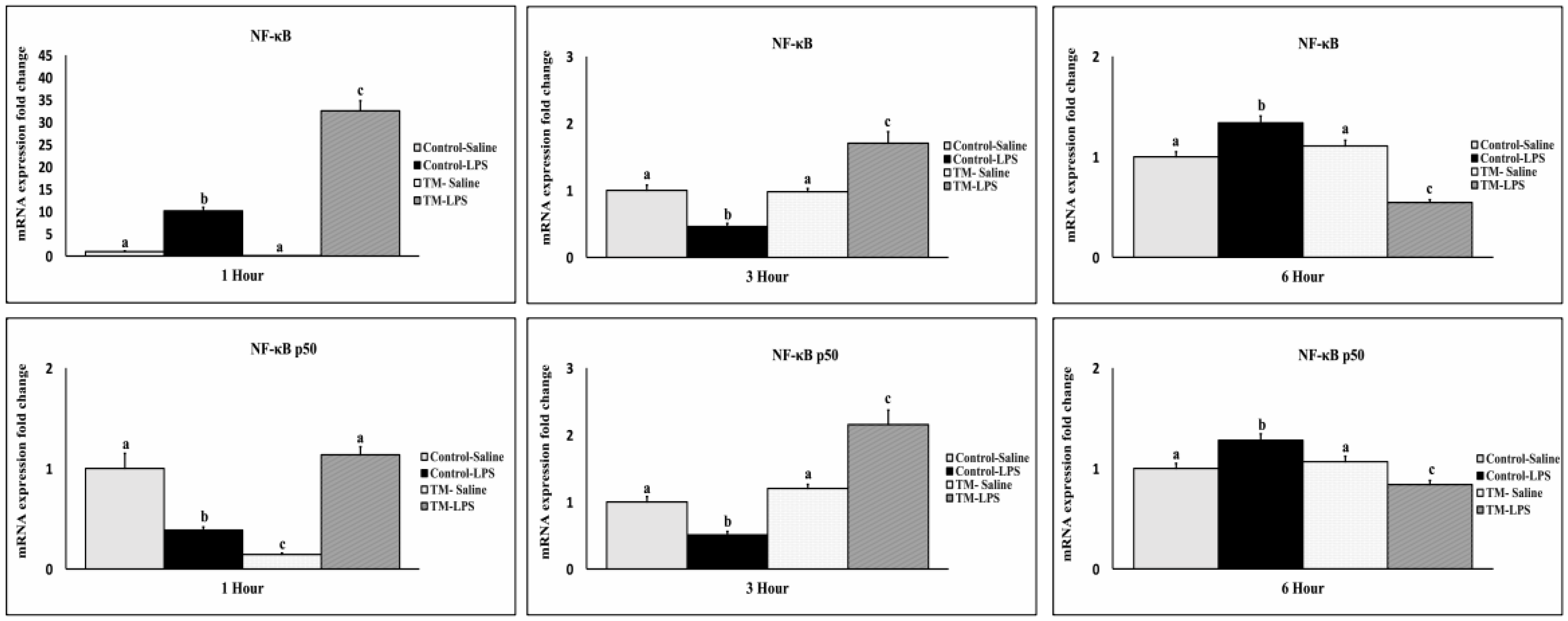
3.5. Impact of TM and LPS Challenge on the mRNA Levels of NF-κB and NF-κBp50 Transcription Factor
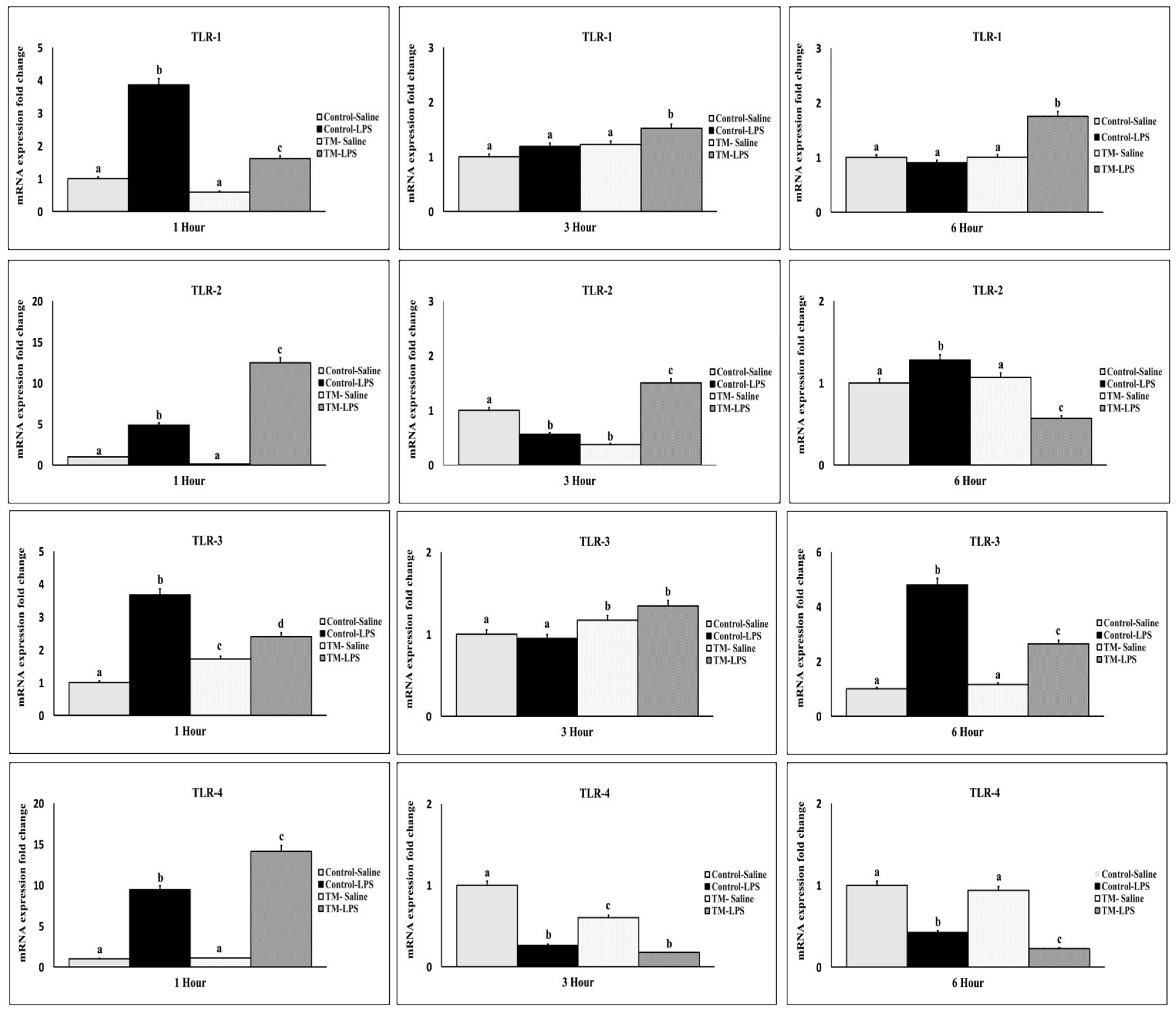
3.6. Impact of TM and LPS Challenge on Toll-like Receptors (TLRs) mRNA Levels (TLR 1, TLR 2, TLR 3, and TLR 4)
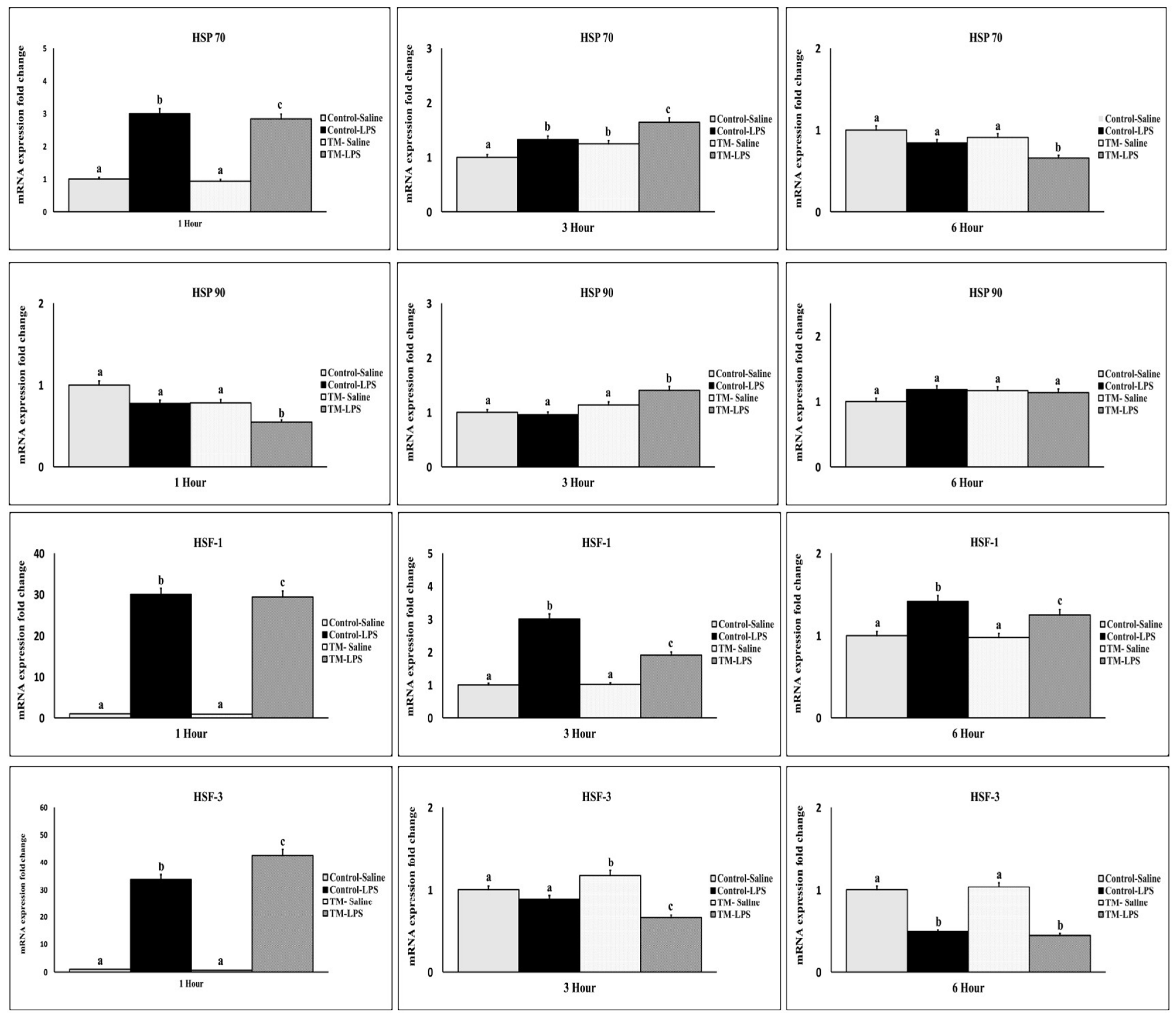
3.7. Impact of TM and LPS Challenge on mRNA Levels of Heat Shock Proteins and Heat Shock Factors (HSP70, HSP90, HSF-1, and HSF-3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADG | Average Daily Gain |
| BW | Body Weight |
| cDNA | Complementary DNA |
| DFI | Daily Feed Intake |
| DNA | Deoxyribonucleic Acid |
| ED | Embryonic Day |
| FCR | Feed Conversion Ratio |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| H&E | Hematoxylin and Eosin |
| HSP | Heat Shock Protein |
| HSP70 | 70-kDa Heat Shock Protein |
| HSP90 | 90-kDa Heat Shock Protein |
| HSF | Heat Shock Factor |
| HSF-1 | Heat Shock Factor 1 |
| HSF-3 | Heat Shock Factor 3 |
| IFN-γ | Interferon Gamma |
| IL | Interleukin |
| IL-1β | Interleukin 1 Beta |
| IL-2 | Interleukin 2 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IP | Intraperitoneal |
| LPS | Lipopolysaccharide |
| mRNA | Messenger Ribonucleic Acid |
| NF-κB | Nuclear Factor Kappa B |
| NF-κBp50 | Nuclear Factor Kappa B p50 Subunit |
| RH | Relative Humidity |
| RNA | Ribonucleic Acid |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction |
| SD | Standard Deviation |
| Tb | Body Temperature |
| TGF-β | Transforming Growth Factor Beta |
| TM | Thermal Manipulation |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- Bennett, C.E.; Thomas, R.; Williams, M.; Zalasiewicz, J.; Edgeworth, M.; Miller, H.; Coles, B.; Foster, A.; Burton, E.J.; Marume, U. The broiler chicken as a signal of a human reconfigured biosphere. R. Soc. Open Sci. 2018, 5, 180325. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Perez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.; D’Angelo, M.; Sola-Oriol, D. Targeted-Release Organic Acids and Essential Oils Improve Performance and Digestive Function in Broilers Under a Necrotic Enteritis Challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Babacan, O.; KaradenİZ, H. Çiğ tavuk etlerinden izole edilen Salmonella spp. suşlarının antibiyotik duyarlılıklarının araştırılması. Vet. Hekim. Der. Derg. 2019, 90, 105–114. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S93–S106. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Sukker, H.; Ababneh, M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019, 98, 991–1001. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Jaradat, Z.W.; Ababneh, M.M.; Okour, M.Z.; Saleh, K.M.M.; Alkofahi, A.; Alboom, M.H. Effects of embryonic thermal manipulation on the immune response to post-hatch Escherichia coli challenge in broiler chicken. Vet. World 2023, 16, 918–928. [Google Scholar] [CrossRef]
- Collin, A.; Berri, C.; Tesseraud, S.; Rodon, F.E.; Skiba-Cassy, S.; Crochet, S.; Duclos, M.J.; Rideau, N.; Tona, K.; Buyse, J.; et al. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 2007, 86, 795–800. [Google Scholar] [CrossRef]
- Piestun, Y.; Shinder, D.; Ruzal, M.; Halevy, O.; Brake, J.; Yahav, S. Thermal manipulations during broiler embryogenesis: Effect on the acquisition of thermotolerance. Poult. Sci. 2008, 87, 1516–1525. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Wick, M.; Lilburn, M.S. Effect of embryonic thermal manipulation on heat shock protein 70 (HSP70) expression and subsequent immune response to post-hatch lipopolysaccharide challenge in Pekin ducklings. Poult. Sci. 2019, 98, 722–733. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Grzesiuk, E. Heat shock response in gastrointestinal tract. J. Physiol. Pharmacol. 2007, 58 (Suppl. S3), 43–62. [Google Scholar]
- Al-Zghoul, M.B.; Ismail, Z.B.; Dalab, A.E.; Al-Ramadan, A.; Althnaian, T.A.; Al-Ramadan, S.Y.; Ali, A.M.; Albokhadaim, I.F.; Al Busadah, K.A.; Eljarah, A.; et al. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Res. Vet. Sci. 2015, 99, 105–111. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Poultry; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Vitorino Carvalho, A.; Hennequet-Antier, C.; Crochet, S.; Bordeau, T.; Courousse, N.; Cailleau-Audouin, E.; Chartrin, P.; Darras, V.M.; Zerjal, T.; Collin, A.; et al. Embryonic thermal manipulation has short and long-term effects on the development and the physiology of the Japanese quail. PLoS ONE 2020, 15, e0227700. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.C.G.; Cristina-Silva, C.; Taxini, C.L.; da Costa Silva, K.S.; Lima, V.T.M.; Macari, M.; Bicego, K.C.; Szawka, R.E.; Gargaglioni, L.H. Embryonic thermal manipulation affects ventilation, metabolism, thermal control and central dopamine in newly hatched and juvenile chicks. Front. Physiol. 2021, 12, 699142. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B. Thermal manipulation during broiler chicken embryogenesis increases basal mRNA levels and alters production dynamics of heat shock proteins 70 and 60 and heat shock factors 3 and 4 during thermal stress. Poult. Sci. 2018, 97, 3661–3670. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Alliftawi, A.R.S.; Saleh, K.M.M.; Jaradat, Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019, 98, 4113–4122. [Google Scholar] [CrossRef]
- Nyuiadzi, D.; Travel, A.; Meda, B.; Berri, C.; Guilloteau, L.A.; Coustham, V.; Wang, Y.; Tona, J.K.; Collin, A. Effect of low incubation temperature and low ambient temperature until 21 days of age on performance and body temperature in fast-growing chickens. Poult. Sci. 2017, 96, 4261–4269. [Google Scholar] [CrossRef]
- Rajkumar, U.; Shanmugam, M.; Rajaravindra, K.S.; Vinoth, A.; Rao, S.V.R. Effect of increased incubation temperature on juvenile growth, immune and serum biochemical parameters in selected chicken populations. Indian J. Anim. Sci. 2015, 85, 1328–1333. [Google Scholar] [CrossRef]
- Vinoth, A.; Thirunalasundari, T.; Shanmugam, M.; Uthrakumar, A.; Suji, S.; Rajkumar, U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones 2018, 23, 235–252. [Google Scholar] [CrossRef]
- Almeida, V.R.; Morita, V.S.; Sgavioli, S.; Vicentini, T.I.; Castiblanco, D.M.; Boleli, I.C. Incubation temperature manipulation during fetal development reduces adiposity of broiler hatchlings. Poult. Sci. 2016, 95, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zaboli, G.R.; Rahimi, S.; Shariatmadari, F.; Torshizi, M.A.; Baghbanzadeh, A.; Mehri, M. Thermal manipulation during pre and post-Hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult. Sci. 2017, 96, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Tainika, B. Thermal Manipulation: Embryonic Development, Hatchability, and Hatching Quality of Broiler Chicks. In Broiler Industry; Téllez-Isaías, G., Latorre, J.D., Martínez-Aguilar, Y., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Al Amaz, S.; Mishra, B. Embryonic thermal manipulation: A potential strategy to mitigate heat stress in broiler chickens for sustainable poultry production. J. Anim. Sci. Biotechnol. 2024, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Amaz, S.A.; Shahid, M.A.H.; Chaudhary, A.; Jha, R.; Mishra, B. Embryonic thermal manipulation reduces hatch time, increases hatchability, thermotolerance, and liver metabolism in broiler embryos. Poult. Sci. 2024, 103, 103527. [Google Scholar] [CrossRef]
- Loyau, T.; Metayer-Coustard, S.; Berri, C.; Crochet, S.; Cailleau-Audouin, E.; Sannier, M.; Chartrin, P.; Praud, C.; Hennequet-Antier, C.; Rideau, N.; et al. Thermal manipulation during embryogenesis has long-term effects on muscle and liver metabolism in fast-growing chickens. PLoS ONE 2014, 9, e105339. [Google Scholar] [CrossRef]
- Elsayed, M.A. Effects of thermal manipulation during late incubation period on post-hatch thermotolerance in ostrich. Czech J. Anim. Sci. 2016, 61, 421–431. [Google Scholar] [CrossRef]
- David, S.A.; Vitorino Carvalho, A.; Gimonnet, C.; Brionne, A.; Hennequet-Antier, C.; Piegu, B.; Crochet, S.; Courousse, N.; Bordeau, T.; Bigot, Y.; et al. Thermal manipulation during embryogenesis impacts H3K4me3 and H3K27me3 histone marks in chicken hypothalamus. Front Genet. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Iraqi, E.; Hady, A.A.; Elsayed, N.; Khalil, H.; El-Saadany, A.; El-Sabrout, K. Effect of thermal manipulation on embryonic development, hatching process, and chick quality under heat-stress conditions. Poult. Sci. 2024, 103, 103257. [Google Scholar] [CrossRef]
- Collin, A.; Joubert, R.; Swennen, Q.; Damon, M.; Coustard, S.; Skiba-Cassy, S.; Everaert, N.; Buyse, J.; Tesseraud, S. Involvement of thyroid hormones in the regulation of mitochondrial oxidations in mammals and birds. In Thyroid Hormones: Functions, Related Diseases and Uses; Nova Science Pub Inc.: Hauppauge, NY, USA, 2009; ISBN 978-160741080-5. [Google Scholar]
- Klandorf, H.; Sharp, P.J.; Macleod, M.G. The relationship between heat production and concentrations of plasma thyroid hormones in the domestic hen. Gen. Comp. Endocrinol. 1981, 45, 513–520. [Google Scholar] [CrossRef]
- Krishnan, G.; Devaraj, C.; Silpa, M.; Sejian, V. Thermoregulation in Birds. In Textbook of Veterinary Physiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 751–764. [Google Scholar]
- Wang, S.; Edens, F.W. Heat-stress response of broiler cockerels to manipulation of the gonadal steroids, testosterone and estradiol. Comp. Biochem. Physiol. B 1993, 106, 629–633. [Google Scholar] [CrossRef]
- Yahav, S.; Collin, A.; Shinder, D.; Picard, M. Thermal manipulations during broiler chick embryogenesis: Effects of timing and temperature. Poult. Sci. 2004, 83, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Piestun, Y.; Druyan, S.; Brake, J.; Yahav, S. Thermal manipulations during broiler incubation alter performance of broilers to 70 days of age. Poult. Sci. 2013, 92, 1155–1163. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Al-Natour, M.; Dalab, A.; Alturki, O.; Althnaian, T.; Al-Ramadan, S.; Hannon, K.; El-Bahr, S. Thermal manipulation mid-term broiler chicken embryogenesis: Effect on muscle growth factors and muscle marker genes. Braz. J. Poult. Sci. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- Walstra, I.; Ten Napel, J.; Kemp, B.; van den Brand, H. Temperature manipulation during layer chick embryogenesis. Poult. Sci. 2010, 89, 1502–1508. [Google Scholar] [CrossRef]
- Piestun, Y.; Shinder, D.; Ruzal, M.; Halevy, O.; Yahav, S. The effect of thermal manipulations during the development of the thyroid and adrenal axes on in-hatch and post-hatch thermoregulation. J. Therm. Biol. 2008, 33, 413–418. [Google Scholar] [CrossRef]
- Christensen, V.L.; Grimes, J.L.; Wineland, M.J.; Davis, G.S. Accelerating embryonic growth during incubation following prolonged egg storage. 2. Embryonic growth and metabolism. Poult. Sci. 2003, 82, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Leksrisompong, N.; Romero-Sanchez, H.; Plumstead, P.W.; Brannan, K.E.; Brake, J. Broiler incubation. 1. Effect of elevated temperature during late incubation on body weight and organs of chicks. Poult. Sci. 2007, 86, 2685–2691. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; El-Bahr, S.M. Thermal manipulation of the broilers embryos: Expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC Vet. Res. 2019, 15, 166. [Google Scholar] [CrossRef]
- Elsayed, N.; Elkomy, A.; El-Saadany, S.; Hassan, E. New suggested schemes for incubation temperature and their effect on embryonic development and hatching power. Asian J. Poult. Sci. 2009, 3, 19–29. [Google Scholar] [CrossRef][Green Version]
- Hundam, S.; Al-Zghoul, M.B.; Ababneh, M.; Alanagreh, L.A.; Dahadha, R.; Mayyas, M.; Alghizzawi, D.; Mustafa, M.A.; Gerrard, D.E.; Dalloul, R.A. Effects of embryonic thermal manipulation on body performance and cecum microbiome in broiler chickens following a post-hatch lipopolysaccharide challenge. Animals 2025, 15, 1149. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhang, J.; Wen, J.; Zhao, G.; Li, Q. Differential mRNA and miRNA profiles reveal the potential roles of genes and miRNAs involved in LPS infection in chicken macrophages. Genes 2021, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, F.B.; Emmans, G.C.; Kyriazakis, I. The effects of pathogen challenges on the performance of naive and immune animals: The problem of prediction. Animal 2007, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- De Boever, S.; Croubels, S.; Meyer, E.; Sys, S.; Beyaert, R.; Ducatelle, R.; De Backer, P. Characterization of an intravenous lipopolysaccharide inflammation model in broiler chickens. Avian Pathol. 2009, 38, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Korosi Molnar, A.; Korosi, L.; Balazs, B.; Gaspardy, A. Effects of heat stress on the immune responses of chickens subjected to thermal manipulation in the pre-hatch period. Acta Vet. Hung. 2021, 69, 67–72. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar] [CrossRef]
- Jing, M.; Li, S.; Zhao, S.; House, J.D. Splenic gene expression of cytokines at multiple time points following lipopolysaccharide challenge in layers. Am. J. Vet. Res. 2024, 85, 1–7. [Google Scholar] [CrossRef]
- Ottaway, C.A.; Fong, I.W.; da Silva, B.; Singer, W.; Karrass, L. Integrative aspects of a human model of endotoxemia. Can. J. Physiol. Pharmacol. 1998, 76, 473–478. [Google Scholar] [CrossRef]
- Oda, S.; Hirasawa, H.; Shiga, H.; Nakanishi, K.; Matsuda, K.; Nakamua, M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 2005, 29, 169–175. [Google Scholar] [CrossRef]
- Freiman, S.V.; Onufriev, M.V.; Stepanichev, M.Y.; Moiseeva, Y.V.; Lazareva, N.A.; Gulyaeva, N.V. The stress effects of a single injection of isotonic saline solution: Systemic (blood) and central (frontal cortex and dorsal and ventral hippocampus). Neurochem. J. 2016, 10, 115–119. [Google Scholar] [CrossRef]
- Deng, C.; Tan, H.; Zhou, H.; Wang, M.; Lu, Y.; Xu, J.; Zhang, H.; Han, L.; Ai, Y. Four cysteine residues contribute to homodimerization of chicken interleukin-2. Int. J. Mol. Sci. 2019, 20, 5744. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Zhu, X.; Luo, Y.; Shang, Y.; Gu, X.-L. The anti-inflammatory and antioxidant effects of leonurine hydrochloride after lipopolysaccharide challenge in broiler chicks. Poult. Sci. 2019, 98, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Gehad, A.E.; Lillehoj, H.S.; Hendricks, G.L.; Mashaly, M.M. Initiation of humoral immunity. I. The role of cytokines and hormones in the initiation of humoral immunity using T-independent and T-dependent antigens. Dev. Comp. Immunol. 2002, 26, 751–759. [Google Scholar] [CrossRef]
- Lauw Fanny, N.; ten Hove, T.; Dekkers Pascale, E.P.; de Jonge, E.; van Deventer Sander, J.H.; van der Poll, T. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect. Immun. 2000, 68, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, S.A.; Slattery, R.; Aud, D.M.; Krishna, M.; Lucian, L.A.; Murray, R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect. Immun. 1996, 64, 3231–3235. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Mishra, M.K.; Basu, A.; Bishayi, B. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology 2010, 215, 443–451. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef]
- Metzger, D.W. IL-12 as an adjuvant for the enhancement of protective humoral immunity. Expert Rev. Vaccines 2009, 8, 515–518. [Google Scholar] [CrossRef]
- Sacks, D.; Sher, A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002, 3, 1041–1047. [Google Scholar] [CrossRef]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. IFN-gamma priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. J. Interferon Cytokine Res. 2005, 25, 73–81. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Qin, F.; Guan, X.; Xu, W.; Xu, L. Prokaryotic expression of chicken interferon-gamma fusion protein and its effect on expression of poultry heat shock protein 70 under heat stress. Anim. Sci. J. 2017, 88, 882–892. [Google Scholar] [CrossRef]
- Leshchinsky, T.V.; Klasing, K.C. Profile of chicken cytokines induced by lipopolysaccharide is modulated by dietary alpha-tocopheryl acetate. Poult. Sci. 2003, 82, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.d.B. Effects of thermal manipulation during embryogenesis and post-hatch acute heat stress on the mRNA expression dynamics of cytokines in broiler chickens. FASEB J. 2019, 33, 621.1. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Saleh, K.M.M.; Al-Zghoul, M.B. Effect of acute heat stress on the mRNA levels of cytokines in broiler chickens subjected to embryonic thermal manipulation. Animals 2019, 9, 499. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Saleh, K.M.; Ababneh, M.M.K. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult. Sci. 2019, 98, 1805–1819. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, T.; Rothwell, L.; Vervelde, L.; Kaiser, P.; Boulton, K.; Nolan, M.J.; Tomley, F.M.; Blake, D.P.; Hume, D.A. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Dev. Comp. Immunol. 2016, 63, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Hu, X.; Zhong, Y.; Wen, F.; Tang, X.; Yang, S.; Zhong, S.; Zhou, Z.; Yuan, X.; et al. The influence on oxidative stress markers, inflammatory factors and intestinal injury-related molecules in Wahui pigeon induced by lipopolysaccharide. PLoS ONE 2021, 16, e0251462. [Google Scholar] [CrossRef]
- Arendt, M.; Elissa, J.; Schmidt, N.; Michael, E.; Potter, N.; Cook, M.; Knoll, L.J. Investigating the role of interleukin 10 on Eimeria intestinal pathogenesis in broiler chickens. Vet. Immunol. Immunopathol. 2019, 218, 109934. [Google Scholar] [CrossRef]
- Piek, E.; Heldin, C.H.; Ten Dijke, P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999, 13, 2105–2124. [Google Scholar] [CrossRef]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-beta in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef]
- Akita, K.; Okuno, M.; Enya, M.; Imai, S.; Moriwaki, H.; Kawada, N.; Suzuki, Y.; Kojima, S. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology 2002, 123, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, Z.; Karin, M. NF-kappaB: A Double-Edged Sword Controlling Inflammation. Biomedicines 2022, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Ansari, A.R.; Li, N.Y.; Sun, Z.J.; Huang, H.B.; Zhao, X.; Cui, L.; Hu, Y.F.; Zhong, J.M.; Karrow, N.A.; Liu, H.Z. Lipopolysaccharide induces acute bursal atrophy in broiler chicks by activating TLR4-MAPK-NF-kappaB/AP-1 signaling. Oncotarget 2017, 8, 108375–108391. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.Z.; Gong, J.G.; Li, J.H.; Hao, Y.S.; Xu, H.J.; Liu, Y.C.; Feng, Z.H. Dietary resveratrol supplementation on growth performance, immune function and intestinal barrier function in broilers challenged with lipopolysaccharide. Poult. Sci. 2023, 102, 102968. [Google Scholar] [CrossRef]
- Li, N.; Ansari, A.R.; Sun, Z.; Huang, H.; Cui, L.; Hu, Y.; Zhao, X.; Zhong, J.; Abdel-Kafy, E.M.; Liu, H. Toll like receptor 4 signaling pathway participated in Salmonella lipopolysaccharide-induced spleen injury in young chicks. Microb. Pathog. 2017, 112, 288–294. [Google Scholar] [CrossRef]
- Capobianco, A.J.; Chang, D.; Mosialos, G.; Gilmore, T.D. p105, the NF-kappa B p50 precursor protein, is one of the cellular proteins complexed with the v-Rel oncoprotein in transformed chicken spleen cells. J. Virol. 1992, 66, 3758–3767. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Elsharkawy, A.M.; Oakley, F.; Lin, F.; Packham, G.; Mann, D.A.; Mann, J. The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J. Hepatol. 2010, 53, 519–527. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kannaki, T.R.; Reddy, M.R.; Verma, P.C.; Shanmugam, M. Differential Toll-like receptor (TLR) mRNA expression patterns during chicken embryological development. Anim. Biotechnol. 2015, 26, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Gelbart, T.; West, C. Synergy between TLR2 and TLR4: A safety mechanism. Blood Cells Mol. Dis. 2001, 27, 728–730. [Google Scholar] [CrossRef]
- Srivastava, T.; Sharma, M.; Yew, K.H.; Sharma, R.; Duncan, R.S.; Saleem, M.A.; McCarthy, E.T.; Kats, A.; Cudmore, P.A.; Alon, U.S.; et al. LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: Changes in TLR profile. J. Cell Commun. Signal. 2013, 7, 49–60. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015, 94, 1504–1511. [Google Scholar] [CrossRef]
- Eren, U.; Kum, S.; Nazligul, A.; Gules, O.; Aka, E.; Yildiz, M.; Zorlu, S. TLR2 and TLR4 molecules and antigen-presenting cell compositions in cecal tonsils of broiler chicks (Gallus gallus domesticus) in the first two weeks of the post-hatch period. Anat. Histol. Embryol. 2022, 51, 125–135. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Saleh, K.M. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult. Sci. 2020, 99, 2727–2735. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef]
- Basaiawmoit, R.V.; Rattan, S.I. Cellular stress and protein misfolding during aging. Methods Mol. Biol. 2010, 648, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008, 22, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, M.; Ostrov, V.; Yurinskaya, M.; Garbuz, D.; Murashev, A.; Antonova, O.; Evgen’ev, M. Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones 2012, 17, 89–101. [Google Scholar] [CrossRef]
- Asea, A.; Kraeft, S.-K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef]
- Miller, L.; Qureshi, M.A. Comparison of heat-shock-induced and lipopolysaccharide-induced protein changes and tumoricidal activity in a chicken mononuclear cell line. Poult. Sci. 1992, 71, 979–987. [Google Scholar] [CrossRef]
- Kaucsar, T.; Bodor, C.; Godo, M.; Szalay, C.; Revesz, C.; Nemeth, Z.; Mozes, M.; Szenasi, G.; Rosivall, L.; Soti, C.; et al. LPS-induced delayed preconditioning is mediated by Hsp90 and involves the heat shock response in mouse kidney. PLoS ONE 2014, 9, e92004. [Google Scholar] [CrossRef]
- Chen, S.; Zuo, X.; Yang, M.; Lu, H.; Wang, N.; Wang, K.; Tu, Z.; Chen, G.; Liu, M.; Liu, K. Severe multiple organ injury in HSF1 knockout mice induced by lipopolysaccharide is associated with an increase in neutrophil infiltration and surface expression of adhesion molecules. J. Leukoc. Biol. 2012, 92, 851–857. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Xi, L.; Liu, H.C.; Odle, J.; Luo, X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS ONE 2014, 9, e102204. [Google Scholar] [CrossRef]
- Bunch, H. HSF1 in RNA polymerase II promoter-proximal pausing and HSP70 transcription. In Heat Shock Proteins in Inflammatory Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 489–508. [Google Scholar]
- Tanabe, M.; Kawazoe, Y.; Takeda, S.; Morimoto, R.I.; Nagata, K.; Nakai, A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J. 1998, 17, 1750–1758. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Dalab, A.E.; Yahya, I.E.; Althnaian, T.A.; Al-Ramadan, S.Y.; Ali, A.M.; Albokhadaim, I.F.; El-Bahr, S.M.; Al Busadah, K.A.; Hannon, K.M. Thermal manipulation during broiler chicken embryogenesis: Effect on mRNA expressions of Hsp108, Hsp70, Hsp47 and Hsf-3 during subsequent post-hatch thermal challenge. Res. Vet. Sci. 2015, 103, 211–217. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (%) | Starter (d1–24) | Grower (d25–35) |
|---|---|---|
| Corn | 60.20 | 62.0 |
| Soybean Meal (CP 40%) | 34.30 | 31.50 |
| Concentrate | 3.20 | 2.70 |
| Vegetable Oil | 1.00 | 2.50 |
| Limestone | 1.10 | 1.10 |
| Vitamin and mineral premix * | 0.20 | 0.20 |
| Total | 100.00 | 100.00 |
| Nutrient Composition (Calculated) ** | ||
| Metabolizable energy, kcal/kg | 3110 | 3155 |
| Crude Protein (%) | 22.52 | 21.53 |
| Lysine (%) | 1.51 | 1.32 |
| Methionine (%) | 0.55 | 0.51 |
| Calcium | 1.08 | 1.0 |
| Available Phosphorus | 0.49 | 0.44 |
| The Gene | Sequence (5′–3′) | TM (°C) |
|---|---|---|
| β-Actin | F: ACCGCAAATGCTTCTAAACC R: ATAAAGCCATGCCAATCTCG | 60 |
| GAPDH | F: TTGTCTCCTGTGACTTCAATGGTG R: ACGGTTGCTGTATCCAAACTCAT | 60 |
| TNF-α | F: GACAGCCTATGCCAACAAGTA R: TTACAGGAAGGGCAACTCATC | 60 |
| IL-1 β | F: CCCGCCTTCCGCTACA R: CACGAAGCACTTCTGGTTGATG | 60 |
| IL-2 | F: GAGAGCATCCGGATAGTGAAT R: TGTGGAGGCTTTGCATAAGAG | 60 |
| IL-6 | F: AAATCCCTCCTCGCCAATCT R: CCCTCACGGTCTTCTCCATAAA | 60 |
| IL-12 | F: CTGTGGCTCGCACTGATAAA R: CAATGACCTCCAGGAACATCTC | 60 |
| IFN-γ | F: ACCTTCCTGATGGCGTGAAG R: GCGCTGGATTCTCAAGTCGT | 60 |
| IL-10 | F: CAGCAATCCAGAGACGATGAA R: AGTGGACTTGCACTGGAATAG | 60 |
| TGF-β | F: GGGTGTCCCATACCATTTAGAG R: CCCTTTAACGCAGAGGGATT | 60 |
| NF-κB | F: GATGTGGAGACAGACAGCTAAC R: CATAAGACGCACCACACTGA | 60 |
| NF-κB p50 | F: CACAGCTGGAGGGAAGTAAAT R: TTGAGTAAGGAAGTGAGGTTGAG | 60 |
| TLR 1 | F: AGTCCATCTTTGTGTTGTCGCC R: ATTGGCTCCAGCAAGATCAGG | 60 |
| TLR 2 | F: GATTGTGGACAACATCATTGACTC R: AGAGCTGCTTTCAAGTTTTCCC | 60 |
| TLR 3 | F: TCAGTACATTTGTAACACCCCGCC R: GGCGTCATAATCAAACACTCC | 60 |
| TLR 4 | F: AGTCTGAAATTGCTGAGCTCAAAT R: GCGACGTTAAGCCATGGAAG | 60 |
| HSP 70 | F: CGGGCAAGTTTGACCTAA R: TTGGCTCCCACCCTATCTCT | 60 |
| HSP 90 | F: TCCTGTCCTGGCTTTAGTTT R: AGGTGGCATCTCCTCGGT | 60 |
| HSF 1 | F: CAGGGAAGCAGTTGGTTCACTACACG R: CCTTGGGTTTGGGTTGCTCAGTC | 60 |
| HSF 3 | F: GAGTTCCAGCACCCTTTCTT R: TCTTTCCACAGGGCCTTATTT | 60 |
| Parameter | CON | TM | p-Value |
|---|---|---|---|
| True hatchability % 1 | 93.03 ± 0.541 a | 93.71 ± 1.092 a | 0.633 |
| Unfertilized eggs % 2 | 4.36 ± 0.316 a | 1.72 ± 0.015 b | 0.014 |
| Hatch time (h) | 496.9 ± 5.3 a | 488.3 ± 4.2 b | 0.040 |
| Embryonic death % 3 | 2.61 ± 0.857 a | 3.72 ± 0.828 a | 0.451 |
| Hatchability of fertile % 4 | 97.27 ±0.888 a | 95.35 ± 1.126 a | 0.311 |
| Chick Abnormalities % 5 (at hatching) | 0.94 ± 0.313 a | 3.36 ± 1.519 a | 0.259 |
| Item | Group | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Overall (Day 1–35) |
|---|---|---|---|---|---|---|---|
| BW | CON | 182 ± 2.13 a | 511.04 ± 4.07 a | 1126.25 ± 10.91 a | 1911.01 ± 18.64 a | 2729.29 ± 38.11 a | 2729.29 ± 38.11 a |
| TM | 183.33 ± 1.4 a | 481.33 ± 5.60 b | 1048.75 ± 11.65 b | 1764.68 ± 15.71 b | 2519.51 ± 32.81 b | 2519.51 ± 32.81 b | |
| ADFI | CON | 25.22 ± 0.18 a | 59.29 ± 1.14 a | 116.39 ± 1.37 a | 170.58 ± 1.56 a | 207.55 ± 2.70 a | 115.81 ± 0.88 a |
| TM | 23.13 ± 0.26 b | 54.67 ± 1.23 b | 105.84 ± 1.46 b | 152.66 ± 1.92 b | 168.53 ± 3.89 b | 100.96 ± 0.90 b | |
| ADG | CON | 20.34 ± 0.32 a | 47 ± 0.49 a | 87.89 ± 1.57 a | 112.11 ± 2.44 a | 116.83 ± 5.29 a | 76.85 ± 1.09 a |
| TM | 20.52 ± 0.30 a | 42.57 ± 0.85 b | 81.06 ± 1.20 b | 102.28 ± 1.99 b | 107.49 ± 5.07 a | 70.85 ± 0.96 b | |
| FCR | CON | 0.97 ± 0.01 a | 1.26 ± 0.03 a | 1.33 ± 0.02 a | 1.53 ± 0.03 a | 1.85 ± 0.12 a | 1.51 ± 0.02 a |
| TM | 0.88 ± 0.01 b | 1.29 ± 0.03 a | 1.31 ± 0.02 a | 1.50 ± 0.03 a | 1.59 ± 0.08 a | 1.40 ± 0.02 b |
| Organ/Tissue | CON | TM | p-Value |
|---|---|---|---|
| Liver | 0.345 | ||
| Absolute weight (g) | 59.77 ± 2.87 a | 51.90 ± 2.52 a | |
| Relative weight | 2.19 ± 0.105 a | 2.06 ± 0.10 a | |
| Spleen | 0.561 | ||
| Absolute weight | 3.55 ± 0.38 a | 3.02 ± 0.23 a | |
| Relative weight | 0.13 ± 0.014 a | 0.12 ± 0.009 a | |
| heart | 0.001 | ||
| Absolute weight | 13.73 ± 0.55 | 9.47 ± 0.66 | |
| Relative weight | 0.503 ± 0.020 a | 0.376 ± 0.026 b | |
| Small Intestine | <0.0001 | ||
| Absolute weight | 76.96 ± 5.05 a | 39.30 ± 2.60 b | |
| Relative weight | 2.82 ± 0.185 a | 1.56 ± 0.103 b | |
| Large Intestine | 0.053 | ||
| Absolute weight | 21.83 ± 2.46 a | 14.87 ± 1.01 a | |
| Relative weight | 0.80 ± 0.09 a | 0.59 ± 0.04 a | |
| digestive System | <0.0001 | ||
| Absolute weight | 265.82 ± 9.83 a | 173.34 ± 11.34 a | |
| Relative weight | 9.74 ± 0.36 a | 6.88 ± 0.45 b | |
| Gizzard weight | 0.0042 | ||
| Absolute weight | 45.58 ± 1.45 a | 32.25 ± 2.67 b | |
| Relative weight | 1.67 ± 0.053 a | 1.28 ± 0.106 b | |
| proventriculus | <0.001 | ||
| Absolute weight | 9.28 ± 0.49 a | 5.80 ± 0.43 b | |
| Relative weight | 0.34 ± 0.018 a | 0.23 ± 0.017 b | |
| gallbladder | 0.644 | ||
| Absolute weight | 0.79 ± 0.08 a | 0.78 ± 0.08 a | |
| Relative weight | 0.029 ± 0.003 a | 0.031 ± 0003 a | |
| Small Intestine length (mm) | 202.0 ± 7.66 a | 161.0 ± 5.39 b | <0.001 |
| Large intestine length (mm) | 27.50 ± 1.73 a | 2.052 ± 6.21 a | 0.287 |
| Before LPS Injection | (°C) | CON-Saline | Control-LPS | TM-Saline | TM-LPS |
| 40.36 ± 0.32 | 40.55 ± 0.11 a | 40.79 ± 0.18 | 40.80 ± 0.22 | ||
| 1 h After LPS injection | (°C) | CON-Saline | Control-LPS | TM-Saline | TM-LPS |
| 40.19 ± 0.25 | 40.19 ± 0.14 b | 40.60 ± 0.15 | 40.34 ± 0.36 | ||
| 3 h After LPS injection | (°C) | CON-Saline | Control-LPS | TM-Saline | TM-LPS |
| 40.43 ± 0.18 | 40.33 ± 0.17 | 40.68 ± 0.20 | 40.86 ± 0.17 | ||
| 6 h After LPS injection | (°C) | CON-Saline | Control-LPS | TM-Saline | TM-LPS |
| 40.40 ± 0.27 | 40.59 ± 0.17 | 40.85 ± 0.29 | 40.61 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zghoul, M.B.; Hundam, S.; Mayyas, M.; Gerrard, D.E.; Dalloul, R.A. Impact of Thermal Manipulation of Broiler Eggs on Growth Performance, Splenic Inflammatory Cytokine Levels, and Heat Shock Protein Responses to Post-Hatch Lipopolysaccharide (LPS) Challenge. Animals 2025, 15, 1736. https://doi.org/10.3390/ani15121736
Al-Zghoul MB, Hundam S, Mayyas M, Gerrard DE, Dalloul RA. Impact of Thermal Manipulation of Broiler Eggs on Growth Performance, Splenic Inflammatory Cytokine Levels, and Heat Shock Protein Responses to Post-Hatch Lipopolysaccharide (LPS) Challenge. Animals. 2025; 15(12):1736. https://doi.org/10.3390/ani15121736
Chicago/Turabian StyleAl-Zghoul, Mohammad Borhan, Seif Hundam, Mohammad Mayyas, David E. Gerrard, and Rami A. Dalloul. 2025. "Impact of Thermal Manipulation of Broiler Eggs on Growth Performance, Splenic Inflammatory Cytokine Levels, and Heat Shock Protein Responses to Post-Hatch Lipopolysaccharide (LPS) Challenge" Animals 15, no. 12: 1736. https://doi.org/10.3390/ani15121736
APA StyleAl-Zghoul, M. B., Hundam, S., Mayyas, M., Gerrard, D. E., & Dalloul, R. A. (2025). Impact of Thermal Manipulation of Broiler Eggs on Growth Performance, Splenic Inflammatory Cytokine Levels, and Heat Shock Protein Responses to Post-Hatch Lipopolysaccharide (LPS) Challenge. Animals, 15(12), 1736. https://doi.org/10.3390/ani15121736






