Effect of Season on Testicular Development and Spermatogenesis in Hu Sheep: Insights from Antioxidant Indices, Oxylipins, and Transcriptomics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Sample Collection
2.3. Testicular Histology
2.4. Measurement of Antioxidant Properties
2.5. Quantitative Real-Time PCR Analysis of Antioxidant Genes
2.6. Metabolomics Analysis of Oxylipins
2.7. RNA Sequence
2.8. Validation of Key Genes
2.9. Integrative Analysis
2.10. Statistical Analyses
3. Results
3.1. Differences in Reproductive Organ Parameters Between the Winter-Born and Summer-Born Groups
3.2. Histological Observations
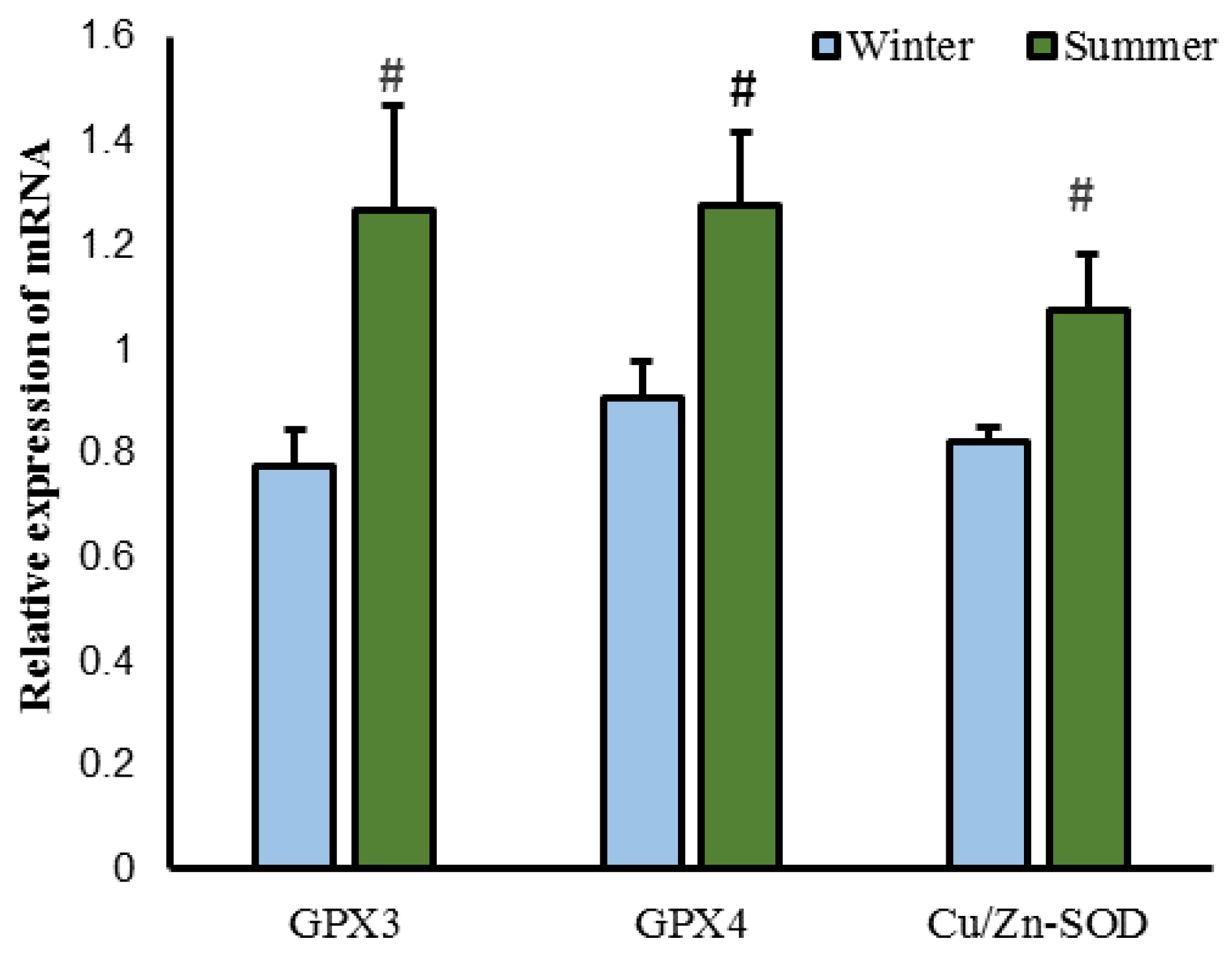
3.3. Effect of Season on Antioxidant Properties
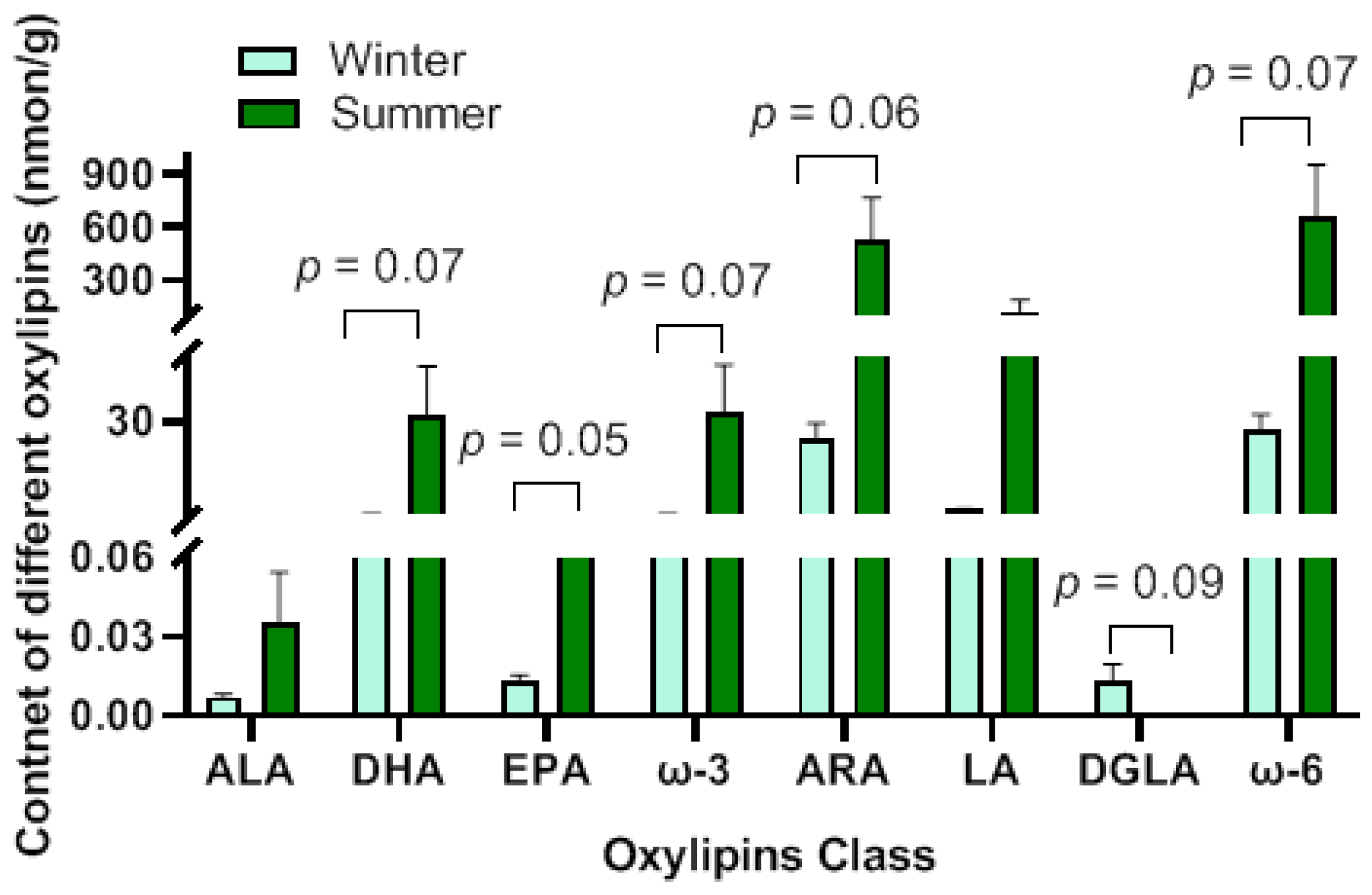
3.4. Metabolic Analysis of Oxylipins
3.5. Identification of Differential Oxylipins
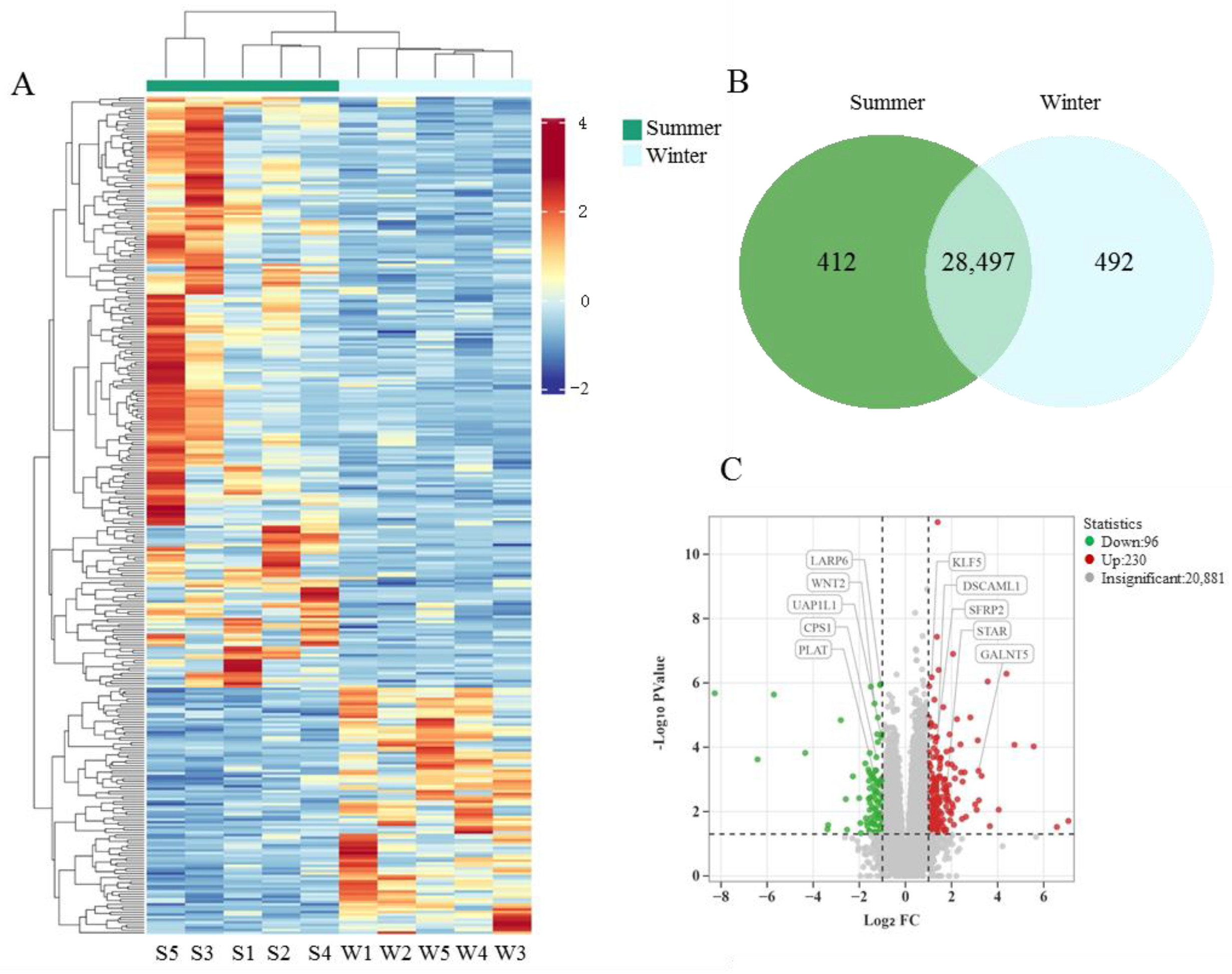
3.6. Analysis of RNA Sequencing Data
3.7. GO and KEGG Analyses of DEGs
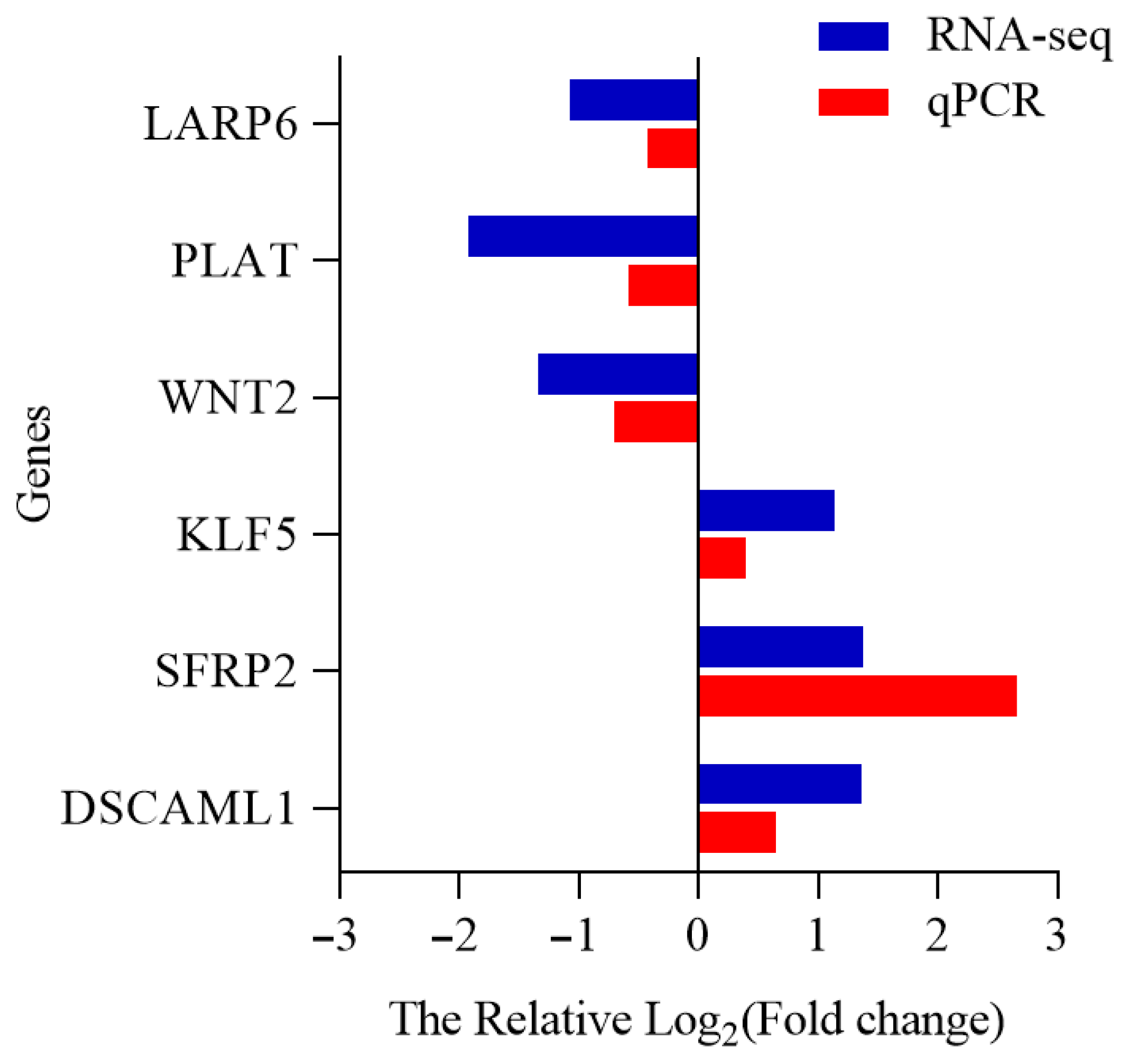
3.8. Verification of DEGs by qRT-PCR
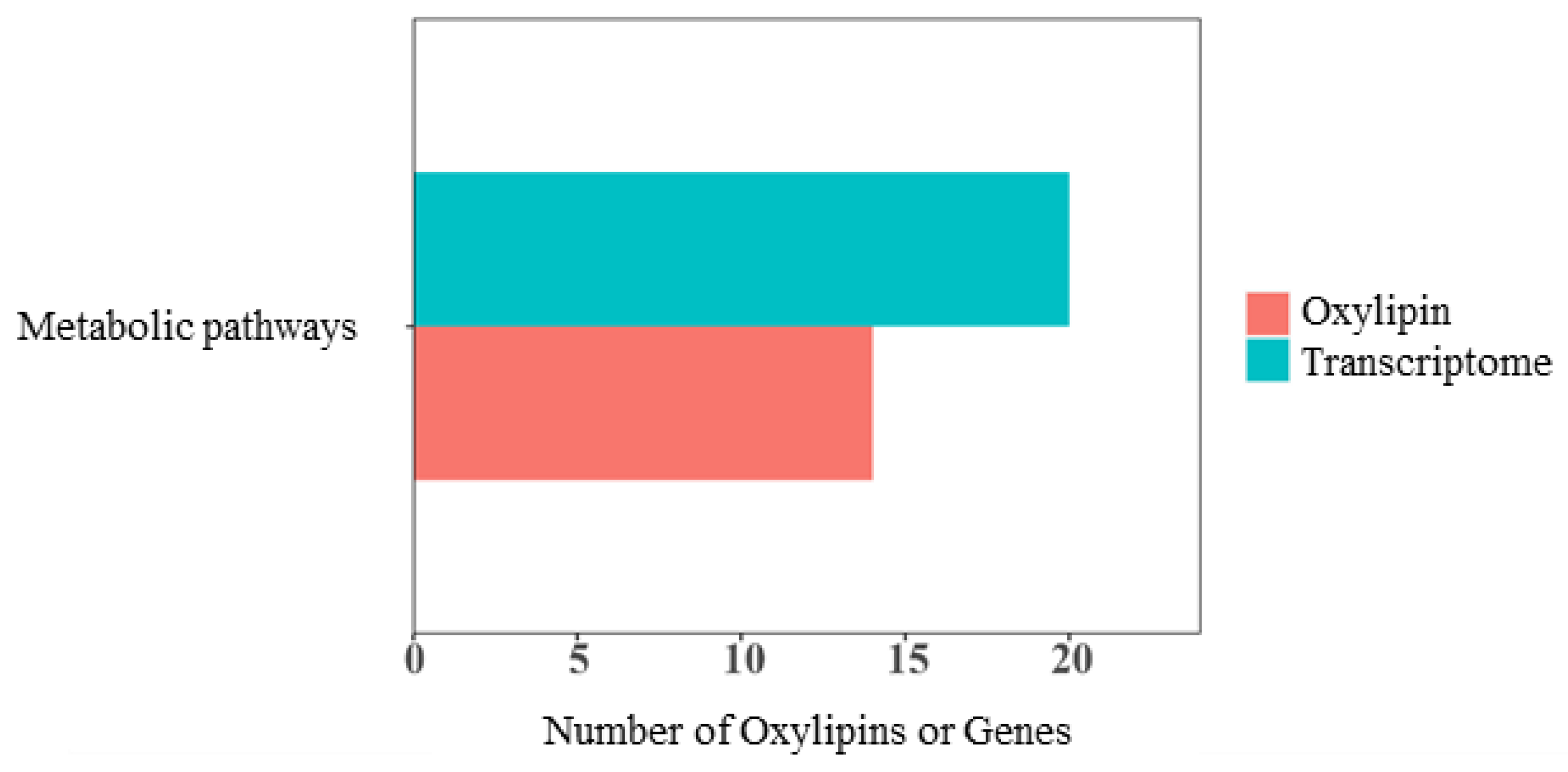
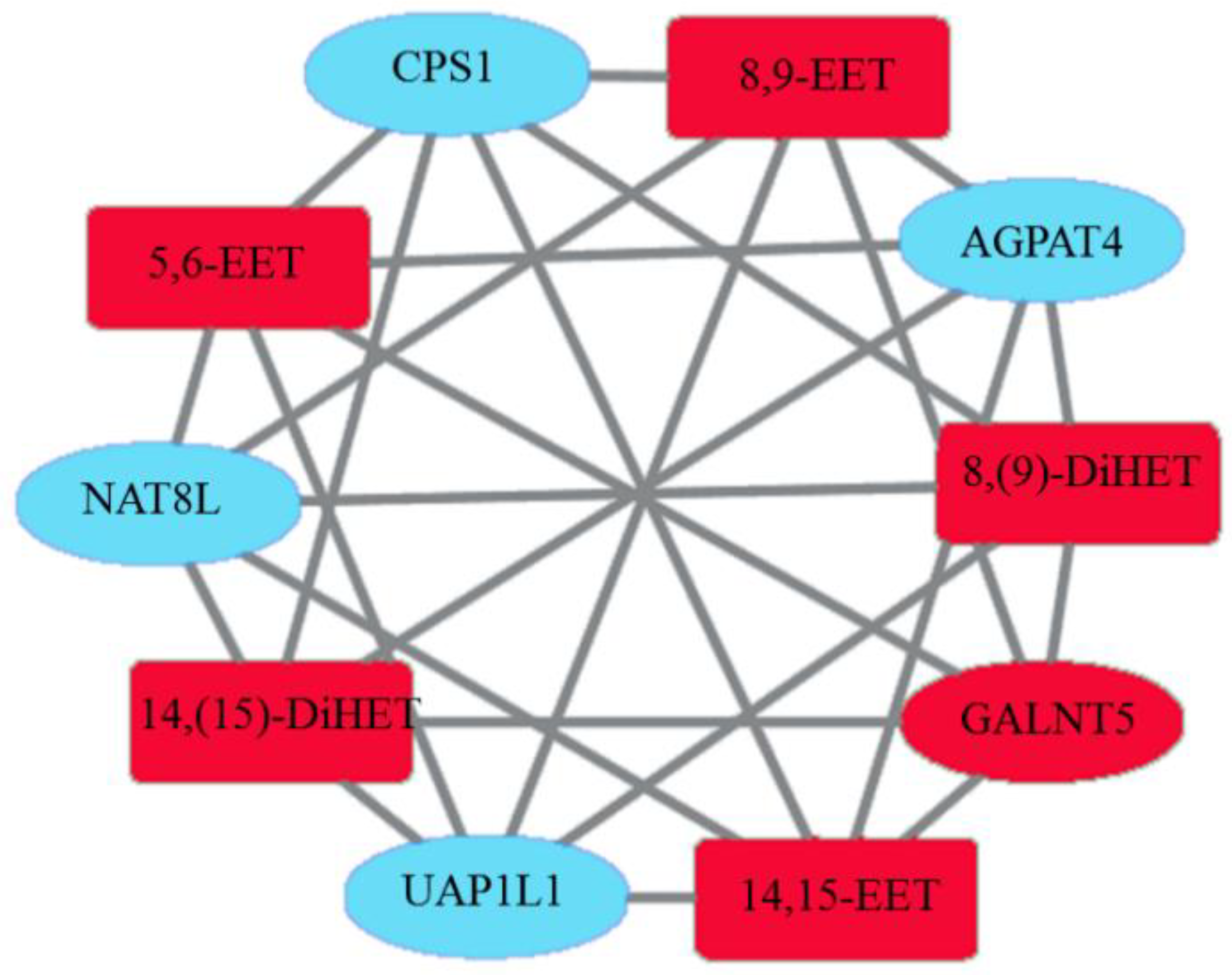
3.9. Combined Analysis of Transcriptomics and Oxylipidomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | α-Linolenic acid (ALA) |
| ARA | Arachidonic acid |
| COX | Cyclooxygenase |
| Cu/Zn-SOD | Cu,Zn-superoxide dismutase |
| CYP450 | Cytochrome P450 |
| DEGs | Differentially expressed genes |
| DGLA | Dohomo-γ-linolenic acid |
| DHA | Docosahexaenoic acid |
| DHETs | Dihydroxyeicosatrienoic acids |
| EET | Epoxyeicosatrienoic acid |
| EPA | Eicosapentaenoic acid |
| FPKM | Fragments per kilobase of transcript per million mapped reads |
| GPX3 | Glutathione Peroxidase 3 |
| GPX4 | Glutathione Peroxidase 4 |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LA | Linoleic acid |
| LOX | Lipoxygenase |
| LXB4 | Lipoxin B4 |
| MDA | Malondialdehyde |
| PCR | Polymerase chain reaction |
| PG | Prostaglandins |
| PGD2 | Prostaglandin D2 |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PUFA | Polyunsaturated fatty acid |
| qRT-PCR | Quantitative real-time reverse transcription polymerase chain reaction |
| ROS: | Reactive oxygen species |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| TX | Thromboxanes |
| UPLC–MS/MS | Ultra-performance liquid chromatography tandem mass spectrometry |
References
- Sukcharoen, N.; Keith, J.; Irvine, D.S.; Aitken, R.J. Prediction of the in-vitro fertilization (IVF) potential of human spermatozoa using sperm function tests: The effect of the delay between testing and IVF. Hum. Reprod. 1996, 11, 1030–1034. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Zhu, J.; Mao, J.; Fan, H. Research progress on the damage mechanism of male reproductive system caused by heat stress. J. Jilin Med. Univ. 2022, 43, 203–206. [Google Scholar] [CrossRef]
- Fan, X.; Xi, H.; Zhang, Z.; Liang, Y.; Li, Q.; He, J. Germ cell apoptosis and expression of Bcl-2 and Bax in porcine testis under normal and heat stress conditions. Acta Histochem. 2017, 119, 198–204. [Google Scholar] [CrossRef]
- Tramontano, F.; Malanga, M.; Farina, B.; Jones, R.; Quesada, P. Heat stress reduces poly(ADPR)polymerase expression in rat testis. Mol. Hum. Reprod. 2000, 6, 575–581. [Google Scholar] [CrossRef]
- He, N. Effect of trace rare earth cerium element on the quality parameters and sperm membrane fluidity of bovine frozen semen. Heilongjiang Anim. Husb. Vet. Med. 2015, 5, 66–69. [Google Scholar] [CrossRef]
- Godia, M.; Estill, M.; Castello, A.; Balasch, S.; Rodriguez-Gil, J.E.; Krawetz, S.A.; Sanchez, A.; Clop, A. A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes. Front. Genet. 2019, 10, 299. [Google Scholar] [CrossRef]
- Garcia-Tomas, M.; Sanchez, J.; Piles, M. Postnatal sexual development of testis and epididymis in the rabbit: Growth and maturity patterns of macroscopic and microscopic markers. Theriogenology 2009, 71, 292–301. [Google Scholar] [CrossRef]
- Claus, R.; Weiler, U.; Wagner, H.G. Photoperiodic influences on reproduction of domestic boars. II. Light influences on semen characteristics and libido. Zentralblatt Veterinarmedizin Reihe A 1985, 32, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Weng, X.X.; Yuan, L.F.; Li, F.; Yue, X.P.; Li, F.D. Effect of feeding linseed diet on testis development, antioxidant capacity, and epididymal cauda sperm concentration in Chinese Hu lamb. Theriogenology 2021, 159, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yao, T.; Zhang, X.; Weng, X.; Li, F.; Yue, X. Oxylipin profiling analyses reveal that ω-3 PUFA is more susceptible to lipid oxidation in sheep testis under oxidative stress. Anim Reprod Sci 2024, 268, 107567. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [CrossRef]
- Roman, R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef]
- Lin, X.; Gu, W.; Tong, X.; Lai, M.; Zhang, Y.; Liu, N.; Jin, X.; Li, C.; Huang, D.; Zhou, F.; et al. EETs Reduction contributes to granulosa cell senescence and endometriosis-associated infertility via the PI3K/AKT/mTOR signaling pathway. Adv. Sci. 2025, e05656. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130. [Google Scholar] [CrossRef]
- Larsen, B.T.; Miura, H.; Hatoum, O.A.; Campbell, W.B.; Hammock, B.D.; Zeldin, D.C.; Falck, J.R.; Gutterman, D.D. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: Implications for soluble epoxide hydrolase inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H491–H499. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Zhang, J.; Luo, Y.; Han, W.; Wang, H.; Xia, H.; Chen, Z.; Yang, Y.; Chen, Q.; et al. Maternal inflammation exaggerates offspring susceptibility to cerebral ischemia-reperfusion injury via the COX-2/PGD2/DP2 pathway activation. Oxidative Med. Cell. Longev. 2022, 2022, 1571705. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Hu, J.; Meng, J.; Lv, H.; Yang, F.; Wang, M.; Liu, R.; Wu, W.; Hou, D.; et al. Plasma oxidative lipidomics reveals signatures for sepsis-associated acute kidney injury. Clin. Chim. Acta 2023, 551, 117616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Q.; Li, J.; Zhong, Y.; Zhang, L.; Huang, Q.; Chen, B.; Mo, M.; Shen, S.; Zhong, Q.; et al. Distinct differences in serum eicosanoids in healthy, enteritis and colorectal cancer individuals. Metabolomics 2017, 14, 4. [Google Scholar] [CrossRef]
- Romano, M.; Recchia, I.; Recchiuti, A. Lipoxin receptors. Sci. World J. 2007, 7, 1393–1412. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Alnajem, A.; Al-Maghrebi, M. The Regulatory Effects of JAK2/STAT3 on Spermatogenesis and the Redox Keap1/Nrf2 Axis in an Animal Model of Testicular Ischemia Reperfusion Injury. Cells 2023, 12, 2292. [Google Scholar] [CrossRef]
- Lin, M.; Liu, X.; Zheng, H.; Huang, X.; Wu, Y.; Huang, A.; Zhu, H.; Hu, Y.; Mai, W.; Huang, Y. IGF-1 enhances BMSC viability, migration, and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway. Stem Cell Res. Ther. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Merino, H.; Singla, D.K. Secreted frizzled-related Protein-2 inhibits doxorubicin-induced apoptosis mediated through the Akt-mTOR pathway in soleus muscle. Oxidative Med. Cell. Longev. 2018, 2018, 6043064. [Google Scholar] [CrossRef] [PubMed]
- Rius-Perez, S.; Torres-Cuevas, I.; Millan, I.; Ortega, A.L.; Perez, S. PGC-1alpha, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, X.; Zheng, H.; Huang, G.; Li, W.; Liu, X.; Liang, J.; Cao, Y.; Hu, Y.; Huang, Y. SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxidative Med. Cell. Longev. 2021, 2021, 9265016. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, Y.; Lu, Y.; Li, Z.; Gao, F. Unraveling the therapeutic potential of carbamoyl phosphate synthetase 1 (CPS1) in human diseases. Bioorg. Chem. 2023, 130, 106253. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Y.; Wu, Z.; Zhou, X.; Wu, T.; Li, P.; Lian, Q.; Xu, S.; Gu, J.; Chen, L.; et al. Deficiency of carbamoyl phosphate synthetase 1 engenders radioresistance in hepatocellular carcinoma via deubiquitinating c-Myc. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1244–1256. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Bi, J.; Li, Z.; Zhang, Z.; Kong, C. Caspase recruitment domain family member 10 regulates carbamoyl phosphate synthase 1 and promotes cancer growth in bladder cancer cells. J. Cell. Mol. Med. 2019, 23, 8128–8138. [Google Scholar] [CrossRef]
- Phan, N.N.; Wang, C.Y.; Li, K.L.; Chen, C.F.; Chiao, C.C.; Yu, H.G.; Huang, P.L.; Lin, Y.C. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget 2018, 9, 6977–6992. [Google Scholar] [CrossRef]
- Dai, C.; Miao, C.X.; Xu, X.M.; Liu, L.J.; Gu, Y.F.; Zhou, D.; Chen, L.S.; Lin, G.; Lu, G.X. Transcriptional activation of human CDCA8 gene regulated by transcription factor NF-Y in embryonic stem cells and cancer cells. J. Biol. Chem. 2015, 290, 22423–22434. [Google Scholar] [CrossRef]
- Wu, X.C.; Yu, Y.Z.; Zuo, Y.Z.; Song, X.L.; Zhou, Z.E.; Xiao, Y.; Luo, D.S.; Yan, W.G.; Zhao, S.C. Identification of UAP1L1 as a critical factor for prostate cancer and underlying molecular mechanism in tumorigenicity. J. Transl. Med. 2022, 20, 91. [Google Scholar] [CrossRef]
- Riccio, G.; Nuzzo, G.; Zazo, G.; Coppola, D.; Senese, G.; Romano, L.; Costantini, M.; Ruocco, N.; Bertolino, M.; Fontana, A.; et al. Bioactivity screening of antarctic sponges reveals anticancer activity and potential cell death via ferroptosis by mycalols. Mar. Drugs 2021, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Kist, M.; Vucic, D. Cell death pathways: Intricate connections and disease implications. EMBO J. 2021, 40, e106700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef]
- Pessentheiner, A.R.; Pelzmann, H.J.; Walenta, E.; Schweiger, M.; Groschner, L.N.; Graier, W.F.; Kolb, D.; Uno, K.; Miyazaki, T.; Nitta, A.; et al. NAT8L (N-acetyltransferase 8-like) accelerates lipid turnover and increases energy expenditure in brown adipocytes. J. Biol. Chem. 2013, 288, 36040–36051. [Google Scholar] [CrossRef]
- Deng, H.; Wu, G.; Zhang, R.; Yin, Q.; Xu, B.; Zhou, L.; Chen, Z. Comparative nutritional and metabolic analysis reveals the taste variations during yellow rambutan fruit maturation. Food Chem. X 2023, 17, 100580. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yan, M.; Wu, S.; Liu, M.; Raza, S.H.A. Supplementary feeding regulates muscle development of Oula sheep (Tibetan Sheep, Ovis aries) through glucose metabolism pathway. Animals 2025, 15, 2626. [Google Scholar] [CrossRef]







| Genes | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| GPX3 XM_015096153.3 | F: TCCATGACATCCGGTGGAAC R: GCATGGGAGTGTGGCATAGT | 250 |
| GPX4 XM_060416444.1 | F: CCACCCTCTGTGGAAATGGAT R: GAGGGACGACTTTTCCCGA | 195 |
| Cu/Zn-SOD XM_004012626.3 | F: GGAGACCTGGGCAATGTGAA R: CCTCCAGCGTTTCCAGTCTT | 137 |
| HPRT1 XM_015105023.2 | F: CGACTGGCTCGAGATGTGAT R: TCACCTGTTGACTGGTCGTT | 197 |
| DSCAML1 XM_027979838.2 | F: GCTCCCAGCATGGTGTTACT R: CGGTGATCTCAAACTTGGCG | 189 |
| SFRP2 NM_001163053.1 | F: ACAACGACCTTTGCATCCCC R: CCTTCTCGGACACTCCGTTC | 249 |
| WNT2 NM_001195319.1 | F: GGTCAGCTCTTCATGGTGGT R: CCAATGGCACGCATCACATC | 132 |
| KLF5 XM_004012185.5 | F: CAGTGCCTCAGTCGTAGACC R: GGCCAGTTCTCAGGTGAGTG | 104 |
| LARP6 XM_027971542.3 | F: GCTGGTGGACCAGATCGAAT R: AGGTGAGCAGCTTGACACTC | 116 |
| PLAT XM_012106011.3 | F: GAATAGGGGTTATGGGGCGG R: CAGCATGTTGTTGGTGACGG | 137 |
| Groups | Winter | Summer | p Value | 95% Confidence Interval | η2 |
|---|---|---|---|---|---|
| Body weight (kg) | 47.23 ± 1.74 | 47.60 ± 1.18 | 0.86 | −4.75–4.01 | 0.0009 |
| Scrotum circumference (cm) | 25.95 ± 0.35 | 26.81 ± 0.50 | 0.17 | −2.10 to 0.39 | 0.05 |
| Testis weight (g) | 241.51 ± 2.68 | 239.16 ± 2.76 | 0.55 | −5.53–10.22 | 0.01 |
| Testis index (g/kg) | 5.11 ± 0.17 | 5.02 ± 0.13 | 0.9995 | −0.42–0.45 | 0.0001 |
| Sperm density (107/g) | 16.86 ± 2.02 | 102.65 ± 9.56 | <0.001 | −105.4–−65.80 | 0.69 |
| Seminiferous tubule diameter (μm) | 208.69 ± 2.59 | 219.22 ± 4.84 | 0.07 | −7.88–−1.17 | 0.31 |
| Thickness of the epithelium (μm) | 69.36 ± 1.12 | 73.88 ± 1.14 | 0.01 | −22.07–1.02 | 0.17 |
| No. | Differential Oxylipins | Classification | VIP | p Value | Fold Change | Type |
|---|---|---|---|---|---|---|
| 1 | 9(S)-HpOTrE | ALA | 1.01 | 0.20 | 4.92 | up |
| 2 | 8(9)-DiHET | ARA | 1.16 | 0.13 | 29.84 | up |
| 3 | 8,9-EET | ARA | 1.25 | 0.10 | 22.32 | up |
| 4 | 13,14-dihydro-15-ketoPGD2 | ARA | 1.41 | 0.07 | 0.00 | down |
| 5 | PGD2 | ARA | 1.10 | 0.13 | 6.03 | up |
| 6 | TXB2 | ARA | 1.04 | 0.13 | 3.46 | up |
| 7 | 5-isoPGF2VI | ARA | 1.39 | 0.05 | 0.24 | down |
| 8 | 13,14-dihydro-15-ketoPGF2α | ARA | 1.41 | 0.02 | 0.22 | down |
| 9 | 5,6-EET | ARA | 1.26 | 0.09 | 21.06 | up |
| 10 | 8(S),15(S)-DiHETE | ARA | 1.62 | 0.01 | 0.29 | down |
| 11 | 5(S),15(S)-DiHETE | ARA | 1.50 | 0.04 | 0.37 | down |
| 12 | 14(15)-DiHET | ARA | 1.17 | 0.13 | 53.55 | up |
| 13 | 14,15-EET | ARA | 1.23 | 0.10 | 28.32 | up |
| 14 | 12-HHT | ARA | 1.76 | 0.01 | 0.27 | down |
| 15 | 5,6-DiHETrE | ARA | 1.34 | 0.06 | 22.42 | up |
| 16 | 11-keto-TXB2 | ARA | 1.10 | 0.11 | 2.82 | up |
| 17 | LXB4 | ARA | 1.04 | 0.18 | Inf | up |
| 18 | 17-HETE | ARA | 1.72 | 0.02 | 3.03 | up |
| 19 | 11,12-EET | ARA | 1.25 | 0.09 | 19.57 | up |
| 20 | 11(12)-DiHET | ARA | 1.16 | 0.13 | 32.73 | up |
| 21 | PGF1α | DGLA | 1.45 | 0.08 | 0.00 | down |
| 22 | PGD1 | DGLA | 1.17 | 0.19 | 0.00 | down |
| 23 | 7(8)-DiHDPE(A) | DHA | 1.19 | 0.12 | Inf | up |
| 24 | 19(20)-EpDPE(A) | DHA | 1.20 | 0.12 | 43.59 | up |
| 25 | 19(20)-DiHDPE(A) | DHA | 1.16 | 0.13 | 31.00 | up |
| 26 | 7,8-EpDPE | DHA | 1.25 | 0.10 | 29.27 | up |
| 27 | 16(17)-EpDPE | DHA | 1.24 | 0.10 | 36.35 | up |
| 28 | PDX | DHA | 1.00 | 0.19 | 14.30 | up |
| 29 | 13(14)-DiHDPE(A) | DHA | 1.17 | 0.13 | 47.10 | up |
| 30 | 8-HDHA | DHA | 1.26 | 0.10 | 2.17 | up |
| 31 | 17(18)-EpETE | EPA | 1.31 | 0.08 | 6.57 | up |
| 32 | 14(15)-EpETE | EPA | 1.24 | 0.10 | 46.19 | up |
| 33 | 14(15)-DiHETE | EPA | 1.79 | 0.01 | 7.35 | up |
| 34 | 5,6-DIHETE | EPA | 1.38 | 0.06 | 36.58 | up |
| 35 | 9-HEPE | EPA | 1.04 | 0.17 | Inf | up |
| 36 | 17(18)-DiHETE | EPA | 1.42 | 0.13 | 2.06 | up |
| 37 | 15-HEPE | EPA | 1.04 | 0.17 | Inf | up |
| 38 | 18-HEPE | EPA | 1.14 | 0.13 | 53.12 | up |
| 39 | 11(12)-DiHETE | EPA | 1.10 | 0.15 | Inf | up |
| 40 | 12(13)-DiHOME | LA | 1.13 | 0.15 | 65.92 | up |
| 41 | 12,13-EpOME | LA | 1.22 | 0.11 | 34.49 | up |
| 42 | 9(S),12(S),13(S)-TriHOME | LA | 1.20 | 0.07 | 2.70 | up |
| 43 | 9,10-EpOME | LA | 1.21 | 0.11 | 39.56 | up |
| 44 | 9,10-DiHOME | LA | 1.15 | 0.14 | 54.98 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, X.; Shen, J.; Weng, X. Effect of Season on Testicular Development and Spermatogenesis in Hu Sheep: Insights from Antioxidant Indices, Oxylipins, and Transcriptomics. Animals 2025, 15, 2824. https://doi.org/10.3390/ani15192824
Li W, Zhang X, Shen J, Weng X. Effect of Season on Testicular Development and Spermatogenesis in Hu Sheep: Insights from Antioxidant Indices, Oxylipins, and Transcriptomics. Animals. 2025; 15(19):2824. https://doi.org/10.3390/ani15192824
Chicago/Turabian StyleLi, Wanhong, Xinyue Zhang, Jie Shen, and Xiuxiu Weng. 2025. "Effect of Season on Testicular Development and Spermatogenesis in Hu Sheep: Insights from Antioxidant Indices, Oxylipins, and Transcriptomics" Animals 15, no. 19: 2824. https://doi.org/10.3390/ani15192824
APA StyleLi, W., Zhang, X., Shen, J., & Weng, X. (2025). Effect of Season on Testicular Development and Spermatogenesis in Hu Sheep: Insights from Antioxidant Indices, Oxylipins, and Transcriptomics. Animals, 15(19), 2824. https://doi.org/10.3390/ani15192824







