Analysis of Relative Abundance Distribution and Environmental Differences for Blue Mackerel (Scomber australasicus) and Chub Mackerel (Scomber japonicus) on the High Seas of the North Pacific Ocean
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
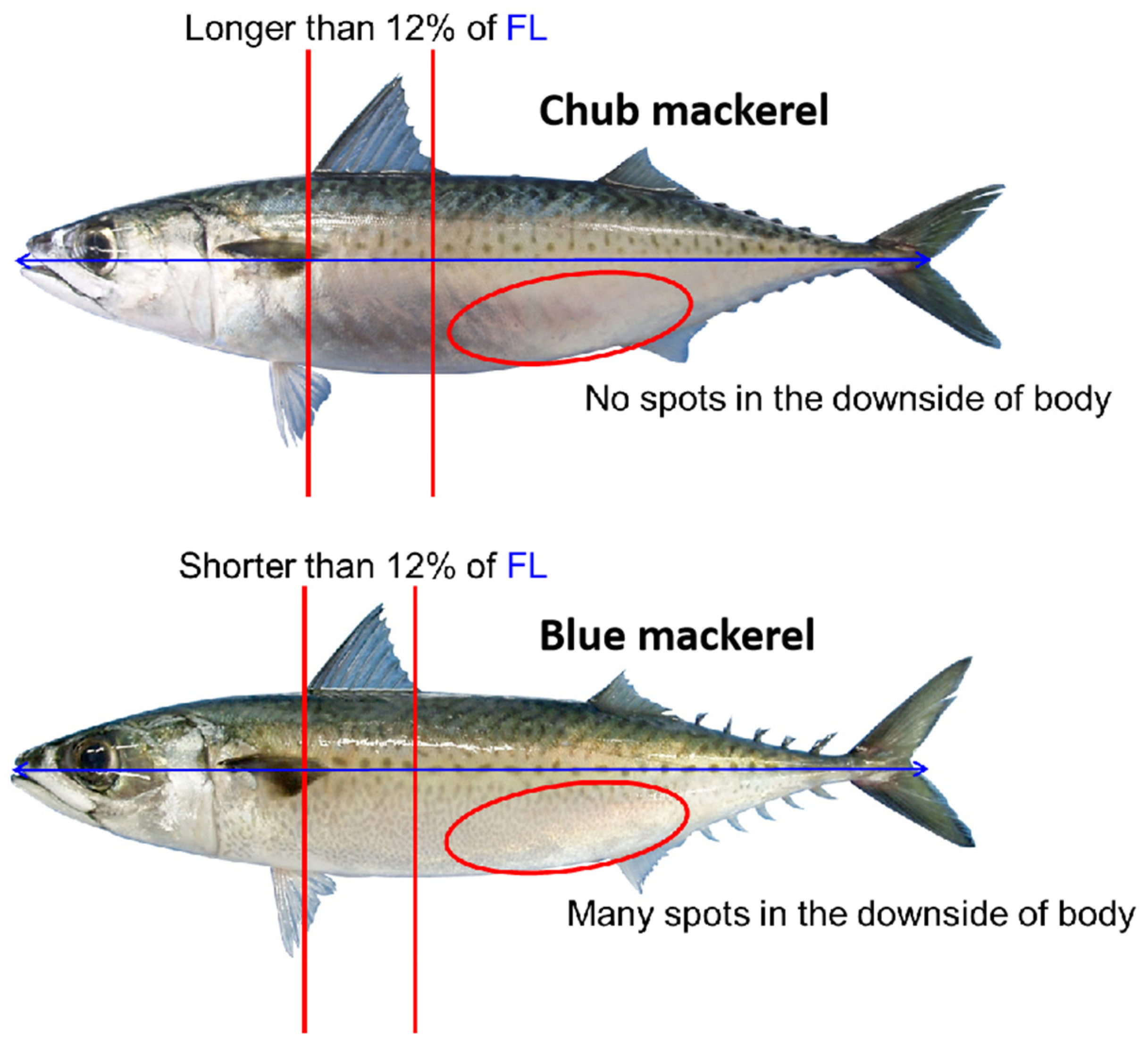
2.1.1. Fishery Data Sources
2.1.2. Acquisition and Selection of Environmental Data
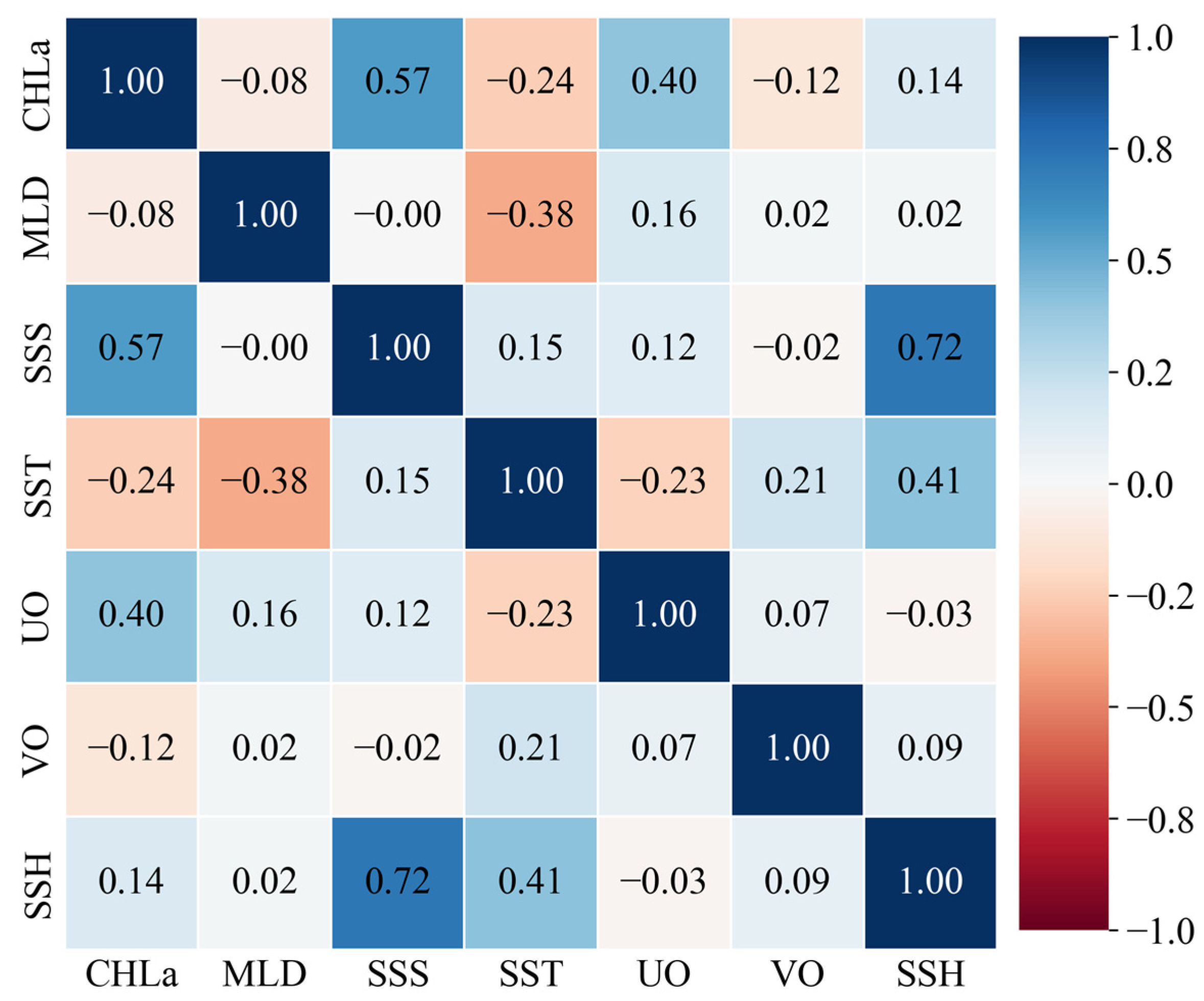
2.1.3. Environmental Data Processing
2.2. Construction of the ZOIBM
2.3. ZOIBM Evaluation Metrics
2.3.1. MCMC Convergence Diagnostics
2.3.2. Posterior Predictive Checks
2.3.3. Quantification of Goodness-of-Fit
2.4. GAM Construction
2.5. Annual Variation in the Centroid for Blue Mackerel and Chub Mackerel
3. Results
3.1. ZOIBM Accuracy Evaluation
3.1.1. Convergence and Parameter Estimation
3.1.2. Posterior Predictive Assessment of ZOIBM Adequacy
3.1.3. Goodness-of-Fit Assessment
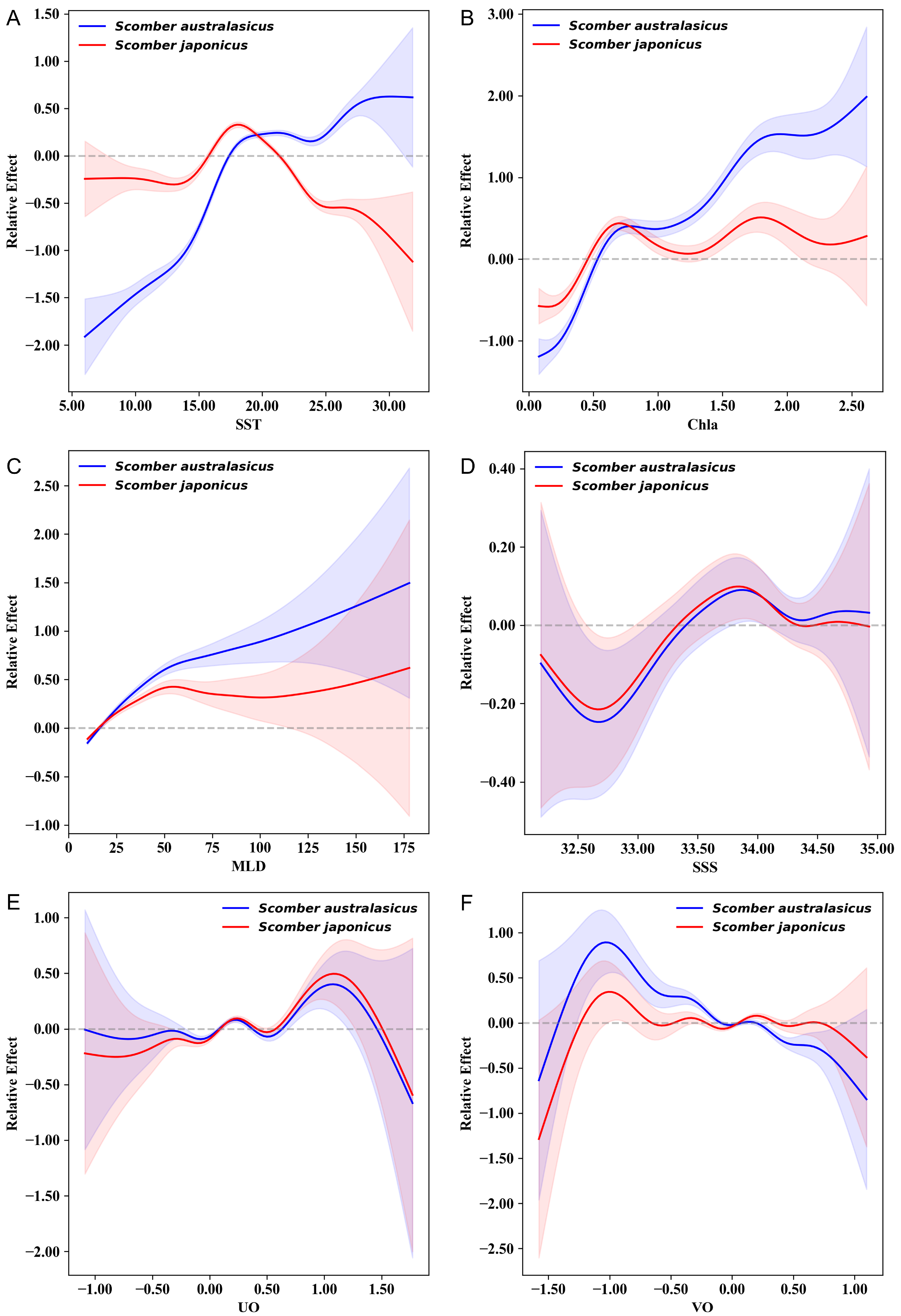
3.2. Effects of Environmental Factors on the Relative Abundance Distribution of the Two Mackerel Species
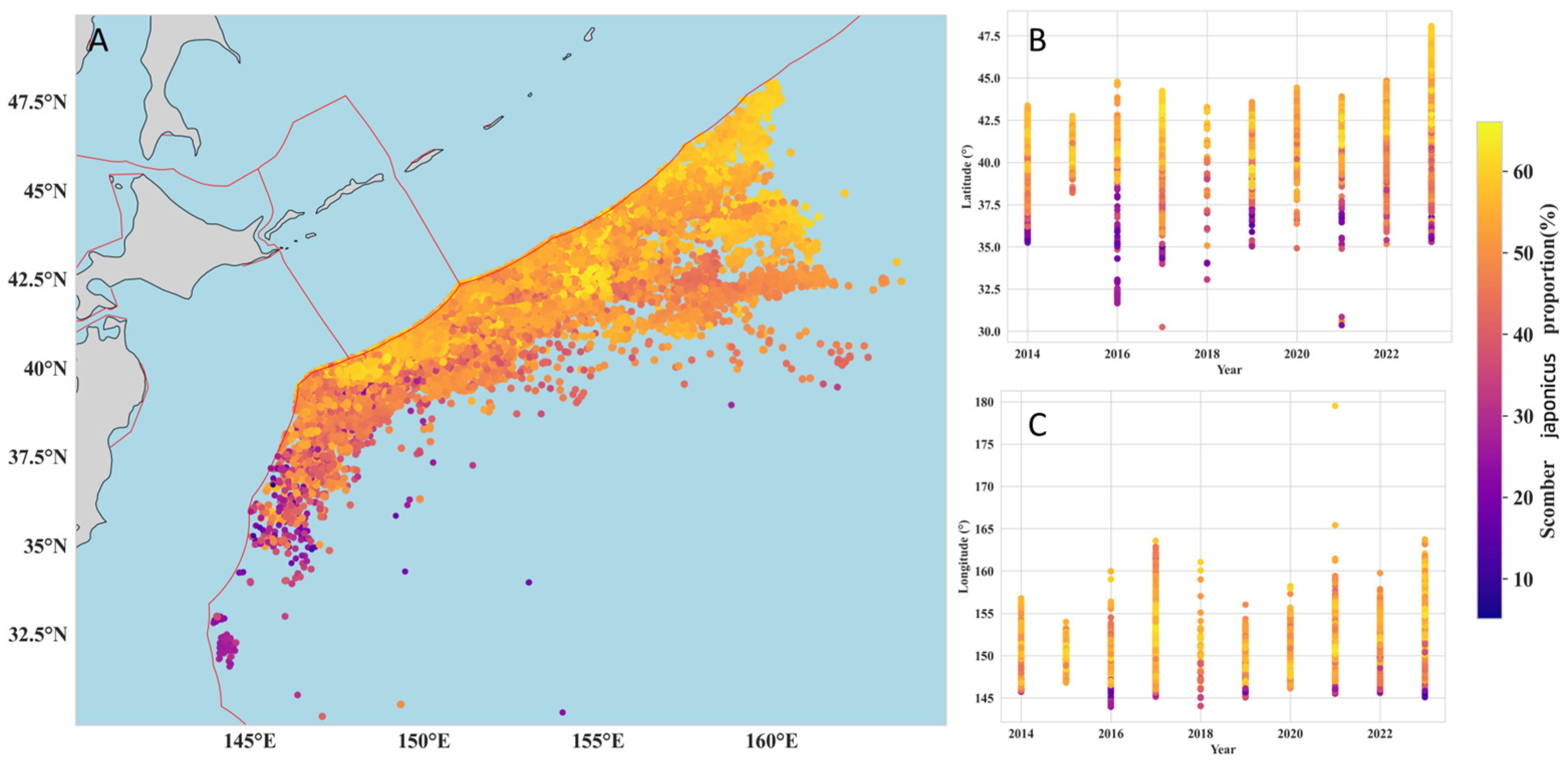
3.3. Relative Abundance Distribution of Blue Mackerel and Chub Mackerel
3.4. Dynamics of the Centroid of Abundance for Blue Mackerel and Chub Mackerel
4. Discussion
4.1. Evaluation of ZOBIM Performance
4.2. Environmental Driving Mechanisms of Spatiotemporal Distributional Differences
4.3. Dynamics of Fishing Ground Centroids
4.4. Management Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearcy, W.G.; Fisher, J.P.; Anma, G.; Meguro, T. Species associations of epipelagic nekton of the North Pacific Ocean, 1978–1993. Fish. Oceanogr. 2010, 5, 1–20. [Google Scholar] [CrossRef]
- Yasuda, I. Hydrographic Structure and Variability in the Kuroshio-Oyashio Transition Area. J. Oceanogr. 2003, 59, 389–402. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.; Jiang, B.; Shang, C.; Sun, Y.; Zhao, X.; Xiang, D.; Zhang, H.; Shi, Y. Construction of chub mackerel (Scomber japonicus) fishing ground prediction model in the northwestern Pacific Ocean based on deep learning and marine environmental variables. Mar. Pollut. Bull. 2023, 193, 115158. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; He, Y.; Fan, W.; Tang, F. Stock assessment using length-based Bayesian evaluation method for three small pelagic species in the Northwest pacific ocean. Front. Mar. Sci. 2022, 9, 775180. [Google Scholar] [CrossRef]
- Yang, C.; Han, H.; Zhang, H.; Shi, Y.; Su, B.; Jiang, P.; Xiang, D.; Sun, Y.; Li, Y. Assessment and management recommendations for the status of Japanese sardine Sardinops melanostictus population in the Northwest Pacific. Ecol. Indic. 2023, 148, 110111. [Google Scholar] [CrossRef]
- Andrews, N.; Bennett, N.J.; Billon, P.L.; Green, S.J.; Sumaila, U.R. Oil, fisheries and coastal communities: A review of impacts on the environment, livelihoods, space and governance. Energy Res. Soc. Sci. 2021, 75, 102009. [Google Scholar] [CrossRef]
- Bates, N.R.; Johnson, R.J. Acceleration of ocean warming, salinification, deoxygenation and acidification in the surface subtropical North Atlantic Ocean. Commun. Earth Environ. 2020, 1, 33. [Google Scholar] [CrossRef]
- Johnson, G.C.; Lyman, J.M. Warming trends increasingly dominate global ocean. Nat. Clim. Change 2020, 10, 757–761. [Google Scholar] [CrossRef]
- Sassa, C.; Tsukamoto, Y. Distribution and growth of Scomber japonicus and S. australasicus larvae in the southern East China Sea in response to oceanographic conditions. Mar. Ecol. Prog. Ser. 2010, 419, 185–199. [Google Scholar] [CrossRef]
- Kume, G.; Shigemura, T.; Okanishi, M.; Hirai, J.; Shiozaki, K.; Ichinomiya, M.; Komorita, T.; Habano, A.; Makino, F.; Kobari, T. Distribution, feeding habits, and growth of chub mackerel, Scomber japonicus, larvae during a high-stock period in the northern Satsunan area, southern Japan. Front. Mar. Sci. 2021, 8, 725227. [Google Scholar] [CrossRef]
- Frederiksen, M.; Edwards, M.; Richardson, A.J.; Halliday, N.C.; Wanless, S. From plankton to top predators: Bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 2006, 75, 1259–1268. [Google Scholar] [CrossRef]
- Queiros, Q.; Fromentin, J.M.; Gasset, E.; Dutto, G.; Saraux, C. Food in the Sea: Size Also Matters for Pelagic Fish. Front. Mar. Sci. 2019, 6, 385. [Google Scholar] [CrossRef]
- Kitasato, A.; Miyazaki, T.; Sugaya, Y.; Omachi, S. Automatic discrimination between Scomber japonicus and Scomber australasicus by geometric and texture features. Fishes 2018, 3, 26. [Google Scholar] [CrossRef]
- McMillan, M.N.; Leahy, S.M.; Hillcoat, K.B.; Wickens, M.; Roberts, E.M.; Daniell, J.J. Untangling multi-species fisheries data with species distribution models. Rev. Fish Biol. Fish. 2024, 34, 1133–1148. [Google Scholar] [CrossRef]
- García Criado, M.; Myers-Smith, I.H.; Bjorkman, A.D.; Elmendorf, S.C.; Normand, S.; Aastrup, P.; Aerts, R.; Alatalo, J.M.; Baeten, L.; Björk, R.G. Plant diversity dynamics over space and time in a warming Arctic. Nature 2025, 642, 653–661. [Google Scholar] [CrossRef]
- Ospina, R.; Ferrari, S.L. Inflated beta distributions. Stat. Pap. 2010, 51, 111–126. [Google Scholar] [CrossRef]
- Tang, B.; Frye, H.A.; Gelfand, A.E.; Silander, J.A. Zero-Inflated Beta Distribution Regression Modeling. J. Agric. Biol. Environ. Stat. 2023, 28, 117–137. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Jiang, K.; Xiang, D.; Shi, Y.; Huang, S.; Li, Y.; Han, H. Simulating the changes of the habitats suitability of chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 116873. [Google Scholar] [CrossRef]
- Yukami, R.; Ohshimo, S.; Yoda, M.; Hiyama, Y. Estimation of the spawning grounds of chub mackerel Scomber japonicus and spotted mackerel Scomber australasicus in the East China Sea based on catch statistics and biometric data. Fish. Sci. 2009, 75, 167–174. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, D.; Wang, J.; Jiang, K.; Zhu, H.; Huang, S.S.; Zhang, F.; Li, Y.; Zhang, H. Comparative analysis of modeling methods and prediction accuracy for Japanese sardine habitat under three climate scenarios with differing greenhouse emission pathways. Mar. Pollut. Bull. 2025, 215, 117867. [Google Scholar] [CrossRef]
- Chavez, F.P.; Ryan, J.; Lluch-Cota, S.E.; Miguel, N.C. From Anchovies to Sardines and Back: Multidecadal Change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Zhang, K.; Li, J.; Xu, Y.; Sun, M.; Jiang, J.; Xu, S.; Cai, Y.; Qiu, Y.; Chen, Z. Impacts of climate events on life history parameters of major commercial fishes in the Beibu Gulf, South China Sea in the last 15 years. Front. Mar. Sci. 2023, 10, 1234772. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, C.; Wang, Y.; Xian, W.; Palomares, M.L. Stock Status Assessments for 12 Exploited Fishery Species in the Tsushima Warm Current Region, Southwest Japan and East China, Using the CMSY and BSM Methods. Front. Mar. Sci. 2020, 7, 640. [Google Scholar] [CrossRef]
- Su, M.; Liu, N.; Zhang, Z.; Zhang, J. Osmoregulatory strategies of estuarine fish Scatophagus argus in response to environmental salinity changes. BMC Genom. 2022, 23, 545. [Google Scholar] [CrossRef]
- Lyu, S.; Tong, J.; Zhu, Z.; Xue, M.; Qiu, Y.; Li, B.; Liu, B. Spatiotemporal distribution characteristics of small pelagic fish in the Northwest Pacific Ocean in summer of 2023 based on acoustics methods. J. Shanghai Ocean Univ. 2025, 34, 394–402. [Google Scholar] [CrossRef]
- An, Y.-Z.; Zhang, R.; Wang, H.-Z.; Chen, J.; Chen, Y.-D. Study on calculation and spatio-temporal variations of global ocean mixed layer depth. Chin. J. Geophys. 2012, 55, 2249–2258. [Google Scholar] [CrossRef]
- Gregorich, M.; Strohmaier, S.; Dunkler, D.; Heinze, G. Regression with Highly Correlated Predictors: Variable Omission Is Not the Solution. Int. J. Environ. Res. Public Health 2021, 18, 4259. [Google Scholar] [CrossRef]
- Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Ospina, R.; Ferrari, S.L.P. A general class of zero-or-one inflated beta regression models. Comput. Stat. Data Anal. 2011, 56, 1609–1623. [Google Scholar] [CrossRef]
- Vuorre, M. How to Analyze Visual Analog (Slider) Scale Data? Available online: https://vuorre.com/posts/2019-02-18-analyze-analog-scale-ratings-with-zero-one-inflated-beta-models/ (accessed on 16 July 2025).
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Rubin, D.B. Bayesian Data Analysis; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from Iterative Simulation Using Multiple Sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Gelman, A.; Meng, X.L.; Stern, H. Posterior Predictive Assessment of Model Fitness Via Realized Discrepancies. Stat. Sin. 1997, 106, 265–297. [Google Scholar] [CrossRef]
- Youngflesh, C.; Montgomery, G.A.; Saracco, J.F.; Miller, D.A.; Guralnick, R.P.; Hurlbert, A.H.; Siegel, R.B.; LaFrance, R.; Tingley, M.W. Demographic consequences of phenological asynchrony for North American songbirds. Proc. Natl. Acad. Sci. USA 2023, 120, e2221961120. [Google Scholar] [CrossRef]
- Xiao, X.; He, Q.; Ma, S.; Liu, J.; Sun, W.; Lin, Y.; Yi, R. Environmental variables improve the accuracy of remote sensing estimation of soil organic carbon content. Sci. Rep. 2024, 14, 18964. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Zhu, Z.; Tong, J.; Xue, M.; Sarr, O.; Gao, T. Assessing the influence of abiotic factors on small pelagic fish distribution across diverse water layers in the Northwest Pacific Ocean through acoustic methods. Ecol. Indic. 2024, 158, 11. [Google Scholar] [CrossRef]
- Chen, S.; Han, H.; Yu, J.; Sun, T.; Zhou, J. Response of diatoms to environmental changes in the Porphyra cultivation system in Haizhou Bay using GBT model and GAM. Mar. Pollut. Bull. 2025, 215, 117846. [Google Scholar] [CrossRef] [PubMed]
- Smithson, M.; Verkuilen, J. A Better Lemon Squeezer? Maximum-Likelihood Regression With BetaDistributed Dependent Variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X. Forecasting model for spotted mackerel biomass based on grey system theory. J. Shanghai Ocean Univ. 2019, 28, 154–160. [Google Scholar] [CrossRef]
- Melomerino, S.M.; Fath, B.D. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Simões, M.V.; Saeedi, H.; Cobos, M.E.; Brandt, A. Environmental matching reveals non-uniform range-shift patterns in benthic marine Crustacea. Clim. Change 2021, 168, 31. [Google Scholar] [CrossRef]
- Hays, G.C. Ocean currents and marine life. Curr. Biol. 2017, 27, R470–R473. [Google Scholar] [CrossRef] [PubMed]
- Yatsu, A. Review of population dynamics and management of small pelagic fishes around the Japanese Archipelago. Fish. Sci. 2019, 85, 611–639. [Google Scholar] [CrossRef]
- Sugimoto, S.; Hanawa, K.; Watanabe, T.; Suga, T.; Xie, S.-P. Enhanced warming of the subtropical mode water in the North Pacific and North Atlantic. Nat. Clim. Change 2017, 7, 656–658. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; Molinos, J.G.; Sydeman, W.J. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Richardson, A.J.; Schoeman, D.S. Climate impact on plankton ecosystems in the Northeast Atlantic. Science 2004, 305, 1609–1612. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Lin, P.; Cheng, L.; Ge, K.; Liu, H.; Duan, J.; Wang, F. North Atlantic–Pacific salinity contrast enhanced by wind and ocean warming. Nat. Clim. Change 2024, 14, 723–731. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S. Variability of the Kuroshio Extension Jet, Recirculation Gyre, and Mesoscale Eddies on Decadal Time Scales. J. Phys. Oceanogr. 2005, 35, 2090–2103. [Google Scholar] [CrossRef]



| Environmental Variable | Data Source | Initial Temporal Resolution | Initial Spatial Resolution |
|---|---|---|---|
| Sea Surface Temperature (/°C) | GOPR | Daily | 0.083° × 0.083° |
| Chlorophyll-a Concentration (mg/m3) | GOBR | Daily | 0.25° × 0.25° |
| Sea Surface Salinity (‰) | GOPR | Daily | 0.083° × 0.083° |
| Sea Surface Height (m) | GOPR | Daily | 0.083° × 0.083° |
| Eastward sea water velocity (m/s) | GOPR | Daily | 0.083° × 0.083° |
| Northward sea water velocity (m/s) | GOPR | Daily | 0.083° × 0.083° |
| Mixed Layer Depth (m) | GOPR | Daily | 0.083° × 0.083° |
| Number | Parameter | R-Hat Value |
|---|---|---|
| 1 | b_Intercept | 1.0010054 |
| 2 | b_phi_Intercept | 0.9999693 |
| 3 | b_zoi_Intercept | 1.0018218 |
| 4 | b_coi_Intercept | 1.0004148 |
| 5 | Spatial Random Effects (mean ± SD) | 1.0013 ± 0.001 |
| Variable | Species | Effective Degrees of Freedom (edf) | Reference Degrees of Freedom (Ref.df) | p-Value |
|---|---|---|---|---|
| Chla | Blue Mackerel | 8.921019 | 8.998026 | <0.001 |
| Chub Mackerel | 8.926323 | 8.99829 | <0.001 | |
| MLD | Blue Mackerel | 3.687771 | 4.540656 | <0.001 |
| Chub Mackerel | 4.685233 | 5.637041 | <0.001 | |
| SSS | Blue Mackerel | 7.26042 | 8.215804 | <0.001 |
| Chub Mackerel | 7.137682 | 8.122104 | <0.001 | |
| SST | Blue Mackerel | 8.38792 | 8.890325 | <0.001 |
| Chub Mackerel | 8.383943 | 8.888969 | <0.001 | |
| UO | Blue Mackerel | 7.518612 | 8.366241 | <0.001 |
| Chub Mackerel | 7.579307 | 8.407768 | <0.001 | |
| VO | Blue Mackerel | 8.351075 | 8.860459 | <0.001 |
| Chub Mackerel | 8.312896 | 8.84484 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, H.; Zhang, F.; Huang, S.; Wang, J.; Xiang, D.; Li, Y.; Sun, Y. Analysis of Relative Abundance Distribution and Environmental Differences for Blue Mackerel (Scomber australasicus) and Chub Mackerel (Scomber japonicus) on the High Seas of the North Pacific Ocean. Animals 2025, 15, 2822. https://doi.org/10.3390/ani15192822
Zhang H, Zhu H, Zhang F, Huang S, Wang J, Xiang D, Li Y, Sun Y. Analysis of Relative Abundance Distribution and Environmental Differences for Blue Mackerel (Scomber australasicus) and Chub Mackerel (Scomber japonicus) on the High Seas of the North Pacific Ocean. Animals. 2025; 15(19):2822. https://doi.org/10.3390/ani15192822
Chicago/Turabian StyleZhang, Heng, Hanji Zhu, Famou Zhang, Sisi Huang, Jianhua Wang, Delong Xiang, Yang Li, and Yuyan Sun. 2025. "Analysis of Relative Abundance Distribution and Environmental Differences for Blue Mackerel (Scomber australasicus) and Chub Mackerel (Scomber japonicus) on the High Seas of the North Pacific Ocean" Animals 15, no. 19: 2822. https://doi.org/10.3390/ani15192822
APA StyleZhang, H., Zhu, H., Zhang, F., Huang, S., Wang, J., Xiang, D., Li, Y., & Sun, Y. (2025). Analysis of Relative Abundance Distribution and Environmental Differences for Blue Mackerel (Scomber australasicus) and Chub Mackerel (Scomber japonicus) on the High Seas of the North Pacific Ocean. Animals, 15(19), 2822. https://doi.org/10.3390/ani15192822







