Optimizing Sow and Litter Performance via a Comprehensive Service-to-Weaning Feeding Regimen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Housing, and Management
2.2. Dietary Treatments, Feeding, and Feeding System
2.2.1. Diet of Control Group
2.2.2. Modified Diet Group
2.3. Feeding: Feed Curve and Number of Daily Intake
2.4. Body Condition, Hypophagia, and Lactation Curve
2.5. Sow Plasma Metabolites
2.6. Litter and Piglet Production Parameters
2.7. Statistical Methods
3. Results
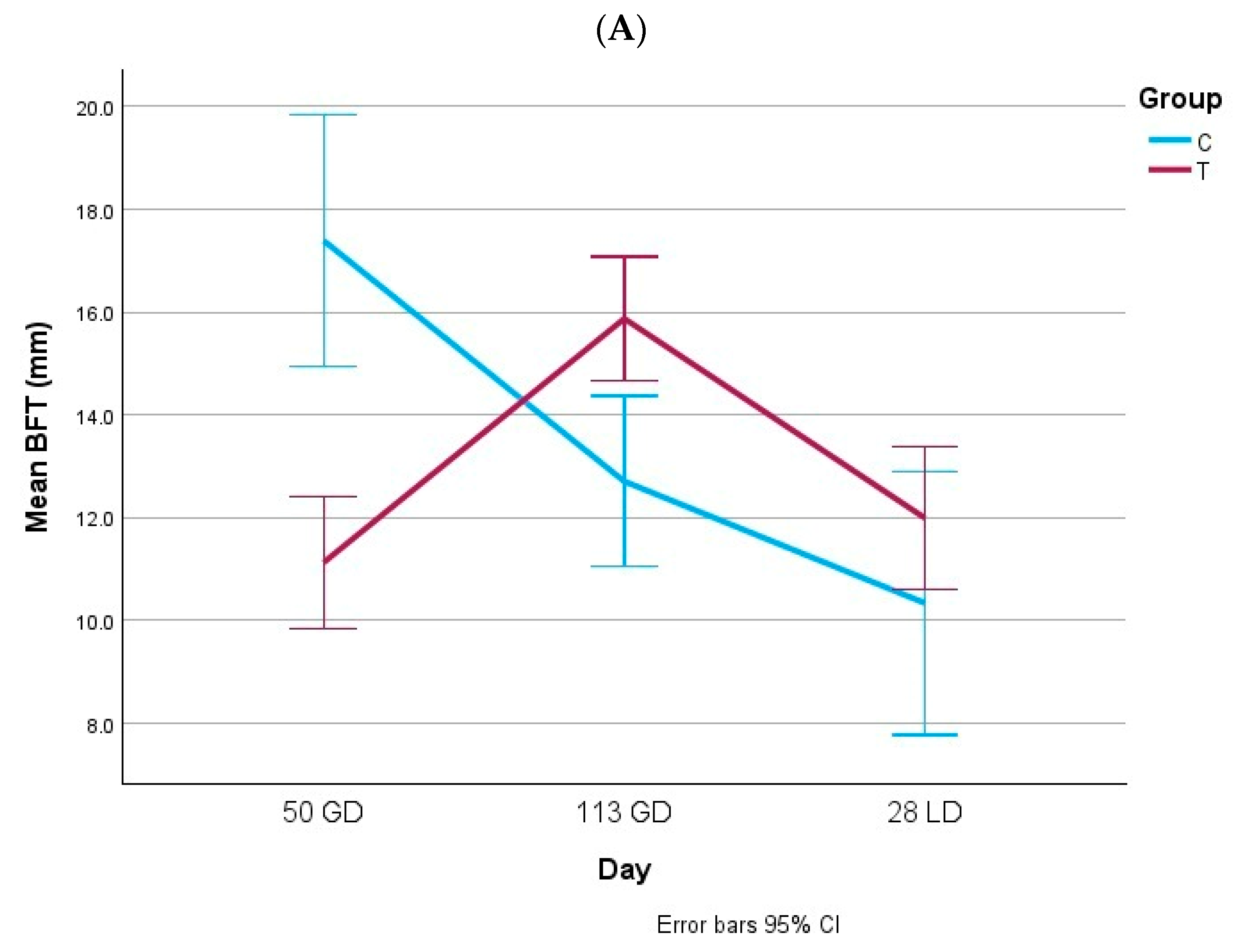
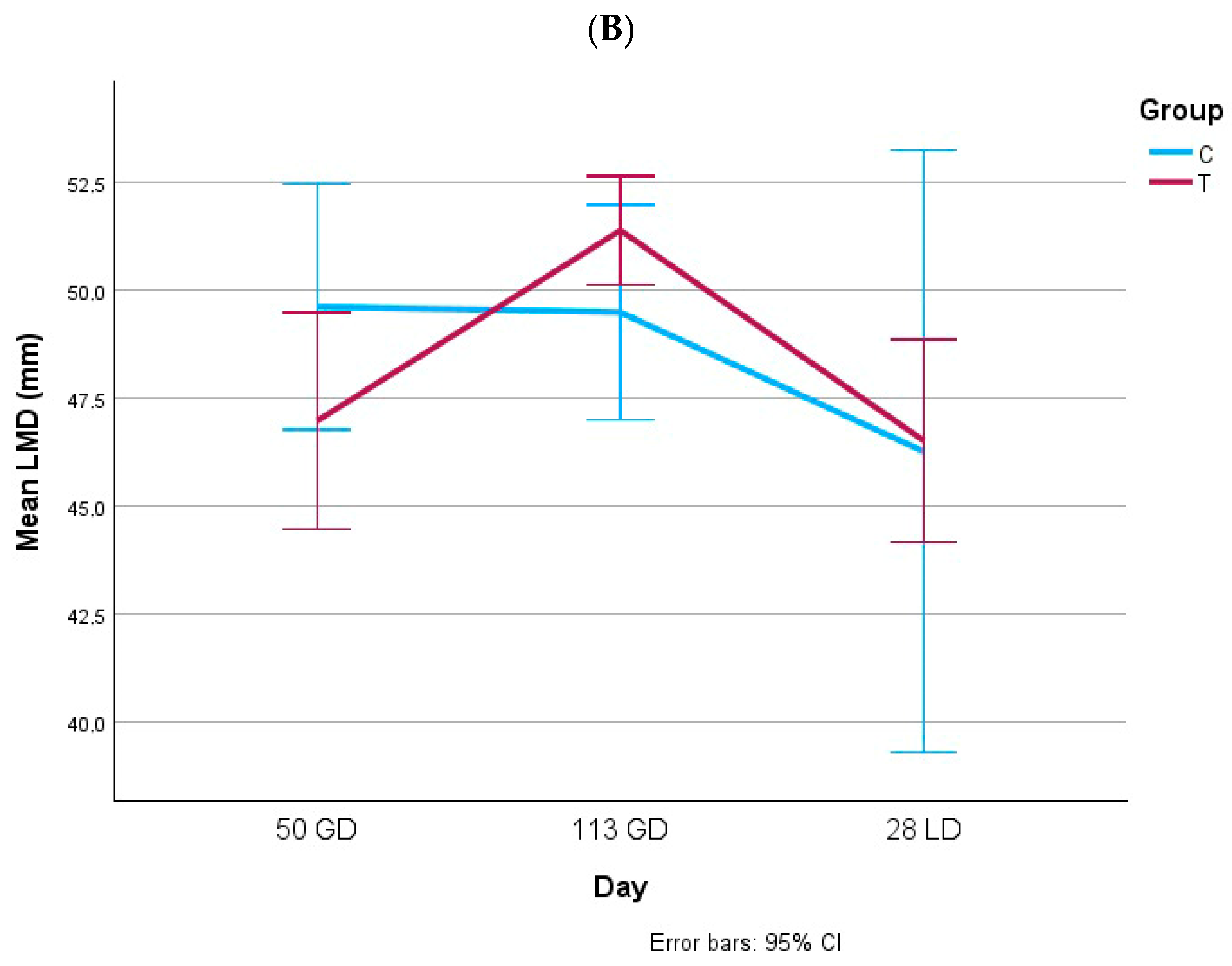
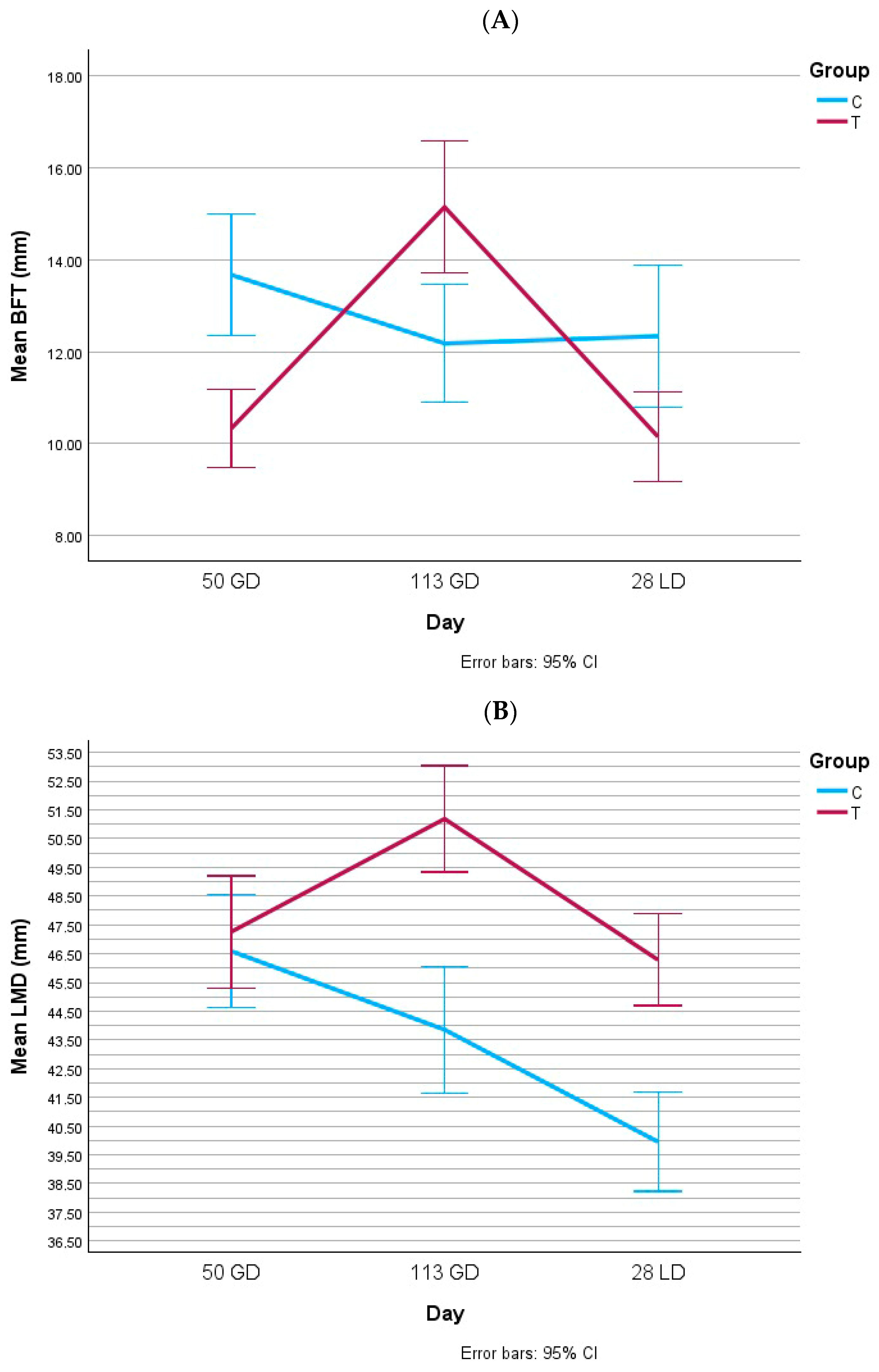
3.1. Body Condition
3.2. Occurrence of Diarrhea, Hypophagia, Positive Lactation Curve, and Low Weight Piglets
3.3. Sow Plasma Metabolites
3.4. Litter Characteristics
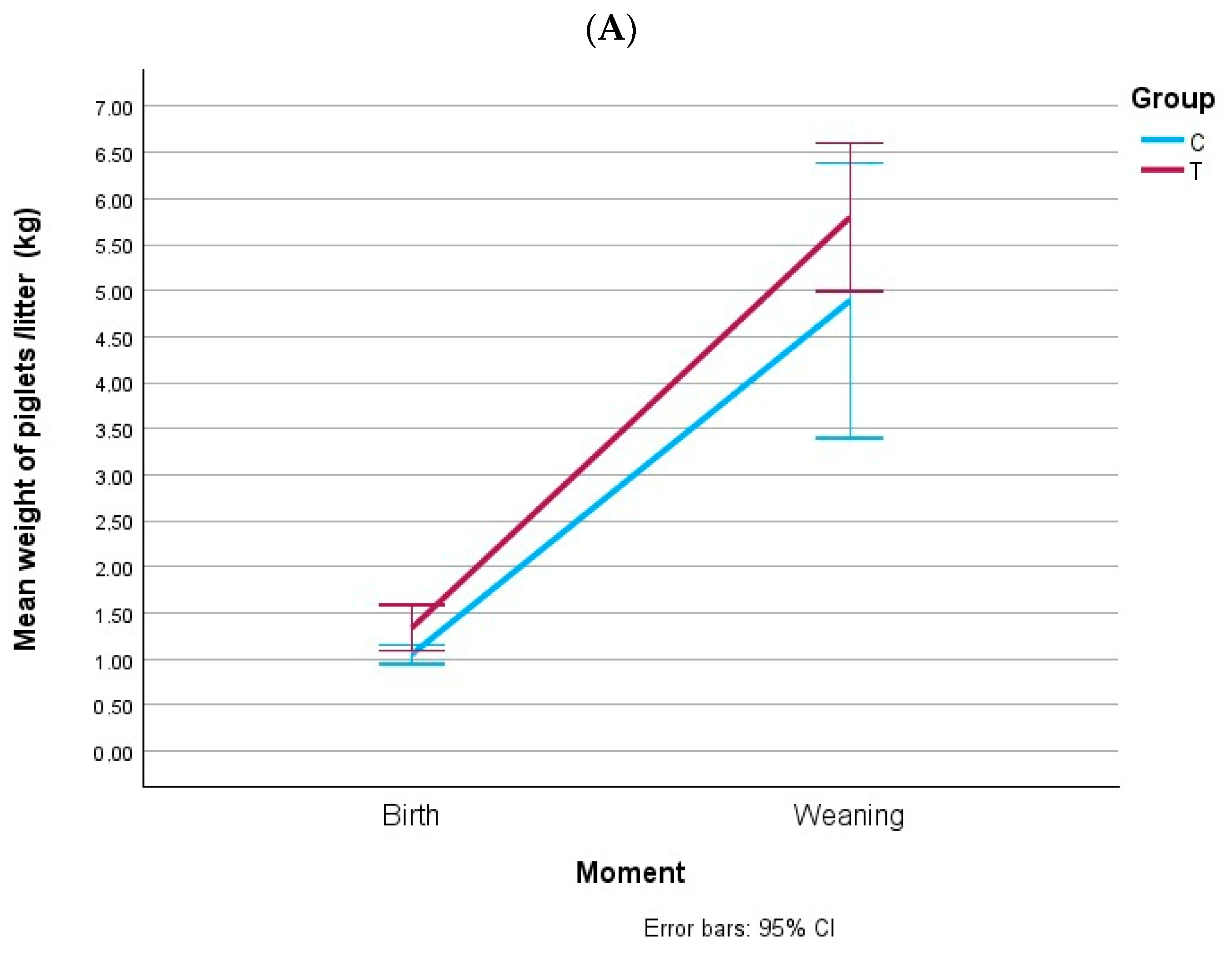
3.5. Weight of Piglets at Birth and at Weaning
4. Discussion
4.1. Maternal Condition
4.2. Parturition Failures/Performance
4.3. Piglet Performance
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C. Offspring of hyper prolific sows: Immunity, birthweight, and heterogeneous litters. Mol. Reprod. Dev. 2023, 90, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Theil, K.; Farmer, C.; Feyera, T. Physiology and nutrition of late gestating and transition sows. J. Anim. Sci. 2022, 100, skac176. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. Recent Adv. Nutr. Sci. 2004, 134, 2169–2172. [Google Scholar]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Farmer, C.; Palin, M.F. Milk production and composition. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 229–250. [Google Scholar]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef]
- Park, M.S.; Yang, W.X.; Shinde, P.L.; Choi, J.Y.; Jo, W.K.; Kim, J.S.; Lohakare, J.D.; Yang, D.K.; Lee, J.K.; Kwon, I.K.; et al. Effects of dietary glucose inclusion on reproductive performance, milk compositions and blood profiles in lactating sows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 677–684. [Google Scholar] [CrossRef]
- Bass, B.E.; Bradley, C.L.; Johnson, Z.B.; Zier-Rush, C.E.; Boyd, R.D.; Usry, J.L.; Frank, J.W. Influence of dietary L-arginine supplementation of sows during late pregnancy on piglet birth weight and sow and litter performance during lactation. J. Anim. Sci. 2017, 95, 248–256. [Google Scholar] [CrossRef]
- Costa, K.A.; Marques, D.B.D.; de Campos, C.F.; Saraiva, A.; Guimarães, J.D.; Guimarães, S.E.F. Nutrition influence on sow reproductive performance and conceptuses nutritional differences and survival: A review about l-arginine supplementation. Livest. Sci. 2019, 228, 97–103. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Marczewski, S.E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012, 13, 109–119. [Google Scholar] [CrossRef]
- Kim, S.W.; Hurley, W.L.; Wu, G.; Ji, F. Ideal amino acid balance for sows during gestation and lactation. J. Anim. Sci. 2009, 87 (Suppl. S14), E123–E132. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.F.; Bruun, T.S.; Feyera, T.; Larsen, U.K.; Theil, P.K. A two-diet feeding regime for lactating sows reduced nutrient deficiency in early lactation and improved milk yield. Livest. Sci. 2016, 191, 165–173. [Google Scholar] [CrossRef]
- Feyera, T.; Højgaard, C.K.; Vinther, J.; Bruun, T.S.; Theil, P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 2017, 95, 5430–5438. [Google Scholar] [CrossRef]
- Feyera, T.; Theil, P.K. Energy and lysine requirements and balances of sows during transition and lactation: A factorial approach. Livest. Sci. 2017, 201, 50–57. [Google Scholar] [CrossRef]
- Père, M.C.; Etienne, M. Uterine blood flow in sows: Effects of pregnancy stage and litter size. Reprod. Nutr. Dev. 2000, 40, 369–382. [Google Scholar] [CrossRef]
- Stewart, V.; Buis, R.Q.; Christensen, B.; Hansen, L.L.; de Lange, C.F.; Mandell, I.B.; Huber, L.A. The effects of precisely meeting estimated daily energy and lysine requirements for gestating sows over three consecutive pregnancies on sow reproductive and lactation performance. Transl. Anim. Sci. 2021, 5, txab226. [Google Scholar] [CrossRef]
- Farmer, C.; Palin, M.F.; Hovey, R.C.; Falt, T.D.; Huber, L.A. Dietary supplementation with lysine (protein) stimulates mammary development in late pregnant gilts. J. Anim. Sci. 2022, 100, skac051. [Google Scholar] [CrossRef]
- Theil, P.K.; Olesen, A.K.; Flummer, C.; Sørensen, G.; Kristensen, N.B. Impact of feeding and postprandial time on plasma ketone bodies in sows during transition and lactation. J. Anim. Sci. 2013, 91, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, B.; Casteràs, A.; Domingo, E.; Nóvoa, F.J.; López, Y.; Cabezas-Agricola, J.M.; Mesa, J. Betahidroxibutirato capilar en la monitorización de la cetoacidosis diabética. Endocrinol. Nutr. 2011, 58, 347–352. [Google Scholar] [CrossRef]
- Klein, S.L.; Scheper, C.; May, K.; König, S. Genetic and nongenetic profiling of milk β-hydroxybutyrate and acetone and their associations with ketosis in Holstein cows. J. Dairy Sci. 2020, 103, 10332–10346. [Google Scholar] [CrossRef]
- Che, L.; Hu, L.; Wu, C.; Xu, Q.; Zhou, Q.; Peng, X.; Wu, D. Effects of increased energy and amino acid intake in late gestation on reproductive performance, milk composition, metabolic, and redox status of sows. J. Anim. Sci. 2019, 97, 2914–2926. [Google Scholar] [CrossRef]
- Xia, M.; Pan, Y.; Guo, L.; Wei, X.; Xiong, J.; Wang, L.; Peng, J.; Wang, C.; Peng, J.; Wei, H. Effect of gestation dietary methionine/lysine ratio on placental angiogenesis and reproductive performance of sows. J. Anim. Sci. 2019, 97, 3487–3497. [Google Scholar] [CrossRef]
- Palencia, J.Y.P.; Lemes, M.A.G.; Garbossa, C.A.P.; Abreu, M.L.T.; Pereira, L.J.; Zangeronimo, M.G. Arginine for gestating sows and foetal development: A systematic review. J. Anim. Physiol. Anim. Nutr. 2017, 102, 204–213. [Google Scholar] [CrossRef]
- Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; Ational Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- FEDNA (Fundación Española para el Desarrollo de la Nutrición Animal). Tablas FEDNA de Composición y Valor Nutritivo de Alimentos Para la Fabricación de Piensos Compuestos, 2nd ed.; De Blas, C., Mateos, G.G., García, P., Eds.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2012. [Google Scholar]
- Wang, W.; Degroote, J.; Van Ginneken, C.; Van Poucke, M.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; De Smet, S.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2016, 30, 863–873. [Google Scholar] [CrossRef]
- Van Tichelen, K.; Prims, S.; Ayuso, M.; Van Kerschaver, C.; Vandaele, M.; Degroote, J.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Handling associated with drenching does not impact survival and general health of low birth weight piglets. Animals 2021, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.J.; Sangild, P.T.; Zhang, Y.Q.; Zhang, S.H. Bioactive compounds in porcine colostrum and milk and their effects on intestinal development in neonatal pigs. In Biology of Growing Animals; Zabielski, R., Gregory, P.C., Weström, B., Salek, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 169–192. [Google Scholar]
- Seoane, S.; De Palo, P.; Lorenzo, J.M.; Maggiolino, A.; González, P.; Pérez-Ciria, L.; Latorre, M.A. Effect of Increasing Dietary Aminoacid Concentration in Late Gestation on Body Condition and Reproductive Performance of Hyperprolific Sows. Animals 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.G.D.; Janssens, G.P.J.; Delputte, P.; Lammertyn, A.; de Kruif, A. Back fat measurements in sows from three commercial pig herds: Relationship with reproductive efficiency and correlation with visual body condition scores. Livest. Prod. Sci. 2004, 91, 57–67. [Google Scholar] [CrossRef]
- Kim, J.S.; Yang, X.; Pangeni, D.; Baidoo, S.K. Relationship between backfat thickness of sows during late gestation and reproductive efficiency at different parities. Acta Agric. Scand. Sect. A Anim. Sci. 2015, 65, 1–8. [Google Scholar] [CrossRef]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef]
- Strathe, A.V.; Bruun, T.S.; Tauson, A.H.; Theil, P.K.; Hansen, C.F. Increased dietary protein for lactating sows affects body composition, blood metabolites and milk production. Animal 2020, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, G.L.; Cline, T.R. A brief review of the 10-year history of the Midwest swine nutrition conference. In Midwest Swine Nutrition Conference Proceedings; Purdue University: West Lafayette, IN, USA, 2010. [Google Scholar]
- Johannsen, J.C.; Sørensen, M.T.; Theil, P.K.; Bruun, T.S.; Farmer, C.; Feyera, T. Optimal protein concentration in diets for sows during the transition period. J. Anim. Sci. 2024, 102, skae082. [Google Scholar] [CrossRef]
- Van den Bosch, M.; Wijnen, J.; Van de Linde, I.B.; Van Wesel, A.A.M.; Melchior, D.; Kemp, B.; Clouard, C. Effects of maternal dietary nitrate supplementation on farrowing and placental characteristics, level of asphyxiation at birth and piglet vitality. Theriogenology 2019, 129, 1–7. [Google Scholar] [CrossRef]
- Miller, E. Nitrogen Retention for Maternal and Fetal Tissues in the Gestating Sow. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2017. [Google Scholar]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Ordaz, G.; Juárez, A.; Valdez, J.J.; Martínez, H.E.; Portillo, L.; Pérez, R.E.; Ortiz, R. Characterization of the metabolic modulation of sows during peripartum and lactation and their association with the lactational physiological hypophagia: A review. Trop. Subtrop. Agroecosyst. 2019, 22, 547–573. [Google Scholar] [CrossRef]
- Bruun, T.S.; Eskildsen, M.; Højgaard, C.K.; Nørskov, N.P.; Knudsen, K.E.B.; Theil, P.K.; Feyera, T. Feeding level during the last week of gestation can influence performance of sows and their litters in the subsequent lactation. J. Anim. Sci. 2023, 101, skad349. [Google Scholar] [CrossRef] [PubMed]
- Bin, P.; Azad, M.A.K.; Liu, G.; Zhu, D.; Kim, S.W.; Yin, Y. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food Funct. 2018, 9, 4979–4988. [Google Scholar] [CrossRef]
- Feyera, T.; Zhou, P.; Nuntapaitoon, M.; Sørensen, K.U.; Krogh, U.; Bruun, T.S.; Theil, P.K. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period. J. Anim. Sci. 2019, 97, 231–245. [Google Scholar] [CrossRef]
- Holanda, D.M.; Marcolla, C.S.; Guimarães, S.E.F.; Neves, M.M.; Hausman, G.J.; Duarte, M.S.; Saraiva, A. Dietary L-arginine supplementation increased mammary gland vascularity of lactating sows. Animal 2019, 13, 790–798. [Google Scholar] [CrossRef]
- Calatayud, M.; Koren, O.; Collado, M.C. Maternal microbiome and metabolic health program microbiome development and health of the offspring. Trends Endocrinol. Metab. 2019, 30, 735–744. [Google Scholar] [CrossRef]
- Duque, P.; Campos, R.; López, A. Evaluation of the lipid metabolic profile and its relation with fetal nutrition in gestating sows. Rev. MVZ Córdoba 2013, 18, 3543–3550. [Google Scholar] [CrossRef]
- Boyd, R.D.; Castro, G.C.; Cabrera, R.A. Nutrition and management of the sow to maximize lifetime productivity. Adv. Pork Prod. 2002, 13, 1–12. [Google Scholar]
- Aherne, F.X.; Kirkwood, R.N. Nutrition and lactation in the sow. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 199–228. [Google Scholar]
- Kaiser, M.; Jacobson, M.; Bækbo, P.; Dahl, J.; Jacobsen, S.; Guo, Y.Z.; Andersen, P.H. Lack of evidence of mastitis as a causal factor for postpartum dysgalactia syndrome in sows. Transl. Anim. Sci. 2020, 4, 250–263. [Google Scholar] [CrossRef]
- Rezaei, R.; Wu, Z.; Hou, Y.; Bazer, F.W.; Wu, G. Amino acids and mammary gland development: Nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 2016, 7, 20. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Johnston, L.J.; Levesque, C.L.; Yin, J.; Dong, B. A systematic review and meta-analysis of dietary fat effects on reproductive performance of sows and growth performance of piglets. J. Anim. Sci. Biotechnol. 2022, 13, 12. [Google Scholar] [CrossRef]
- Aherne, F.X.; Hays, V.W.; Ewan, R.C.; Speer, V.C. Glucose and fructose in the fetal and newborn pig. J. Anim. Sci. 1969, 29, 906–911. [Google Scholar] [CrossRef]





| Cycle | Group C | Group T | Total |
|---|---|---|---|
| 1 | 6 | 3 | 9 |
| 2 | 32 | 16 | 48 |
| 3 | 24 | 16 | 40 |
| 4 | 20 | 26 | 46 |
| 5 | 19 | 36 | 55 |
| 6 | 12 | 27 | 39 |
| 7 | 20 | 10 | 30 |
| 8 | 1 | - | 1 |
| Total | 134 | 134 | 268 |
| Items (Units) | Gestation | Peripartum Feed (Group T) | Lactation | |||
|---|---|---|---|---|---|---|
| Gestation Standard (Group C) | Gestation 1 Feed (Group T) | Gestation 2 Feed (Group T) | Lactation Standard (Group C) | Lactation Feed (Group T) | ||
| Ingredients: | ||||||
| Barley (%) | 48.410 | 15.358 | 26.039 | 15.000 | 22.030 | 18.700 |
| Maize (%) | 0.000 | 24.976 | 25.000 | 24.971 | 19.440 | 30.076 |
| Wheat (%) | 0.000 | 10.800 | 0.000 | 19.700 | 0.000 | 11.200 |
| Soybean meal (47% crude protein) | 0.000 | 0.000 | 1.900 | 17.900 | 18.900 | 15.800 |
| Animal blended fat (%) | 0.000 | 2.450 | 0.000 | 0.000 | 0.000 | 0.000 |
| Beet pulp (%) | 0.000 | 10.000 | 5.000 | 6.000 | 0.000 | 5.100 |
| Full fat soya (%) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 5.000 |
| Rice cylinder (%) | 10.000 | 0.000 | 0.000 | 0.000 | 5.000 | 0.000 |
| By-product biscuit (%) | 0.000 | 0.000 | 0.000 | 0.000 | 7.000 | 0.000 |
| Zootechnical meal (%) | 7.430 | 0.000 | 0.000 | 0.000 | 10.000 | 0.000 |
| Sodium bicarbonate (%) | 0.100 | 0.000 | 0.000 | 0.000 | 0.310 | 0.000 |
| Monocalcium phosphate (%) | 0.090 | 0.000 | 0.000 | 0.000 | 0.580 | 0.000 |
| Dicalcium phosphate (%) | 0.000 | 0.610 | 0.890 | 1.060 | 0.000 | 1.035 |
| Salt (%) | 0.400 | 0.500 | 0.500 | 0.500 | 0.150 | 0.500 |
| L-Lisine 50 (%) | 0.330 | 0.130 | 0.426 | 0.351 | 0.270 | 0.337 |
| L-Threonine (%) | 0.040 | 0.000 | 0.070 | 0.025 | 0.050 | 0.050 |
| L-Methionine (%) | 0.000 | 0.042 | 0.065 | 0.000 | 0.000 | 0.000 |
| Choline chloride (%) | 0.050 | 0.000 | 0.000 | 0.000 | 0.040 | 0.000 |
| Soybean oil (%) | 0.500 | 0.000 | 0.700 | 1.127 | 1.480 | 2.352 |
| Calcium carbonate (%) | 1.620 | 0.625 | 1.110 | 0.000 | 1.720 | 1.350 |
| Sunflower meal (36% crude protein) | 10.000 | 9.210 | 10.000 | 5.066 | 2.700 | 0.000 |
| Wheat bran (%) | 18.980 | 25.000 | 25.000 | 5.000 | 10.000 | 8.300 |
| Alfalfa (%) | 0.480 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sepiolite (%) | 1.320 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Glucogenic precursor (%) | 0.000 | 0.000 | 3.000 | 3.000 | 0.000 | 0.000 |
| Others (%, additives) 1 | 0.240 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Nutritional analyses 2 | ||||||
| Metabolizable energy (Kcal/kg) | 2844.879 | 2935.790 | 2850.032 | 3049.971 | 3174.143 | 3200.385 |
| Fat Matter (%) | 4.106 | 5.138 | 3.440 | 3.252 | 5.008 | 5.502 |
| Crude protein (%) | 12.500 | 12.498 | 13.360 | 17.214 | 17.000 | 16.590 |
| Crude fiber (%) | 8.000 | 7.293 | 7.248 | 5.506 | 5.000 | 4.493 |
| Neutral detergent fiber (%) | 23.460 | 21.499 | 21.428 | 15.809 | 15.953 | 15.710 |
| Arginine (%) | 0.812 | 0.832 | 0.809 | 1.094 | 1.108 | 1.047 |
| Digestible Arginine (%) | 0.672 | 0.740 | 0.718 | 0.997 | 0.978 | 0.494 |
| Lysine (%) | 0.650 | 0.521 | 0.719 | 1.003 | 0.973 | 1.003 |
| Digestible Lysine (%) | 0.508 | 0.400 | 0.600 | 0.870 | 0.811 | 0.879 |
| Methionine (%) | 0.215 | 0.210 | 0.293 | 0.267 | 0.284 | 0.251 |
| Digestive methionine (%) | 0.176 | 0.179 | 0.261 | 0.235 | 0.243 | 0.221 |
| Methionine + cystein (%) | 0.479 | 0.472 | 0.579 | 0.589 | 0.587 | 0.569 |
| Methionine + cysteine Digestive (%) | 0.358 | 0.392 | 0.495 | 0.509 | 0.463 | 0.494 |
| Calcium (%) | 0.900 | 0.901 | 0.910 | 1.000 | 1.000 | 1.000 |
| Phosphorus, Total (%) | 0.616 | 0.567 | 0.638 | 0.584 | 0.613 | 0.575 |
| Phosphorus, Digestive (%) | 0.230 | 0.270 | 0.310 | 0.320 | 0.340 | 0.320 |
| Electrolityc balance meq/Kg 3 | 200.000 | |||||
| Age Group | Cycle | Group C | Group T | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min. | Max. | Median [IQR] | n | Min. | Max. | Median [IQR] | |||
| Gilts | ||||||||||
| 0 | 17 | 0.0 | 0.4 | 0.100 [0.1317] | 21 | 0.0 | 0.1 | 0.00 [0.000] | 0.016 | |
| Multiparous sows | ||||||||||
| 1 | 5 | 0.0 | 0.3 | 0.100 [0.2] | ||||||
| 2 | 15 | 0.0 | 0.2 | 0.000 [0.1] | 9 | 0.0 | 0.0 | 0.000 [0.0] | 0.108 | |
| 3 | 17 | 0.0 | 0.2 | 0.100 [0.1] | 11 | 0.0 | 0.0 | 0.000 [0.0] | 0.019 | |
| 4 | 12 | 0.0 | 0.1 | 0.050 [0.1] | 14 | 0.0 | 0.1 | 0.000 [0.0] | 0.131 | |
| 5 | 10 | 0.0 | 0.1 | 0.000 [0.1] | 22 | 0.0 | 0.0 | 0.000 [0.0] | 0.077 | |
| ≥6 | 19 | 0.0 | 0.3 | 0.000 [0.1] | 19 | 0.0 | 0.0 | 0.000 [0.0] | 0.025 | |
| Global | 73 | 0.0 | 0.3 | 0.000 [0.1] | 75 | 0.0 | 0.1 | 0.000 [0.0] | <0.001 | |
| Age Group | Variable 1 | Variable 2 | ||
|---|---|---|---|---|
| LMD | BFT | Litter Size | ||
| rs | r; rs | r; rs | ||
| p Value | p Value | p Value | ||
| n | n | n | ||
| Gilts | BHBA | −0.451 | −0.529 | −0.074 |
| 0.046 | 0.016 | 0.820 | ||
| 20 | 20 | 12 | ||
| LMD | 0.116 | 0.257 | ||
| 0.625 | 0.473 | |||
| 20 | 10 | |||
| BFT | 0.030 | |||
| 0.935 | ||||
| 10 | ||||
| Multiparous sows | ||||
| BHBA | −0.583 | −0.345 | 0.004 | |
| <0.001 | 0.002 | 0.972 | ||
| 79 | 79 | 84 | ||
| LMD | 0.330 | −0.126 | ||
| 0.003 | 0.297 | |||
| 79 | 70 | |||
| BFT | −0.026 | |||
| 0.839 | ||||
| 70 | ||||
| Age Group | Variable | Group C | Group T | p-Value (Group) | ||
|---|---|---|---|---|---|---|
| n | Mean ± SD; LS ± SE | n | Mean ± SD; LS ± SE | |||
| Gilts | ||||||
| Piglets born alive/litter | 6 | 17.83 ± 1.941 | 6 | 16.33 ± 4.274 | 0.452 | |
| % Stillborn piglets/litter | 6 | 8.28 ± 7.964 | 6 | 9.99 ± 6.529 | 0.693 | |
| Multiparous sows | ||||||
| Piglets born alive/litter | 43 | 16.09 ± 0.543 | 41 | 17.39 ± 0.607 | 0.892 | |
| % Stillborn piglets/litter | 43 | 9.24 ± 0.962 | 41 | 4.54 ± 1.076 | 0.039 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantin, J.; Cantin, C.; Mitjana, O.; Tejedor, M.T.; Gil-Rubio, C.; Garrido, A.M.; Falceto, M.V. Optimizing Sow and Litter Performance via a Comprehensive Service-to-Weaning Feeding Regimen. Animals 2025, 15, 2821. https://doi.org/10.3390/ani15192821
Cantin J, Cantin C, Mitjana O, Tejedor MT, Gil-Rubio C, Garrido AM, Falceto MV. Optimizing Sow and Litter Performance via a Comprehensive Service-to-Weaning Feeding Regimen. Animals. 2025; 15(19):2821. https://doi.org/10.3390/ani15192821
Chicago/Turabian StyleCantin, Julia, Carlos Cantin, Olga Mitjana, Maria Teresa Tejedor, Carlos Gil-Rubio, Ana Maria Garrido, and Maria Victoria Falceto. 2025. "Optimizing Sow and Litter Performance via a Comprehensive Service-to-Weaning Feeding Regimen" Animals 15, no. 19: 2821. https://doi.org/10.3390/ani15192821
APA StyleCantin, J., Cantin, C., Mitjana, O., Tejedor, M. T., Gil-Rubio, C., Garrido, A. M., & Falceto, M. V. (2025). Optimizing Sow and Litter Performance via a Comprehensive Service-to-Weaning Feeding Regimen. Animals, 15(19), 2821. https://doi.org/10.3390/ani15192821







