The Role of Nutrition Across Production Stages to Improve Sow Longevity
Simple Summary
Abstract
1. Introduction
2. Gilt Rearing Period
2.1. Control of Growth Rate
2.2. Gilts’ Mating Weight
2.3. The Role of Micro- and Macrominerals and Vitamins in Limb Health
2.4. Particle Size of Diets
3. Gestation Period
3.1. Body Condition Score
3.2. Feeding Frequency
3.3. Feed Management in Group Housing
3.4. Probiotics Supplementation
3.5. Acidifiers
3.6. Crude Fiber and Sows’ Health
3.7. Bump Feeding
4. Peripartum—Transition Diet
4.1. Negative Dietary Cation–Anion Difference (DCAD) Diets and Calcidiol Supplementation
4.2. Antioxidants
4.3. Crude Protein and Fat, and Their Impact on Heat Stress
4.4. Methyl Donors and Sows’ Health
5. Farrowing and Lactation
5.1. Farrowing
5.2. Lactation and PPDS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Bazer, F.W. Application of New Biotechnologies for Improvements in Swine Nutrition and Pork Production. J. Anim. Sci. Biotechnol. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Kikuti, M.; Preis, G.M.; Deen, J.; Pinilla, J.C.; Corzo, C.A. Sow Mortality in a Pig Production System in the Midwestern USA: Reasons for Removal and Factors Associated with Increased Mortality. Vet. Rec. 2023, 192. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.C.; Moura, C.A.; Thomas, P.; Haberl, B.; Greiner, L.; Rademacher, C.J.; Silva, A.P.S.P.; Trevisan, G.; Linhares, D.C.L.; Silva, G.S. Risk Factors Associated with Sow Mortality in Breeding Herds under One Production System in the Midwestern United States. Prevenvtive Vet. Med. 2023, 213, 105883. [Google Scholar] [CrossRef]
- Koketsu, Y.; Iida, R. Farm Data Analysis for Lifetime Performance Components of Sows and Their Predictors in Breeding Herds. Porc. Health Manag. 2020, 6, 24. [Google Scholar] [CrossRef]
- Belkova, J.; Rozkot, M. Gilt Rearing Impacts on Sow Performance and Longevity–A Review. J. Swine Health Prod. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Engblom, L.; Calderón Díaz, J.A.; Nikkilä, M.; Gray, K.; Harms, P.; Fix, J.; Tsuruta, S.; Mabry, J.; Stalder, K. Genetic Analysis of Sow Longevity and Sow Lifetime Reproductive Traits Using Censored Data. J. Anim. Breed. Genet. 2016, 133, 138–144. [Google Scholar] [CrossRef]
- Małopolska, M.M.; Tuz, R.; Lambert, B.D.; Nowicki, J.; Schwarz, T. The replacement gilt: Current strategies for improvement of the breeding herd. J. Swine Health Prod. 2018, 26, 208–214. [Google Scholar] [CrossRef]
- Knauer, M.T.; Cassady, J.P.; Newcom, D.W.; See, M.T. Phenotypic and Genetic Correlations between Gilt Estrus, Puberty, Growth, Composition, and Structural Conformation Traits with First-Litter Reproductive Measures. J. Anim. Sci. 2011, 89, 935–942. [Google Scholar] [CrossRef]
- Lavery, A.; Lawlor, P.G.; Magowan, E.; Miller, H.M.; O’Driscoll, K.; Berry, D.P. An Association Analysis of Sow Parity, Live-Weight and Back-Fat Depth as Indicators of Sow Productivity. Animal 2019, 13, 622–630. [Google Scholar] [CrossRef]
- Moeller, G.A.; Stalder, K.J. Sow Longevity. In Advances in Pig Welfare; Elsevier: Amsterdam, The Netherlands, 2024; pp. 163–184. [Google Scholar]
- Faccin, J.E.G.; Tokach, M.D.; Goodband, R.D.; DeRouchey, J.M.; Woodworth, J.C.; Gebhardt, J.T. Gilt Development to Improve Offspring Performance and Survivability. J. Anim. Sci. 2022, 100, skac128. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Matias, D.N.; Poor, A.P.; Dutra, M.C.; Moreno, L.Z.; Parra, B.M.; Silva, A.P.S.; Matajira, C.E.C.; de Moura Gomes, V.T.; Barbosa, M.R.F.; et al. Causes of Sow Mortality and Risks to Post-Mortem Findings in a Brazilian Intensive Swine Production System. Animals 2022, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.H.R.F.; Silva, B.A.N.; Donzele, J.L.; Oliveira, R.F.M.; Knol, E.F. Effects of Sow Nutrition during Gestation on Within-Litter Birth Weight Variation: A Review. Animal 2012, 6, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, A.K.; Nyachoti, C.M. Nutritional and Metabolic Consequences of Feeding High-Fiber Diets to Swine: A Review. Engineering 2017, 3, 716–725. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal Piglet Survival: Impact of Sow Nutrition around Parturition on Fetal Glycogen Deposition and Production and Composition of Colostrum and Transient Milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Mallmann, A.L.; Arend, L.S.; Oliveira, G.S.; Mellagi, A.P.G.; Ulguim, R.R.; Bernardi, M.L.; Bortolozzo, F.P.; Knox, R. V Effects of Flush Feeding Strategy before Breeding on Reproductive Performance of Modern Replacement Gilts: Impacts on Ovulation Rate and Litter Traits. J. Anim. Sci. 2020, 98, skaa186. [Google Scholar] [CrossRef]
- Kim, S.W. Recent Advances in Sow Nutrition. Rev. Bras. Zootec. 2010, 39, 303–310. [Google Scholar] [CrossRef]
- Monteiro, M.; Poor, A.; Muro, B.; Carnevale, R.; Leal, D.; Garbossa, C.; Moreno, A.; Almond, G. The Sow Microbiome: Current and Future Perspectives to Maximize the Productivity in Swine Herds. J. Swine Health Prod. 2022, 30, 238–250. [Google Scholar] [CrossRef]
- National Research Council (NRC). Division on Earth, Life Studies, & Committee on Nutrient Requirements of Swine. In Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Zhu, L.; Marjani, S.L.; Jiang, Z. The Epigenetics of Gametes and Early Embryos and Potential Long-Range Consequences in Livestock Species—Filling in the Picture with Epigenomic Analyses. Front. Genet. 2021, 12, 557934. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Wu, D.; Guo, H.-Y.; Zheng, A.-R.; Zhang, G. The Level of Feed Intake Affects Embryo Survival and Gene Expression During Early Pregnancy in Gilts. Reprod. Domest. Anim. 2009, 45, 685–693. [Google Scholar] [CrossRef]
- Heerwagen, M.J.R.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal Obesity and Fetal Metabolic Programming: A Fertile Epigenetic Soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R711–R722. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Arentson-Lantz, E.J.; Donkin, S.S. Excessive Gestational Calorie Intake in Sows Regulates Early Postnatal Adipose Tissue Development in the Offspring. BMC Nutr. 2016, 2, 29. [Google Scholar] [CrossRef]
- Muro, B.B.; Carnevale, R.F.; Leal, D.F.; Almond, G.W.; Monteiro, M.S.; Poor, A.P.; Schinckel, A.P.; Garbossa, C.A. The Importance of Optimal Body Condition to Maximise Reproductive Health and Perinatal Outcomes in Pigs. Nutr. Res. Rev. 2022, 36, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Foxcroft, G. Gilt Management for Fertility and Longevity. Animals 2019, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Bartol, F.F.; Wiley, A.A.; George, A.F.; Miller, D.J.; Bagnell, C.A. Physiology and endocrinology symposium: Postnatal Reproductive Development and the Lactocrine Hypothesis12. J. Anim. Sci. 2017, 95, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.R.C.L.; Laurenssen, B.; Pereira, L.X.; Teerds, K.J.; Soede, N.M. Effects of Birthweight on Reproductive System Development and Onset of Puberty in Gilts. Reprod. Fertil. Dev. 2017, 29, 254. [Google Scholar] [CrossRef]
- Magnabosco, D.; Bernardi, M.L.; Wentz, I.; Cunha, E.C.P.; Bortolozzo, F.P. Low Birth Weight Affects Lifetime Productive Performance and Longevity of Female Swine. Livest. Sci. 2016, 184, 119–125. [Google Scholar] [CrossRef]
- Boyle, L.; Björklund, L. Effects of Fattening Boars in Mixed or Single Sex Groups and Split Marketing on Pig Welfare. Anim. Welf. 2007, 16, 259–262. [Google Scholar] [CrossRef]
- Dourmad, J.Y.; Etienne, M.; Noblet, J. Reconstitution of Body Reserves in Multiparous Sows during Pregnancy: Effect of Energy Intake during Pregnancy and Mobilization during the Previous Lactation. J. Anim. Sci. 1996, 74, 2211. [Google Scholar] [CrossRef]
- Theil, P.K.; Krogh, U.; Bruun, T.S.; Feyera, T. Feeding the Modern Sow to Sustain High Productivity. Mol. Reprod. Dev. 2023, 90, 517–532. [Google Scholar] [CrossRef]
- Klaaborg, J.; Carl, T.N.; Bruun, T.S.; Strathe, A.V.; Bache, J.K.; Kristensen, A.R.; Amdi, C. The Effect of Feeding Strategy during Rearing in a Commercial Setting on Gilt Body Condition, Lactation Performance and Culling Rate in Modern Sows Nursing Large Litters. Livest. Sci. 2019, 228, 144–150. [Google Scholar] [CrossRef]
- Cia, M.C.; Edwards, S.A.; Glasgow, V.L.; Shanks, M.; Fraser, H. Modification of Body Composition by Altering the Dietary Lysine to Energy Ratio during Rearing and the Effect on Reproductive Performance of Gilts. Anim. Sci. 1998, 66, 457–463. [Google Scholar] [CrossRef]
- Gill, B.P. Body Composition of Breeding Gilts in Response to Dietary Protein and Energy Balance from Thirty Kilograms of Body Weight to Completion of First Parity1. J. Anim. Sci. 2006, 84, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Strathe, A.V.; Hales, J.; Brandt, P.; Bruun, T.S.; Amdi, C.; Hansen, C.F. Effects of Dietary Protein Level and Energy Intake from 50 to 120 kg on Body Weight, Back Fat Thickness and Body Composition in Gilts. Livest. Sci. 2019, 227, 11–16. [Google Scholar] [CrossRef]
- Farmer, C. Nutritional Impact on Mammary Development in Pigs: A Review. J. Anim. Sci. 2018, 96, 3748–3756. [Google Scholar] [CrossRef]
- Farmer, C.; Petitclerc, D.; Sorensen, M.T.; Vignola, M.; Dourmad, J.Y. Impacts of Dietary Protein Level and Feed Restriction during Prepuberty on Mammogenesis in Gilts12. J. Anim. Sci. 2004, 82, 2343–2351. [Google Scholar] [CrossRef]
- Sørensen, M.T.; Farmer, C.; Vestergaard, M.; Purup, S.; Sejrsen, K. Mammary Development in Prepubertal Gilts Fed Restrictively or Ad Libitum in Two Sub-Periods between Weaning and Puberty. Livest. Sci. 2006, 99, 249–255. [Google Scholar] [CrossRef]
- Helm, E.T.; Patience, J.F.; Romoser, M.R.; Johnson, C.D.; Ross, J.W.; Gabler, N.K. Evaluation of Increased Fiber, Decreased Amino Acids, or Decreased Electrolyte Balance as Dietary Approaches to Slow Finishing Pig Growth Rates. J. Anim. Sci. 2021, 99, skab164. [Google Scholar] [CrossRef]
- Gregory, N.; Farmer, C.; Friendship, R.M.; Huber, L.-A. The Effect of Moderate Energy and Protein Restriction during Gilt Development on Changes in Body Weight and Backfat Depth and Subsequent Lactation Performance. J. Anim. Sci. 2023, 101, skac351. [Google Scholar] [CrossRef]
- Bruun, T.; Julie, K.; Strathe, A.V. Effects of Age, Weighting and Backfat Thickness at First Service on Litter Size in First Parity and Proportion of Sows Serviced for Second Parity in Danish Sows. In Proceedings of the 11th ICPR, Ghent, Belgium, 5–8 June 2022. [Google Scholar]
- Filha, W.S.A.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Reproductive Performance of Gilts According to Growth Rate and Backfat Thickness at Mating. Anim. Reprod. Sci. 2010, 121, 139–144. [Google Scholar] [CrossRef]
- Bruun, T.S.; Strathe, A.V.; Krogsdahl, J. Feeding of Growing Gilts—Part 3: Effects on Litter Size and Proportion of Sows Remated for Their Second Parity. In Danish: Fodring Af Polte i Opvækstperioden—Del 3: Effekter På Kuldstørrelse Og Andel Af Søer Der Løbes i Andet Kuld. SEGES Svineproduktion Den Rullende Afprøvning 2022, 1206. Available online: https://svineproduktion.dk/publikationer/kilder/lu_medd/2020/1206 (accessed on 7 December 2024).
- Bortolozzo, F.P.; Bernardi, M.L.; Kummer, R.; Wentz, I. Growth, Body State and Breeding Performance in Gilts and Primiparous Sows. Soc Reprod Fertil Suppl 2009, 66, 281–291. [Google Scholar] [CrossRef]
- Williams, N.H.; Patterson, J.; Foxcroft, G. Non-Negotiables in Gilt Development. Adv. Pork Prod. 2005, 16, 281. [Google Scholar]
- Mahan, D.C. Mineral Nutrition of the Sow: A Review. J. Anim. Sci. 1990, 68, 573. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.; Oravainen, J.; Orro, T.; Seppä-Lassila, L.; Ala-Kurikka, E.; Virolainen, J.; Tast, A.; Peltoniemi, O.A.T. Lameness and Fertility of Sows and Gilts in Randomly Selected Loose-Housed Herds in Finland. Vet. Rec. 2006, 159, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.E.; Wachmann, H. Osteochondrosis of the Elbow Joint in Finishing Pigs from Three Herds: Associations among Different Types of Joint Changes and between Osteochondrosis and Growth Rate. Vet J. 2011, 188, 197–203. [Google Scholar] [CrossRef]
- Kraeling, R.R.; Webel, S.K. Current Strategies for Reproductive Management of Gilts and Sows in North America. J. Anim. Sci. Biotechnol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Dewey, C.E.; Friendship, R.M.; Wilson, M.R. Clinical and Postmortem Examination of Sows Culled for Lameness. Can. Vet. J. 1993, 34, 555–556. [Google Scholar]
- Pluym, L.M.; Van Nuffel, A.; Van Weyenberg, S.; Maes, D. Prevalence of Lameness and Claw Lesions during Different Stages in Thereproductive Cycle of Sows and the Impact on Reproduction Results. Animal 2013, 7, 1174–1181. [Google Scholar] [CrossRef]
- Kirk, R.K.; Svensmark, B.; Ellegaard, L.P.; Jensen, H.E. Locomotive Disorders Associated with Sow Mortality in Danish Pig Herds. J. Vet. Med. Ser. A 2005, 52, 423–428. [Google Scholar] [CrossRef]
- Schwertz, C.I.; Bianchi, R.M.; Cecco, B.S.; Pavarini, S.P.; Driemeier, D. Causes of Death of Sows in Three Brazilian Pig Farms. Pesqui. Veterinária Bras. 2021, 41, e06857. [Google Scholar] [CrossRef]
- Vier, C.M.; Dritz, S.S.; Tokach, M.D.; DeRouchey, J.M.; Goodband, R.D.; Gonçalves, M.A.D.; Orlando, U.A.D.; Bergstrom, J.R.; Woodworth, J.C. Calcium to Phosphorus Ratio Requirement of 26- to 127-Kg Pigs Fed Diets with or without Phytase. J. Anim. Sci. 2019, 97, 4041–4052. [Google Scholar] [CrossRef]
- Frantz, N.Z.; Andrews, G.A.; Tokach, M.D.; Nelssen, J.L.; Goodband, R.D.; DeRouchey, J.M.; Dritz, S.S. Effect of Dietary Nutrients on Osteochondrosis Lesions and Cartilage Properties in Pigs. Am. J. Vet. Res. 2008, 69, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.S.; Anil, L.; Deen, J. Effect of Lameness on Sow Longevity. J. Am. Vet. Med. Assoc. 2009, 235, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Ferket, P.R.; Oviedo-Rondón, E.O.; Mente, P.L.; Bohórquez, D.V.; Santos, A.A.; Grimes, J.L.; Richards, J.D.; Dibner, J.J.; Felts, V. Organic Trace Minerals and 25-Hydroxycholecalciferol Affect Performance Characteristics, Leg Abnormalities, and Biomechanical Properties of Leg Bones of Turkeys. Poult. Sci. 2009, 88, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Lisgara, Μ.; Skampardonis, V.; Leontides, L. Effect of Diet Supplementation with Chelated Zinc, Copper and Manganese on Hoof Lesions of Loose Housed Sows. Porc. Health Manag. 2016, 2, 6. [Google Scholar] [CrossRef]
- van Riet, M.M.J.; Millet, S.; Aluwé, M.; Janssens, G.P.J. Impact of Nutrition on Lameness and Claw Health in Sows. Livest. Sci. 2013, 156, 24–35. [Google Scholar] [CrossRef]
- Hostetler, C.E.; Kincaid, R.L.; Mirando, M.A. The Role of Essential Trace Elements in Embryonic and Fetal Development in Livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef]
- Richards, J.D.; Zhao, J.; Harrell, R.J.; Atwell, C.A.; Dibner, J.J. Trace Mineral Nutrition in Poultry and Swine. Asian-Australas J. Anim. Sci. 2010, 23, 1527–1534. [Google Scholar] [CrossRef]
- Fabà, L.; Gasa, J.; Tokach, M.D.; Varella, E.; Solà-Oriol, D. Effects of Supplementing Organic Microminerals and Methionine during the Rearing Phase of Replacement Gilts on Lameness, Growth, and Body Composition1. J. Anim. Sci. 2018, 96, 3274–3287. [Google Scholar] [CrossRef]
- Cerutti, G.A.; Kramer, T.; Ramella, K.D.C.L.; Ramos, A.S. de Uso de Microminerais Complexados Na Redução de Lesões de Casco Em Reprodutoras Suínas/Use of Complexed Trace Minerals to Improve Claw Lesions in Swine Sows. Braz. J. Dev. 2021, 7, 101064–101076. [Google Scholar] [CrossRef]
- Silva, G.A.; Fernandez, F.G.; Backes, A.P.; Donin, D.G.; Fernandes, S.R.; Fireman, A.K.; Kramer, T.; Alberton, G.C. Effect of the Organic Minerals Zinc, Manganese, and Copper on Growth Performance and the Locomotor System of Finishing Pigs. Semin. Cienc. Agrar. 2019, 40, 3209. [Google Scholar] [CrossRef]
- Souza, T.C.G.D.; Pierozan, E.A. Minerais Complexados Na Prevenção e Tratamento de Problemas Locomotores Em Porcas. Rev. Acadêmica Ciência Anim. 2015, 13, 159–165. [Google Scholar] [CrossRef]
- Mülling, C.K.W.; Bragulla, H.H.; Reese, S.; Budras, K.-D.; Steinberg, W. How Structures in Bovine Hoof Epidermis Are Influenced by Nutritional Factors. Anat. Histol. Embryol. 1999, 28, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Rabiee, A.R. Effect of Feeding Biotin on Milk Production and Hoof Health in Lactating Dairy Cows: A Quantitative Assessment. J. Dairy Sci. 2011, 94, 1465–1476. [Google Scholar] [CrossRef]
- Misir, R.; Blair, R. Effect of Biotin Supplementation of a Barley-Wheat Diet on Restoration of Healthy Feet, Legs and Skin of Biotin Deficient Sows. Res. Vet. Sci. 1986, 40, 212–218. [Google Scholar] [CrossRef]
- Simmins, P.; Brooks, P. Supplementary Biotin for Sows: Effect on Claw Integrity. Vet. Rec. 1988, 122, 431–435. [Google Scholar] [CrossRef]
- Penny, R.; Cameron, R.; Johnson, S.; Kenyon, P.; Smith, H.; Bell, A.; Cole, J.; Taylor, J. Foot Rot of Pigs: The Influence of Biotin Supplementation on Foot Lesions in Sows. Vet. Rec. 1980, 107, 350–351. [Google Scholar] [CrossRef]
- Bruun, T.S.; Jensen, S.K.; Larsen, T.; Nielsen, M.B.F.; Roger, L.; Feyera, T. Effect of Dietary Calcium and Vitamin D Supplements on Plasma Bone Turnover Biomarkers, Bone Mineralization, Bone Strength, and Lameness Score in Gilts. J. Anim. Sci. 2024, 102, skae310. [Google Scholar] [CrossRef]
- Lauridsen, C.; Halekoh, U.; Larsen, T.; Jensen, S.K. Reproductive Performance and Bone Status Markers of Gilts and Lactating Sows Supplemented with Two Different Forms of Vitamin D1. J. Anim. Sci. 2010, 88, 202–213. [Google Scholar] [CrossRef]
- Lütke-Dörhoff, M.; Schulz, J.; Westendarp, H.; Visscher, C.; Wilkens, M.R. Dietary Supplementation of 25-hydroxycholecalciferol as an Alternative to Cholecalciferol</b> in Swine Diets: A Review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1288–1305. [Google Scholar] [CrossRef]
- Jakobsen, S.S.; Jakobsen, J.; Nielsen, J.P. Vitamin D Levels in Sows from Five Danish Outdoor Herds. Animals 2022, 12, 299. [Google Scholar] [CrossRef]
- Cybulski, P.; Woźniak, A.; Larska, M.; Jabłoński, A.; Stadejek, T. Gastric Ulcers in Finishing Pigs: The Evaluation of Selected Non-Dietary Risk Factors and Impact on Production Performance. Porc. Health Manag. 2024, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Vearick, G.; Mellagi, A.P.G.; Bortolozzo, F.P.; Wentz, I.; Bernarndi, M.L. Causas associadas à morte de matrizes suínas. Arch. Vet. Sci. 2008, 13, 126–132. [Google Scholar] [CrossRef][Green Version]
- Ala-Kurikka, E.; Munsterhjelm, C.; Bergman, P.; Laine, T.; Pekkarinen, H.; Peltoniemi, O.; Valros, A.; Heinonen, M. Pathological Findings in Spontaneously Dead and Euthanized Sows—A Descriptive Study. Porc. Health Manag. 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Peralvo-Vidal, J.M.; Weber, N.R.; Nielsen, J.P.; Bache, J.K.; Haugegaard, S.; Pedersen, A.Ø. Risk Factors for Gastric Ulceration in Nursery Pigs. Prev. Vet. Med. 2021, 189, 105298. [Google Scholar] [CrossRef] [PubMed]
- Ayles, H.L.; Friendship, R.M.; Bubenik, G.A.; Ball, R.O. Effect of Feed Particle Size and Dietary Melatonin Supplementation on Gastric Ulcers in Swine. Can. J. Anim. Sci. 1999, 79, 179–185. [Google Scholar] [CrossRef]
- Wondra, K.J.; Hancock, J.D.; Behnke, K.C.; Hines, R.H.; Stark, C.R. Effects of Particle Size and Pelleting on Growth Performance, Nutrient Digestibility, and Stomach Morphology in Finishing Pigs. J. Anim. Sci. 1995, 73, 757–763. [Google Scholar] [CrossRef]
- Cybulski, P.; Larska, M.; Woźniak, A.; Jabłoński, A.; Stadejek, T. The Dietary Risk Factors of Gastric Ulcers in Finishing Pigs from 16 Polish Farms. Agriculture 2021, 11, 719. [Google Scholar] [CrossRef]
- Rojas, O.J.; Stein, H.H. Processing of Ingredients and Diets and Effects on Nutritional Value for Pigs. J. Anim. Sci. Biotechnol. 2017, 8, 48. [Google Scholar] [CrossRef]
- Dirkzwager, A.; Elbers, A.R.W.; van der Aar, P.J.; Vos, J.H. Effect of Particle Size and Addition of Sunflower Hulls to Diets on the Occurrence of Oesophagogastric Lesions and Performance in Growing-Finishing Pigs. Livest. Prod. Sci. 1998, 56, 53–60. [Google Scholar] [CrossRef]
- Dourmad, J.-Y.; Étienne, M.; Valancogne, A.; Dubois, S.; van Milgen, J.; Noblet, J. InraPorc: A Model and Decision Support Tool for the Nutrition of Sows. Anim. Feed Sci. Technol. 2008, 143, 372–386. [Google Scholar] [CrossRef]
- Strathe, A.V.; Strathe, A.B.; Theil, P.K.; Hansen, C.F.; Kebreab, E. Determination of Protein and Amino Acid Requirements of Lactating Sows Using a Population-Based Factorial Approach. Animal 2015, 9, 1319–1328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millet, S.; Kumar, S.; De Boever, J.; Meyns, T.; Aluwé, M.; De Brabander, D.; Ducatelle, R. Effect of Particle Size Distribution and Dietary Crude Fibre Content on Growth Performance and Gastric Mucosa Integrity of Growing–Finishing Pigs. Vet. J. 2012, 192, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Biksi, I.; Takács, N.; Vetési, F. Association between Endometritis and Urocystitis in Culled Sows. Acta Vet. Hung. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Bench, C.J.; Rioja-Lang, F.C.; Hayne, S.M.; Gonyou, H.W. Group Gestation Housing with Individual Feeding—I: How Feeding Regime, Resource Allocation, and Genetic Factors Affect Sow Welfare. Livest. Sci. 2013, 152, 208–217. [Google Scholar] [CrossRef]
- Maes, D.; Pluym, L.; Peltoniemi, O. Impact of Group Housing of Pregnant Sows on Health. Porc. Health Manag. 2016, 2, 17. [Google Scholar] [CrossRef]
- Jensen, T.B.; Toft, N.; Bonde, M.K.; Kongsted, A.G.; Kristensen, A.R.; Sørensen, J.T. Herd and Sow-Related Risk Factors for Mortality in Sows in Group-Housed Systems. Prev. Vet. Med. 2012, 103, 31–37. [Google Scholar] [CrossRef]
- Jin, S.S.; Jin, Y.H.; Jang, J.C.; Hong, J.S.; Jung, S.W.; Kim, Y.Y. Effects of Dietary Energy Levels on Physiological Parameters and Reproductive Performance of Gestating Sows over Three Consecutive Parities. J. Anim. Sci. 2018, 31, 410–420. [Google Scholar] [CrossRef]
- Nikkilä, M.T.; Stalder, K.J.; Mote, B.E.; Rothschild, M.F.; Gunsett, F.C.; Johnson, A.K.; Karriker, L.A.; Boggess, M.V.; Serenius, T.V. Genetic Associations for Gilt Growth, Compositional, and Structural Soundness Traits with Sow Longevity and Lifetime Reproductive Performance1. J. Anim. Sci. 2013, 91, 1570–1579. [Google Scholar] [CrossRef]
- Drolet, R.; D’Allaire, S.; Chagnon, M. Some Observations on Cardiac Failure in Sows. Can. Vet. J. 1992, 33, 325. [Google Scholar]
- Solà-Oriol, D.; Gasa, J. Feeding Strategies in Pig Production: Sows and Their Piglets. Anim. Feed Sci. Technol. 2017, 233, 34–52. [Google Scholar] [CrossRef]
- Leal, D.F.; Muro, B.B.D.; Nichi, M.; Almond, G.W.; Viana, C.H.C.; Vioti, G.; Carnevale, R.F.; Garbossa, C.A.P. Effects of Post-Insemination Energy Content of Feed on Embryonic Survival in Pigs: A Systematic Review. Anim. Reprod. Sci. 2019, 205, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Athorn, R.Z.; Stott, P.; Bouwman, E.G.; Chen, T.Y.; Kennaway, D.J.; Langendijk, P. Effect of Feeding Level on Luteal Function and Progesterone Concentration in the Vena Cava during Early Pregnancy in Gilts. Reprod. Fertil. Dev. 2013, 25, 531. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.C.; Sørensen, M.T.; Feyera, T.; Pelck, J.S.; Bruun, T.S. Effect of Dietary Protein for Gestating Sows on Re-Establishment of Body Reserves and Impact on Reproductive Performance. Livest. Sci. 2024, 286, 105521. [Google Scholar] [CrossRef]
- Johannsen, J.C.; Sørensen, M.T.; Theil, P.K.; Bruun, T.S.; Farmer, C.; Feyera, T. Optimal Protein Concentration in Diets for Sows during the Transition Period. J. Anim. Sci. 2024, 102, skae082. [Google Scholar] [CrossRef]
- Soede, N.M.; Kemp, B. Best Practices in the Lactating and Weaned Sow to Optimize Reproductive Physiology and Performance. In The Gestating and Lactating Sow; Brill Wageningen Academic: Wageningen, The Netherlands, 2015; pp. 377–407. [Google Scholar]
- Wang, Z.; Liu, B.S.; Wang, X.Y.; Wei, Q.H.; Tian, H.; Wang, L.Q. Effects of Altrenogest on Reproductive Performance of Gilts and Sows: A Meta-Analysis. Anim. Reprod. Sci. 2018, 197, 10–21. [Google Scholar] [CrossRef]
- Corezzolla, J.L.; Ulguim, R. da R.; Gasperin, B.G.; Rauber, L.P.; Bianchi, I. Altrenogest Treatment Effects on the Reproductive Performance of Sows during Transition to Batch Farrowing. Ciência Rural 2020, 50, e20190806. [Google Scholar] [CrossRef]
- Araújo, V. de O.; de Oliveira, R.A.; Vieira, M. de F.A.; Silveira, H.; Fonseca, L. da S.; Alves, L.K.S.; Guimarães, E.B.B.; Schinckel, A.P.; Garbossa, C.A.P. Bump Feed for Gestating Sows Is Really Necessary? Livest. Sci. 2020, 240, 104184. [Google Scholar] [CrossRef]
- Rutherford, K.M.D.; Thompson, C.S.; Thomson, J.R.; Lawrence, A.B.; Nielsen, E.O.; Busch, M.E.; Haugegaard, S.; Sandøe, P. A Study of Associations between Gastric Ulcers and the Behaviour of Finisher Pigs. Livest. Sci. 2018, 212, 45–51. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, H.; Xu, J.; Su, Y.; Zhu, W. Feeding Frequency Affects the Growth Performance, Nutrient Digestion and Absorption of Growing Pigs with the Same Daily Feed Intake. Livest. Sci. 2021, 250, 104558. [Google Scholar] [CrossRef]
- Manu, H.; Lee, S.; Keyes, M.C.; Cairns, J.; Baidoo, S.K. Behavioral and Stress Responses to Feeding Time in Pregnant Sows under Limit-Fed Regime. J. Anim. Sci. 2021, 99, skab108. [Google Scholar] [CrossRef]
- Reynolds, C.B.; Elias, A.N.; Whisnant, C.S. Effects of Feeding Pattern on Ghrelin and Insulin Secretion in Pigs. Domest. Anim. Endocrinoly 2010, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Holenweger, F.; Schüpbach, G.; Hofer, A.; Sidler, X.; Grahofer, A. Housing and Management Factors and Breed Predisposition for Haemorrhagic Bowel Syndrome in Swine. Porc. Health Manag. 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Melnichouk, S.I. Mortality Associated with Gastric Ulceration in Swine. Can. Vet. J. 2002, 43, 223–225. [Google Scholar] [PubMed]
- Carnevale, R.F.; Muro, B.B.; Pierozan, C.R.; Monteiro, M.S.; Leal, D.F.; Poor, A.P.; Alves, L.K.; Gomes, N.A.; Silva, C.A.; Maes, D.; et al. Peripheral Glycemia and Farrowing Traits in Pigs: An Observational Study. Livest. Sci. 2023, 270, 105203. [Google Scholar] [CrossRef]
- Björkman, S.; Oliviero, C.; Kauffold, J.; Soede, N.M.; Peltoniemi, O.A.T. Prolonged Parturition and Impaired Placenta Expulsion Increase the Risk of Postpartum Metritis and Delay Uterine Involution in Sows. Theriogenology 2018, 106, 87–92. [Google Scholar] [CrossRef]
- Olsson, A.-C.; Andersson, M.; Botermans, J.; Rantzer, D.; Svendsen, J. Animal Interaction and Response to Electronic Sow Feeding (ESF) in 3 Different Herds and Effects of Function Settings to Increase Capacity. Livest. Sci. 2011, 137, 268–272. [Google Scholar] [CrossRef]
- Jang, J.-C.; Oh, S.-H. Management Factors Affecting Gestating Sows’ Welfare in Group Housing Systems—A Review. Anim. Biosci. 2022, 35, 1817–1826. [Google Scholar] [CrossRef]
- Brouns, F.; Edwards, S.A. Social Rank and Feeding Behaviour of Group-Housed Sows Fed Competitively or Ad Libitum. Appl. Anim. Behav. Sci. 1994, 39, 225–235. [Google Scholar] [CrossRef]
- Vargovic, L.; Hermesch, S.; Athorn, R.Z.; Bunter, K.L. Feed Intake and Feeding Behavior Traits for Gestating Sows Recorded Using Electronic Sow Feeders. J. Anim. Sci. 2021, 99, skaa395. [Google Scholar] [CrossRef]
- Danielsen, V.; Vestergaard, E.-M. Dietary Fibre for Pregnant Sows: Effect on Performance and Behaviour. Anim. Feed Sci. Technol. 2001, 90, 71–80. [Google Scholar] [CrossRef]
- Meunier-Salaün, M.C.; Edwards, S.A.; Robert, S. Effect of Dietary Fibre on the Behaviour and Health of the Restricted Fed Sow. Anim. Feed Sci. Technol. 2001, 90, 53–69. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Modification of Brain Serotonin by the Diet. Annu. Rev. Med. 1974, 25, 7089. [Google Scholar] [CrossRef] [PubMed]
- Sève, B. Physiological Roles of Tryptophan in Pig Nutrition. Adv. Exp. Med. Biol. 1999, 467, 729–741. [Google Scholar] [PubMed]
- Nelson, R.J.; Chiavegatto, S. Molecular Basis of Aggression. Trends Neurosci. 2001, 24, 713–719. [Google Scholar] [CrossRef]
- Linder, A.E.; Ni, W.; Diaz, J.L.; Szasz, T.; Burnett, R.; Watts, S.W. Serotonin (5-HT) in Veins: Not All in Vain. J. Pharmacol. Exp. Ther. 2007, 323, 415–421. [Google Scholar] [CrossRef]
- Poletto, R.; Meisel, R.L.; Richert, B.T.; Cheng, H.-W.; Marchant, J.N. Aggression in Replacement Grower and Finisher Gilts Fed a Short-Term High-Tryptophan Diet and the Effect of Long-Term Human–Animal Interaction. Appl. Anim. Behav. Sci. 2010, 122, 98–110. [Google Scholar] [CrossRef]
- Poletto, R.; Kretzer, F.C.; Hötzel, M.J. Minimizing Aggression during Mixing of Gestating Sows with Supplementation of a Tryptophan-Enriched Diet. Physiol. Behav. 2014, 132, 36–43. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic Syndrome During Perinatal Period in Sows and the Link With Gut Microbiota and Metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef]
- Ma, C.; Azad, A.K.; Tang, W.; Zhu, Q.; Wang, W.; Gao, Q.; Kong, X. Maternal Probiotics Supplementation Improves Immune and Antioxidant Function in Suckling Piglets via Modifying Gut Microbiota. J. Appl. Microbiol. 2022, 133, 515–528. [Google Scholar] [CrossRef]
- Björkman, S.; Kauffold, J.; Kaiser, M.Ø. Reproductive Health of the Sow during Puerperium. Mol. Reprod. Dev. 2023, 90, 561–579. [Google Scholar] [CrossRef]
- Tsuruta, T.; Inoue, R.; Tsushima, T.; Watanabe, T.; Tsukahara, T.; Ushida, K. Oral Administration of EC-12 Increases the Baseline Gene Expression of Antiviral Cytokine Genes, IFN-γ and TNF-α, in Splenocytes and Mesenteric Lymph Node Cells of Weaning Piglets. Biosci. Microbiota Food Health 2013, 32, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Guan, K.; Su, Q.; Wang, X.; Yan, Z.; Kuang, K.; Wang, Y.; Zhang, Q.; Zhou, X.; Liu, B. Change of Gut Microbiota in PRRSV-Resistant Pigs and PRRSV-Susceptible Pigs from Tongcheng Pigs and Large White Pigs Crossed Population upon PRRSV Infection. Animals 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Castillo, R.; Ding, X. Effect of Oral Administration with Lactobacillus Plantarum CAM6 Strain on Sows during Gestation-Lactation and the Derived Impact on Their Progeny Performance. Mediat. Inflamm. 2021, 2021, 6615960. [Google Scholar] [CrossRef] [PubMed]
- Menegat, M.B.; DeRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D. Effects of Bacillus Subtilis C-3102 on Sow and Progeny Performance, Fecal Consistency, and Fecal Microbes during Gestation, Lactation, and Nursery Periods. J. Anim. Sci. 2019, 97, 3920–3937. [Google Scholar] [CrossRef] [PubMed]
- Saladrigas-García, M.; Solà-Oriol, D.; López-Vergé, S.; D’Angelo, M.; Collado, M.C.; Nielsen, B.; Faldyna, M.; Pérez, J.F.; Martín-Orúe, S.M. Potential Effect of Two Bacillus Probiotic Strains on Performance and Fecal Microbiota of Breeding Sows and Their Piglets. J. Anim. Sci. 2022, 100, skac163. [Google Scholar] [CrossRef]
- Böhmer, B.M.; Kramer, W.; Roth-Maier, D.A. Dietary Probiotic Supplementation and Resulting Effects on Performance, Health Status, and Microbial Characteristics of Primiparous Sows. J. Anim. Physiol. Anim. Nutr. 2006, 90, 309–315. [Google Scholar] [CrossRef]
- Sampath, V.; Cho, S.; Jeong, J.; Mun, S.; Lee, C.H.; Hermes, R.G.; Taechavasonyoo, A.; Smeets, N.; Kirwan, S.; Han, K.; et al. Dietary Bacillus Spp. Supplementation to Both Sow and Progenies Improved Post-Weaning Growth Rate, Gut Function, and Reduce the pro-Inflammatory Cytokine Production in Weaners Challenged with Escherichia Coli K88. Anim. Microbiome 2024, 6, 3. [Google Scholar] [CrossRef]
- Konieczka, P.; Ferenc, K.; Jørgensen, J.N.; Hansen, L.H.B.; Zabielski, R.; Olszewski, J.; Gajewski, Z.; Mazur-Kuśnirek, M.; Szkopek, D.; Szyryńska, N.; et al. Feeding Bacillus-Based Probiotics to Gestating and Lactating Sows Is an Efficient Method for Improving Immunity, Gut Functional Status and Biofilm Formation by Probiotic Bacteria in Piglets at Weaning. Anim. Nutr. 2023, 13, 361–372. [Google Scholar] [CrossRef]
- Hu, J.; Kim, Y.H.; Kim, I.H. Effects of Two Bacillus Strains Probiotic Supplement on Reproduction Performance, Nutrient Digestibility, Blood Profile, Fecal Score, Excreta Odor Contents and Fecal Microflora in Lactation Sows, and Growth Performance in Sucking Piglets. Livest. Sci. 2021, 244, 104293. [Google Scholar] [CrossRef]
- Cao, M.; Li, Y.; Wu, Q.J.; Zhang, P.; Li, W.T.; Mao, Z.Y.; Wu, D.M.; Jiang, X.M.; Zhuo, Y.; Fang, Z.F.; et al. Effects of Dietary Clostridium Butyricum Addition to Sows in Late Gestation and Lactation on Reproductive Performance and Intestinal Microbiota. J. Anim. Sci. 2019, 97, 3426–3439. [Google Scholar] [CrossRef]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Z.; Kerr, K.A.; Moeser, A.J. Chronic Social Stress in Pigs Impairs Intestinal Barrier and Nutrient Transporter Function, and Alters Neuro-Immune Mediator and Receptor Expression. PLoS ONE 2017, 12, e0171617. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, D.V.; Liddle, R.A. The Gut Connectome: Making Sense of What You Eat. J. Clin. Investig. 2015, 125, 888–890. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Pereira, M.M.C.; Andretta, I.; Franceschi, C.H.; Kipper, M.; Mariani, A.; Stefanello, T.; Carvalho, C.; Vieira, J.; Moura Rocha, L.; Ribeiro, A.M.L. Effects of Multistrain Probiotic Supplementation on Sows’ Emotional and Cognitive States and Progeny Welfare. Animals 2024, 14, 847. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, Y.; Chen, W.; Li, Y.; Han, Y.; Zhang, B.; Pineda, L.; Li, X.; Jiang, X. Effect of an Organic Acid Blend as an Antibiotic Alternative on Growth Performance, Antioxidant Capacity, Intestinal Barrier Function, and Fecal Microbiota in Weaned Piglets. J. Anim. Sci. 2024, 102, skae149. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V.A. Review of the Effects of Dietary Organic Acids Fed to Swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Liu, C.; Mo, X.-J.; Chen, M.; Zhao, X.-L.; Liu, M.-Z.; Wang, S.-B.; Zhou, B.; Zhao, C.-X. Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants 2022, 11, 809. [Google Scholar] [CrossRef]
- Nhara, R.B.; Marume, U.; Nantapo, C.W.T. Potential of Organic Acids, Essential Oils and Their Blends in Pig Diets as Alternatives to Antibiotic Growth Promoters. Animals 2024, 14, 762. [Google Scholar] [CrossRef]
- Ferronato, G.; Prandini, A. Dietary Supplementation of Inorganic, Organic, and Fatty Acids in Pig: A Review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef]
- Montoya, C.A.; Rohleff, I.; Hodgkinson, S.; Stoklosinski, H.M.; Moughan, P.J. Type and Amount of Dietary Fiber Influence the Hindgut Synthesis of Organic Acids from Fermentable Material of Both Total and Nondietary Origin in a Pig Model of the Adult Human. J. Nutr. 2023, 153, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, B.; Jiang, Q.; Han, M.; Zhao, Y.; Long, L.; Fu, C.; Yao, K. Alpha-Ketoglutarate in Low-Protein Diets for Growing Pigs: Effects on Cecal Microbial Communities and Parameters of Microbial Metabolism. Front. Microbiol. 2018, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, M.; Zhang, Y.; Yu, K.; Zhu, W. Succinate Modulates Intestinal Barrier Function and Inflammation Response in Pigs. Biomolecules 2019, 9, 486. [Google Scholar] [CrossRef]
- Rathnayake, D.; Mun, H.S.; Dilawar, M.A.; Baek, K.S.; Yang, C.J. Time for a Paradigm Shift in Animal Nutrition Metabolic Pathway: Dietary Inclusion of Organic Acids on the Production Parameters, Nutrient Digestibility, and Meat Quality Traits of Swine and Broilers. Life 2021, 11, 476. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Wang, J.; Hu, C.; Ji, F.; Xie, J.; Yang, Y.; Yu, X.; Diao, X.; Lv, R. Effects of Dietary Probiotics and Acidifiers on the Production Performance, Colostrum Components, Serum Antioxidant Activity and Hormone Levels, and Gene Expression in Mammary Tissue of Lactating Sows. Animals 2023, 13, 1536. [Google Scholar] [CrossRef]
- Ebert, M.N. Expression of Glutathione S-Transferases (GSTs) in Human Colon Cells and Inducibility of GSTM2 by Butyrate. Carcinogenesis 2003, 24, 1637–1644. [Google Scholar] [CrossRef]
- Yano, S.; Tierney, D.F. Butyrate Increases Catalase Activity and Protects Rat Pulmonary Artery Smooth Muscle Cells against Hyperoxia. Biochem. Biophys. Res. Commun. 1989, 164, 1143–1148. [Google Scholar] [CrossRef]
- Toden, S.; Bird, A.R.; Topping, D.L.; Conlon, M.A. Dose-Dependent Reduction of Dietary Protein-Induced Colonocyte DNA Damage by Resistant Starch in Rats Correlates More Highly with Caecal Butyrate than with Other Short Chain Fatty Acids. Cancer Biol. Ther. 2007, 6, 253–258. [Google Scholar] [CrossRef]
- Rosignoli, P. Protective Activity of Butyrate on Hydrogen Peroxide-Induced DNA Damage in Isolated Human Colonocytes and HT29 Tumour Cells. Carcinogenesis 2001, 22, 1675–1680. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.-L.; Deng, X.; Zhu, T.-T.; Xiong, J.-F.; Xu, Y.-H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, H.; Chen, B. DJ-1 Protects Retinal Pericytes against High Glucose-Induced Oxidative Stress through the Nrf2 Signaling Pathway. Sci. Rep. 2020, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Lee, K.Y.; Kim, I.H. Analysis of the Effect of Dietary Protected Organic Acid Blend on Lactating Sows and Their Piglets. Rev. Bras. Zootec. 2016, 45, 39–47. [Google Scholar] [CrossRef]
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D.-K. Probiotic Roles of Lactobacillus Sp. in Swine: Insights from Gut Microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, D.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Hua, L.; et al. Maternal Tributyrin Supplementation in Late Pregnancy and Lactation Improves Offspring Immunity, Gut Microbiota, and Diarrhea Rate in a Sow Model. Front. Microbiol. 2023, 14, 1142174. [Google Scholar] [CrossRef]
- DeRouchey, J.M.; Hancock, J.D.; Hines, R.H.; Cummings, K.R.; Lee, D.J.; Maloney, C.A.; Dean, D.W.; Park, J.S.; Cao, H. Effects of Dietary Electrolyte Balance on the Chemistry of Blood and Urine in Lactating Sows and Sow Litter Performance. J. Anim. Sci. 2003, 81, 3067–3074. [Google Scholar] [CrossRef]
- Kluge, H.; Broz, J.; Eder, K. Effects of Dietary Benzoic Acid on Urinary PH and Nutrient Digestibility in Lactating Sows. Livest. Sci. 2010, 134, 119–121. [Google Scholar] [CrossRef]
- Poor, A.P.; Moreno, L.Z.; Monteiro, M.S.; Matajira, C.E.C.; Dutra, M.C.; Leal, D.F.; Silva, A.P.S.; Gomes, V.T.M.; Barbosa, M.R.F.; Sato, M.I.Z.; et al. Vaginal Microbiota Signatures in Healthy and Purulent Vulvar Discharge Sows. Sci. Rep. 2022, 12, 9106. [Google Scholar] [CrossRef]
- Poor, A.P.; Moreno, L.Z.; Monteiro, M.S.; Matajira, C.E.C.; Dutra, M.C.; Leal, D.F.; Silva, A.P.S.; Gomes, V.T.M.; de Souza, I.O.; Araújo, K.M.; et al. Characterization of Escherichia Coli Isolated from Sows Presenting Purulent Vulvar Discharge. Microorganisms 2024, 12, 123. [Google Scholar] [CrossRef]
- Walsh, M.C.; Sholly, D.M.; Hinson, R.B.; Saddoris, K.L.; Sutton, A.L.; Radcliffe, J.S.; Odgaard, R.; Murphy, J.; Richert, B.T. Effects of Water and Diet Acidification with and without Antibiotics on Weanling Pig Growth and Microbial Shedding. J. Anim. Sci. 2007, 85, 1799–1808. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-Chain Fatty Acids and Monoglycerides as Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Naseri, K.; Kheravii, S.K.; Li, L.; Wu, S.-B. Buffered Formic Acid and a Monoglyceride Blend Coordinately Alleviate Subclinical Necrotic Enteritis Impact in Broiler Chickens. Poult. Sci. 2021, 100, 101214. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Saliu, E.-M.; Martínez-Vallespín, B.; Aschenbach, J.R.; Brockmann, G.A.; Fulde, M.; Hartmann, S.; Kuhla, B.; Lucius, R.; Metges, C.C.; et al. Dietary Fiber and Its Role in Performance, Welfare, and Health of Pigs. Anim. Health Res. Rev. 2022, 23, 165–193. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F.D. Dietary Fiber and Protein Fermentation in the Intestine of Swine and Their Interactive Effects on Gut Health and on the Environment: A Review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Jarrett, S.; Ashworth, C.J. The Role of Dietary Fibre in Pig Production, with a Particular Emphasis on Reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- de Leeuw, J.A.; Jongbloed, A.W.; Verstegen, M.W.A. Dietary Fiber Stabilizes Blood Glucose and Insulin Levels and Reduces Physical Activity in Sows (Sus Scrofa). J. Nutr. 2004, 134, 1481–1486. [Google Scholar] [CrossRef]
- Souza da Silva, C.; Bolhuis, J.E.; Gerrits, W.J.J.; Kemp, B.; van den Borne, J.J.G.C. Effects of Dietary Fibers with Different Fermentation Characteristics on Feeding Motivation in Adult Female Pigs. Physiol. Behav. 2013, 110–111, 148–157. [Google Scholar] [CrossRef]
- Blackwood, A.D.; Salter, J.; Dettmar, P.W.; Chaplin, M.F. Dietary Fibre, Physicochemical Properties and Their Relationship to Health. J. R. Soc. Promot. Health 2000, 120, 242–247. [Google Scholar] [CrossRef]
- McRorie, J.W. Dietary Fiber: All Fibers Are Not Alike. In Nutrition Guide for Physicians and Related Healthcare Professionals; Springer International Publishing: Cham, Switzerland, 2017; pp. 229–239. [Google Scholar]
- McRorie, J.W. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, H.; Yang, Y.; He, J.; Cao, M.; Yang, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; et al. Effects of the Ratio of Insoluble Fiber to Soluble Fiber in Gestation Diets on Sow Performance and Offspring Intestinal Development. Animals 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Serena, A.; Jørgensen, H.; Bach Knudsen, K.E. Absorption of Carbohydrate-Derived Nutrients in Sows as Influenced by Types and Contents of Dietary Fiber. J. Anim. Sci. 2009, 87, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Ashworth, C.J.; Hunter, M.G.; Penny, P.; Slevin, J.; Edwards, S.A. The Effect of Feeding a High Fibre Diet from Mid Lactation until Breeding on Subsequent Litter Size of Sows. BSAP Occas. Publ. 2004, 31, 175–179. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Slevin, J.; Hunter, M.G.; Edwards, S.A.; Ashworth, C.J. Beneficial Effects of a High Fibre Diet on Oocyte Maturity and Embryo Survival in Gilts. Reproduction 2007, 133, 433–439. [Google Scholar] [CrossRef]
- Huang, P.; Mou, Q.; Yang, Y.; Li, J.; Xu, M.; Huang, J.; Li, J.; Yang, H.; Liang, X.; Yin, Y. Effects of Supplementing Sow Diets during Late Gestation with Pennisetum Purpureum on Antioxidant Indices, Immune Parameters and Faecal Microbiota. Vet. Med. Sci. 2021, 7, 1347–1358. [Google Scholar] [CrossRef]
- Oh, S.M.; Hosseindoust, A.; Ha, S.H.; Mun, J.Y.; Moturi, J.; Tajudeen, H.; Choi, Y.H.; Lee, S.H.; Kim, J.S. Importance of Dietary Supplementation of Soluble and Insoluble Fibers to Sows Subjected to High Ambient Temperatures during Late Gestation and Effects on Lactation Performance. Anim. Nutr. 2024, 16, 73–83. [Google Scholar] [CrossRef]
- Robert, S.; Matte, J.J.; Farmer, C.; Girard, C.L.; Martineau, G.P. High-Fibre Diets for Sows: Effects on Stereotypies and Adjunctive Drinking. Appl. Anim. Behav. Sci. 1993, 37, 297–309. [Google Scholar] [CrossRef]
- Priester, M.; Visscher, C.; Fels, M.; Rohn, K.; Dusel, G. Fibre Supply for Breeding Sows and Its Effects on Social Behaviour in Group-Housed Sows and Performance during Lactation. Porc. Health Manag. 2020, 6, 15. [Google Scholar] [CrossRef]
- Moturi, J.; Hosseindoust, A.; Tajudeen, H.; Mun, J.Y.; Ha, S.H.; Kim, J.S. Influence of Dietary Fiber Intake and Soluble to Insoluble Fiber Ratio on Reproductive Performance of Sows during Late Gestation under Hot Climatic Conditions. Sci. Rep. 2022, 12, 19749. [Google Scholar] [CrossRef]
- Pearodwong, P.; Muns, R.; Tummaruk, P. Prevalence of Constipation and Its Influence on Post-Parturient Disorders in Tropical Sows. Trop. Anim. Health Prod. 2016, 48, 525–531. [Google Scholar] [CrossRef]
- Dumniem, N.; Boonprakob, R.; Panvichitra, C.; Thongmark, S.; Laohanarathip, N.; Parnitvoraphoom, T.; Changduangjit, S.; Boonmakaew, T.; Teshanukroh, N.; Tummaruk, P. Impacts of Fiber Supplementation in Sows during the Transition Period on Constipation, Farrowing Duration, Colostrum Production, and Pre-Weaning Piglet Mortality in the Free-Farrowing System. Animals 2024, 14, 854. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and Sow-Related Factors Affecting the Duration of Farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, J. Variations on Gut Health and Energy Metabolism in Pigs and Humans by Intake of Different Dietary Fibers. Food Sci. Nutr. 2021, 9, 4639–4654. [Google Scholar] [CrossRef] [PubMed]
- Molist, F.; de Segura, A.G.; Gasa, J.; Hermes, R.G.; Manzanilla, E.G.; Anguita, M.; Pérez, J.F. Effects of the Insoluble and Soluble Dietary Fibre on the Physicochemical Properties of Digesta and the Microbial Activity in Early Weaned Piglets. Anim. Feed Sci. Technol. 2009, 149, 346–353. [Google Scholar] [CrossRef]
- Owusu-Asiedu, A.; Patience, J.F.; Laarveld, B.; Van Kessel, A.G.; Simmins, P.H.; Zijlstra, R.T. Effects of Guar Gum and Cellulose on Digesta Passage Rate, Ileal Microbial Populations, Energy and Protein Digestibility, and Performance of Grower Pigs. J. Anim. Sci. 2006, 84, 843–852. [Google Scholar] [CrossRef]
- Ampode, K.M.B.; Mun, H.-S.; Lagua, E.B.; Chem, V.; Park, H.-R.; Kim, Y.-H.; Yang, C.-J. Bump Feeding Improves Sow Reproductive Performance, Milk Yield, Piglet Birth Weight, and Farrowing Behavior. Animals 2023, 13, 3148. [Google Scholar] [CrossRef]
- Gonçalves, M.A.D.; Gourley, K.M.; Dritz, S.S.; Tokach, M.D.; Bello, N.M.; DeRouchey, J.M.; Woodworth, J.C.; Goodband, R.D. Effects of Amino Acids and Energy Intake during Late Gestation of High-Performing Gilts and Sows on Litter and Reproductive Performance under Commercial Conditions. J. Anim. Sci. 2016, 94, 1993–2003. [Google Scholar] [CrossRef]
- Mallmann, A.L.; Betiolo, F.B.; Camilloti, E.; Mellagi, A.P.G.; Ulguim, R.R.; Wentz, I.; Bernardi, M.L.; Gonçalves, M.A.D.; Kummer, R.; Bortolozzo, F.P. Two Different Feeding Levels during Late Gestation in Gilts and Sows under Commercial Conditions: Impact on Piglet Birth Weight and Female Reproductive Performance. J. Anim. Sci. 2018, 96, 4209–4219. [Google Scholar] [CrossRef]
- Moreira, R.H.R.; Pérez Palencia, J.Y.; Moita, V.H.C.; Caputo, L.S.S.; Saraiva, A.; Andretta, I.; Ferreira, R.A.; de Abreu, M.L.T. Variability of Piglet Birth Weights: A Systematic Review and Meta-Analysis. J. Anim. Physiol. Anim. Nutr. 2020, 104, 657–666. [Google Scholar] [CrossRef]
- Ferreira, S.V.; Rodrigues, L.A.; Ferreira, M.A.; Alkmin, D.V.; Dementshuk, J.M.; Almeida, F.R.C.L.; Fontes, D.O. Plane of Nutrition during Gestation Affects Reproductive Performance and Retention Rate of Hyperprolific Sows under Commercial Conditions. Animal 2021, 15, 100153. [Google Scholar] [CrossRef]
- Chipman, A.; Rademacher, C.; Johnson, C.; Stalder, K.; Johnson, A.; Keating, A.; Patience, J.; Gabler, N.; Linhares, D.; Schwartz, K. Pelvic Organ Prolapse: An Industry-Wide Collaboration to Identify Putative Contributing Factors; Iowa State University: Ames, IA, USA, 2018. [Google Scholar]
- Feyera, T.; Theil, P.K. Energy and Lysine Requirements and Balances of Sows during Transition and Lactation: A Factorial Approach. Livest. Sci. 2017, 201, 50–57. [Google Scholar] [CrossRef]
- Schoos, A.; Muro, B.B.D.; Carnevale, R.F.; Chantziaras, I.; Biebaut, E.; Janssens, G.P.J.; Maes, D. Relationship between Piglets’ Survivability and Farrowing Kinetics in Hyper-Prolific Sows. Porc. Health Manag. 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.F.; Muro, B.B.D.; Leal, D.F.; Alves, L.K.S.; Monteiro, M.S.; Gomes, N.A.C.; Pereira, F.A.; Ferreira, F.N.A.; Neta, C.S.S.; Watanabe, T.T.N.; et al. The Effects of Feeding Sows at Onset of Farrowing Supplemental Energy (Blend of Carbohydrates and Glycerol) on Farrowing Kinetics and Piglet Vitality. Animal 2024, 18, 101104. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N. Update on Postpartum Dysgalactia Syndrome in Sows. J. Anim. Sci. 2020, 98, S117–S125. [Google Scholar] [CrossRef]
- Weaver, A.C.; Braun, T.C.; Braun, J.A.; Golder, H.M.; Block, E.; Lean, I.J. Effects of Negative Dietary Anion Cation Difference and Calcidiol Supplementation in Transition Diets Fed to Sows on Piglet Survival, Piglet Weight, and Sow Metabolism. J. Anim. Sci. 2024, 102, skae027. [Google Scholar] [CrossRef]
- Langendijk, P.L.; Soede, N.M. Physiology and Management of the Peri-Parturient Sow in the Context of Changing Production Conditions. Reprod. Domest. Anim. 2023, 58, 84–92. [Google Scholar] [CrossRef]
- Wray, S. Uterine Contraction and Physiological Mechanisms of Modulation. Am. J. Physiol. Cell Physiol. 1993, 264, C1–C18. [Google Scholar] [CrossRef]
- Schonewille, J.T.; Van’t Klooster, A.T.; Wouterse, H.; Beynen, A.C. Hypocalcemia Induced by Intravenous Administration of Disodium Ethylenediaminotetraacetate and Its Effects on Excretion of Calcium in Urine of Cows Fed a High Chloride Diet. J. Dairy Sci. 1999, 82, 1317–1324. [Google Scholar] [CrossRef]
- Bikker, P.; Blok, M.C. Phosphorus and Calcium Requirements of Growing Pigs and Sows; Wageningen Livestock Research: Wageningen, The Netherland, 2017. [Google Scholar]
- Houe, H.; Østergaard, S.; Thilsing-Hansen, T.; Jørgensen, R.J.; Larsen, T.; Sørensen, J.T.; Agger, J.F.; Blom, J.Y. Milk Fever and Subclinical Hypocalcaemia--an Evaluation of Parameters on Incidence Risk, Diagnosis, Risk Factors and Biological Effects as Input for a Decision Support System for Disease Control. Acta Vet. Scand. 2001, 42, 1–29. [Google Scholar]
- Beck, N.; Webster, S. Effects of Acute Metabolic Acidosis on Parathyroid Hormone Action and Calcium Mobilization. Am. J. Physiol. Leg. Content 1976, 230, 127–131. [Google Scholar] [CrossRef]
- Guo, J.Y.; Pasquetti, T.J.; Kim, S.W. Lowering Dietary Cation-Anion Difference Increases Sow Blood and Milk Calcium Concentrations. J. Anim. Sci. 2019, 97, 2927–2939. [Google Scholar] [CrossRef] [PubMed]
- Glosson, K.M.; Zhang, X.; Zanzalari, K.P.; Bascom, S.S.; Rowson, A.D.; Wang, Z.; Drackley, J.K. Negative Dietary Cation-Anion Difference and Amount of Calcium in Prepartum Diets: Effects on Urine and Serum Minerals. JDS Commun. 2023, 4, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.A.; Hiew, M.W.H.; El Badawy, S.A.; Constable, P.D. Plasma Calcium Concentrations Are Decreased at Least 9 Hours before Parturition in Multiparous Holstein-Friesian Cattle in a Herd Fed an Acidogenic Diet during Late Gestation. J. Dairy Sci. 2018, 101, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Henman, D.; Lean, I.J.; Block, E.; Golder, H.M. Data on the Effects of the Anionic Protein Meal BioChlor® on Sows before and after Farrowing. Data Brief 2023, 48, 109168. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.L.; Johnston, S.L.; Lirette, R.D.; Bidner, T.D.; Southern, L.L.; Jardon, P.W. The Effect of Diets Varying in Dietary Cation-Anion Difference Fed in Late Gestation and in Lactation on Sow Productivity. Prof. Anim. Sci. 2008, 24, 149–155. [Google Scholar] [CrossRef]
- Langendijk, P. Latest Advances in Sow Nutrition during Early Gestation. Animals 2021, 11, 1720. [Google Scholar] [CrossRef]
- Darriet, C.; Axe, D.E.; Crenshaw, T.D. Acidogenic Mineral Additions Increased Ca Mobilization in Prepartum Sows. J. Anim. Sci. 2017, 95, 212–225. [Google Scholar] [CrossRef]
- Weber, G.M.; Witschi, A.-K.M.; Wenk, C.; Martens, H. TRIENNIAL GROWTH SYMPOSIUM— Effects of Dietary 25-Hydroxycholecalciferol and Cholecalciferol on Blood Vitamin D and Mineral Status, Bone Turnover, Milk Composition, and Reproductive Performance of Sows. J. Anim. Sci. 2014, 92, 899–909. [Google Scholar] [CrossRef]
- Meuter, A.; Thoby, J.-M.; Lichou, J.-Y.; Renouf, B. Effet d’une Supplémentation En 25-Hydroxycholécalciférol Sur Les Performances Des Truies Gestantes et Allaitantes et de Leurs Portées. Journées Recherche Porcine 2016, 48, 153–154. Available online: https://www.journees-recherche-porcine.com/texte/2016/alimentation/ap28.pdf (accessed on 1 August 2024).
- Lapointe, J. Mitochondria as Promising Targets for Nutritional Interventions Aiming to Improve Performance and Longevity of Sows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 809–821. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.-J.; Ferreira, A.L.A. Oxidative Stress Status of Highly Prolific Sows during Gestation and Lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Betteridge, D.J. What Is Oxidative Stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kim, S.W. Oxidative Stress Status and Reproductive Performance of Sows during Gestation and Lactation under Different Thermal Environments. Asian-Australas J. Anim. Sci. 2020, 33, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional Strategies to Alleviate Oxidative Stress in Sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef]
- Reyes-Camacho, D.; Vinyeta, E.; Pérez, J.F.; Aumiller, T.; Criado, L.; Palade, L.M.; Taranu, I.; Folch, J.M.; Calvo, M.A.; Van der Klis, J.D.; et al. Phytogenic Actives Supplemented in Hyperprolific Sows: Effects on Maternal Transfer of Phytogenic Compounds, Colostrum and Milk Features, Performance and Antioxidant Status of Sows and Their Offspring, and Piglet Intestinal Gene Expression. J. Anim. Sci. 2020, 98, skz390. [Google Scholar] [CrossRef]
- Durmic, Z.; Blache, D. Bioactive Plants and Plant Products: Effects on Animal Function, Health and Welfare. Anim. Feed Sci. Technol. 2012, 176, 150–162. [Google Scholar] [CrossRef]
- Palade, L.M.; Habeanu, M.; Marin, D.E.; Chedea, V.S.; Pistol, G.C.; Grosu, I.A.; Gheorghe, A.; Ropota, M.; Taranu, I. Effect of Dietary Hemp Seed on Oxidative Status in Sows during Late Gestation and Lactation and Their Offspring. Animals 2019, 9, 194. [Google Scholar] [CrossRef]
- Sun, J.; Wang, P.; Chen, G.; Luo, J.; Xi, Q.; Cai, G.; Wu, J.; Zeng, B.; Xie, Y.; Jiang, Q.; et al. Effect of Moringa Oleifera Supplementation on Productive Performance, Colostrum Composition and Serum Biochemical Indexes of Sow. J. Anim. Physiol. Anim. Nutr. 2020, 104, 291–299. [Google Scholar] [CrossRef]
- Xie, C.; Wu, X.; Long, C.; Wang, Q.; Fan, Z.; Li, S.; Yin, Y. Chitosan Oligosaccharide Affects Antioxidant Defense Capacity and Placental Amino Acids Transport of Sows. BMC Vet. Res. 2016, 12, 243. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Lyu, H.; Gu, X.; Song, Z.; He, X.; Fan, Z. Effects of Dietary Inulin during Late Gestation on Sow Physiology, Farrowing Duration and Piglet Performance. Anim. Reprod. Sci. 2020, 219, 106531. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Cheng, C.; Cui, J.; Ji, Y.; Hao, X.; Li, Q.; Ren, W.; Deng, B.; Yin, Y.; et al. Unraveling the Association of Fecal Microbiota and Oxidative Stress with Stillbirth Rate of Sows. Theriogenology 2019, 136, 131–137. [Google Scholar] [CrossRef]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’Doherty, J.V. Effect of Purified β-Glucans Derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on Piglet Performance, Selected Bacterial Populations, Volatile Fatty Acids and pro-Inflammatory Cytokines in the Gastrointestinal Tract of Pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.; Bauer, L.; Swanson, K.; Tappenden, K.; Fahey, G.; De Godoy, M. Soluble Fiber Dextrin and Soluble Corn Fiber Supplementation Modify Indices of Health in Cecum and Colon of Sprague-Dawley Rats. Nutrients 2013, 5, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wu, B.; Liu, Z.; Zhang, T. Effects of Different Selenium Sources on Sow Reproductive Performance and Piglet Development: A Meta-Analysis. J. Anim. Feed Sci. 2021, 30, 260–270. [Google Scholar] [CrossRef]
- Pinto, A.; Juniper, D.T.; Sanil, M.; Morgan, L.; Clark, L.; Sies, H.; Rayman, M.P.; Steinbrenner, H. Supranutritional Selenium Induces Alterations in Molecular Targets Related to Energy Metabolism in Skeletal Muscle and Visceral Adipose Tissue of Pigs. J. Inorg. Biochem. 2012, 114, 47–54. [Google Scholar] [CrossRef]
- Halliwell, B. Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Aznar, B.; Grandia, J.; Tejedor, M.T.; Falceto, M.V. Effect of Vitamin C and Iron Supplementation in Pregnant Hyper-Prolific Sows. Livest. Sci. 2024, 280, 105404. [Google Scholar] [CrossRef]
- Feng, T.; Bai, J.; Xu, X.; Guo, Y.; Huang, Z.; Liu, Y. Supplementation with N-Carbamylglutamate and Vitamin C: Improving Gestation and Lactation Outcomes in Sows under Heat Stress. Anim. Prod. Sci. 2018, 58, 1854. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, J.; Hu, R.; Li, Y.; Wang, Y.; Chen, H.; Hou, D.-X.; He, J.; Wu, S. Modulation of Gut Microbiota and Oxidative Status by β-Carotene in Late Pregnant Sows. Front. Nutr. 2020, 7, 612875. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Hong, C.; Huang, Y.; Cao, S.; Wang, L.; Yang, X.; Hu, S.; Gao, K.; Jiang, Z.; Xiao, H. Accurate Models and Nutritional Strategies for Specific Oxidative Stress Factors: Does the Dose Matter in Swine Production? J. Anim. Sci. Biotechnol. 2024, 15, 11. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Y.; Yin, C.; Deng, M.; Tang, T.; Deng, B.; Ren, W.; Deng, J.; Yin, Y.; Tan, C. Differential Analysis of Gut Microbiota Correlated with Oxidative Stress in Sows With High or Low Litter Performance During Lactation. Front. Microbiol. 2018, 9, 1665. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Jacobsen, S.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Theil, P.K.; Jacobson, M. Hormonal and Metabolic Indicators before and after Farrowing in Sows Affected with Postpartum Dysgalactia Syndrome. BMC Vet. Res. 2018, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.J.; Liu, F.; Hung, A.T.; DiGiacomo, K.; Chauhan, S.S.; Leury, B.J.; Furness, J.B.; Celi, P.; Dunshea, F.R. Nutritional Strategies to Alleviate Heat Stress in Pigs. Anim. Prod. Sci. 2015, 55, 1391. [Google Scholar] [CrossRef]
- Bjerg, B.; Brandt, P.; Pedersen, P.; Zhang, G. Sows’ Responses to Increased Heat Load–A Review. J. Therm. Biol. 2020, 94, 102758. [Google Scholar] [CrossRef]
- van Essen, G.J.; te Lintel Hekkert, M.; Sorop, O.; Heinonen, I.; van der Velden, J.; Merkus, D.; Duncker, D.J. Cardiovascular Function of Modern Pigs Does Not Comply with Allometric Scaling Laws. Sci. Rep. 2018, 8, 792. [Google Scholar] [CrossRef]
- Ross, J.W.; Hale, B.J.; Seibert, J.T.; Romoser, M.R.; Adur, M.K.; Keating, A.F.; Baumgard, L.H. Physiological Mechanisms Through Which Heat Stress Compromises Reproduction in Pigs. Mol. Reprod. Dev. 2017, 84, 934–945. [Google Scholar] [CrossRef]
- Byrd, C.J.; McConn, B.R.; Gaskill, B.N.; Schinckel, A.P.; Green-Miller, A.R.; Lay, D.C.; Johnson, J.S. Characterizing the Effect of Incrementally Increasing Dry Bulb Temperature on Linear and Nonlinear Measures of Heart Rate Variability in Nonpregnant, Mid-Gestation, and Late-Gestation Sows. J. Anim. Sci. 2022, 100, skac004. [Google Scholar] [CrossRef]
- Zhang, S.; Johnson, J.S.; Trottier, N.L. Effect of Dietary near Ideal Amino Acid Profile on Heat Production of Lactating Sows Exposed to Thermal Neutral and Heat Stress Conditions. J. Anim. Sci. Biotechnol. 2020, 11, 75. [Google Scholar] [CrossRef]
- Kim, H.; Jin, X.; Kim, C.; Pan, N.; Kim, Y.Y. Effects of Different Levels of Dietary Crude Protein on the Physiological Response, Reproductive Performance, Blood Profiles, Milk Composition and Odor Emission in Gestating Sows. Anim. Biosci. 2023, 36, 1263–1273. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Peng, Y.; Zhang, Y.; Liu, Y.; Liu, Y.; Yin, Y. Research Progress on Anti-Stress Nutrition Strategies in Swine. Anim. Nutr. 2023, 13, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Johnston, L.J.; Levesque, C.L.; Yin, J.; Dong, B. A Systematic Review and Meta-Analysis of Dietary Fat Effects on Reproductive Performance of Sows and Growth Performance of Piglets. J. Anim. Sci. Biotechnol. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Roszkos, R.; Tóth, T.; Mézes, M. Review: Practical Use of n-3 Fatty Acids to Improve Reproduction Parameters in the Context of Modern Sow Nutrition. Animals 2020, 10, 1141. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I. Effects of Organic Acid and Medium Chain Fatty Acid Blends on the Performance of Sows and Their Piglets. Anim. Sci. J. 2018, 89, 1673–1679. [Google Scholar] [CrossRef]
- Świątkiewicz, M.; Hanczakowska, E.; Okoń, K.; Kowalczyk, P.; Grela, E.R. Effect of Maternal Diet and Medium Chain Fatty Acids Supplementation for Piglets on Their Digestive Tract Development, Structure, and Chyme Acidity as Well as Performance and Health Status. Animals 2020, 10, 834. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The Opposing Effects of N−3 and N−6 Fatty Acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Roberts, R.M. Interferon-Tau, a Type 1 Interferon Involved in Maternal Recognition of Pregnancy. Cytokine Growth Factor Rev. 2007, 18, 403–408. [Google Scholar] [CrossRef]
- Cronje, P.B. Essential Role of Methyl Donors in Animal Productivity. Anim. Prod. Sci. 2018, 58, 655. [Google Scholar] [CrossRef]
- Sharma, P.; Senthilkumar, R.; Brahmachari, V.; Sundaramoorthy, E.; Mahajan, A.; Sharma, A.; Sengupta, S. Mining Literature for a Comprehensive Pathway Analysis: A Case Study for Retrieval of Homocysteine Related Genes for Genetic and Epigenetic Studies. Lipids Health Dis. 2006, 5, 1. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Lalla, E.; Lu, Y.; Gleason, M.R.; Wolf, B.M.; Tanji, N.; Ferran, L.J.; Kohl, B.; Rao, V.; Kisiel, W.; et al. Hyperhomocysteinemia Enhances Vascular Inflammation and Accelerates Atherosclerosis in a Murine Model. J. Clin. Investig. 2001, 107, 675–683. [Google Scholar] [CrossRef]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal Heat Stress Reduces Pig Intestinal Integrity and Increases Endotoxin Translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Sánchez, V.; Pérez, B.; Camacho, R.L.; Arce, N.; Avelar, E.; González-Vega, J.-C.; Htoo, J.K.; Cervantes, M. Effect of Methionine Supplementation above Requirement on Performance; Intestinal Morphology, Antioxidant Activity, and Gene Expression; and Serum Concentration of Amino Acids in Heat Stressed Pigs. J. Anim. Sci. 2023, 101, skac379. [Google Scholar] [CrossRef] [PubMed]
- Del Vesco, A.P.; Khatlab, A. de S.; Santana, T.P.; Pozza, P.C.; Menck Soares, M.A.; Brito, C.O.; Barbosa, L.T.; Gasparino, E. Heat Stress Effect on the Intestinal Epithelial Function of Broilers Fed Methionine Supplementation. Livest. Sci. 2020, 240, 104152. [Google Scholar] [CrossRef]
- Mendoza, S.M.; Boyd, R.D.; Remus, J.; Wilcock, P.; Martinez, G.E.; van Heugten, E. Sow Performance in Response to Natural Betaine Fed during Lactation and Post-Weaning during Summer and Non-Summer Months. J. Anim. Sci. Biotechnol. 2020, 11, 69. [Google Scholar] [CrossRef]
- van Wettere, W.H.E.J.; Herde, P.; Hughes, P.E. Supplementing Sow Gestation Diets with Betaine during Summer Increases Litter Size of Sows with Greater Numbers of Parities. Anim. Reprod. Sci. 2012, 132, 44–49. [Google Scholar] [CrossRef]
- Cabezón, F.A.; Stewart, K.R.; Schinckel, A.P.; Richert, B.T. Effects of Betaine and Heat Stress on Lactation and Postweaning Reproductive Performance of Sows. Prof Anim Sci 2017, 33, 241–253. [Google Scholar] [CrossRef]
- He, Q.; Zou, T.; Chen, J.; He, J.; Jian, L.; Xie, F.; You, J.; Wang, Z. Methyl-Donor Micronutrient for Gestating Sows: Effects on Gut Microbiota and Metabolome in Offspring Piglets. Front. Nutr. 2021, 8, 675640. [Google Scholar] [CrossRef]
- Jin, C.; Zhuo, Y.; Wang, J.; Zhao, Y.; Xuan, Y.; Mou, D.; Liu, H.; Zhou, P.; Fang, Z.; Che, L.; et al. Methyl Donors Dietary Supplementation to Gestating Sows Diet Improves the Growth Rate of Offspring and Is Associating with Changes in Expression and DNA Methylation of Insulin-Like Growth Factor-1 Gene. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1340–1350. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Zhuo, Y.; Feng, B.; Fang, Z.; Xu, S.; Li, J.; Zhao, H.; Wu, D.; Hua, L.; et al. Effects of Maternal Methyl Donor Intake during Pregnancy on Ileum Methylation and Function in an Intrauterine Growth Restriction Pig Model. J. Anim. Sci. Biotechnol. 2024, 15, 19. [Google Scholar] [CrossRef]
- Giudicelli, F.; Brabant, A.-L.; Grit, I.; Parnet, P.; Amarger, V. Excess of Methyl Donor in the Perinatal Period Reduces Postnatal Leptin Secretion in Rat and Interacts with the Effect of Protein Content in Diet. PLoS ONE 2013, 8, e68268. [Google Scholar] [CrossRef]
- Van Dijk, A.J.; Van Rens, B.T.T.M.; Van Der Lende, T.; Taverne, M.A.M. Factors Affecting Duration of the Expulsive Stage of Parturition and Piglet Birth Intervals in Sows with Uncomplicated, Spontaneous Farrowings. Theriogenology 2005, 64, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Muro, B.B.D.; Carnevale, R.F.; Andretta, I.; Leal, D.F.; Monteiro, M.S.; Poor, A.P.; Almond, G.W.; Garbossa, C.A.P. Effects of Uterotonics on Farrowing Traits and Piglet Vitality: A Systematic Review and Meta-Analysis. Theriogenology 2021, 161, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.S.; Muro, B.B.D.; Poor, A.P.; Leal, D.F.; Carnevale, R.F.; Shiroma, M.P.; Almond, G.W.; Garbossa, C.A.P.; Moreno, A.M.; Viana, C.H.C. Effects of Farrowing Induction with Prostaglandins on Farrowing Traits and Piglet Performance: A Systematic Review and Meta-Analysis. Theriogenology 2022, 180, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Neves, J.S.; Castro, D.S.; Lopes, S.O.; Santos, S.L.; Silva, S.V.C.; Araújo, V.O.; Vieira, M.F.A.; Muro, B.B.D.; Leal, D.F.; et al. Supplying Sows Energy on the Expected Day of Farrowing Improves Farrowing Kinetics and Newborn Piglet Performance in the First 24 h after Birth. Animal 2020, 14, 2271–2276. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig Blastocyst–Uterine Interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Feyera, T.; Pedersen, T.F.; Krogh, U.; Foldager, L.; Theil, P.K. Impact of Sow Energy Status during Farrowing on Farrowing Kinetics, Frequency of Stillborn Piglets, and Farrowing Assistance1. J. Anim. Sci. 2018, 96, 2320–2331. [Google Scholar] [CrossRef]
- Gourley, K.M.; Swanson, A.J.; Royall, R.Q.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Hastad, C.W.; Woodworth, J.C. Effects of Timing and Size of Meals Prior to Farrowing on Sow and Litter Performance. Transl. Anim. Sci. 2020, 4, 724–736. [Google Scholar] [CrossRef]
- Grahofer, A.; Plush, K. Lactation in Swine: Review Article. Anim. Front. 2023, 13, 112–118. [Google Scholar] [CrossRef]
- Varley, M.A.; Foxcroft, G.R. Endocrinology of the Lactating and Weaned Sow. J. Reprod. Fertil. Suppl 1990, 40, 47–61. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Wang, S.; Zhang, X.; Hou, J.; Xu, F.; Wang, Z.; Xu, L.; Diao, X. Effects of Soybean Isoflavone and Astragalus Polysaccharide Mixture on Colostrum Components, Serum Antioxidant, Immune and Hormone Levels of Lactating Sows. Animals 2021, 11, 132. [Google Scholar] [CrossRef]
- Capasso, R.; Aviello, G.; Capasso, F.; Savino, F.; Izzo, A.A.; Lembo, F.; Borrelli, F. Silymarin BIO-C®, an Extract from Silybum Marianum Fruits, Induces Hyperprolactinemia in Intact Female Rats. Phytomedicine 2009, 16, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Tava, A.; Galletti, S.; Tameni, M.; Varisco, G.; Costa, A.; Steidler, S. Effects of Silymarin, a Natural Hepatoprotector, in Periparturient Dairy Cows. J. Dairy Sci. 2004, 87, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Lapointe, J.; Palin, M.-F. Effects of the Plant Extract Silymarin on Prolactin Concentrations, Mammary Gland Development, and Oxidative Stress in Gestating Gilts. J. Anim. Sci. 2014, 92, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, H.S.; Albino, L.F.T.; Calderano, A.A.; Hannas, M.I.; Sakomura, N.K.; Perazzo, F.G.; Rocha, G.C.; Saraiva, A.; Abreu, M.L.T.; Genova, J.L.; et al. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais; Departamento de Zootecnia-UFV: Viçosa, Brazil, 2024; p. 576. [Google Scholar]
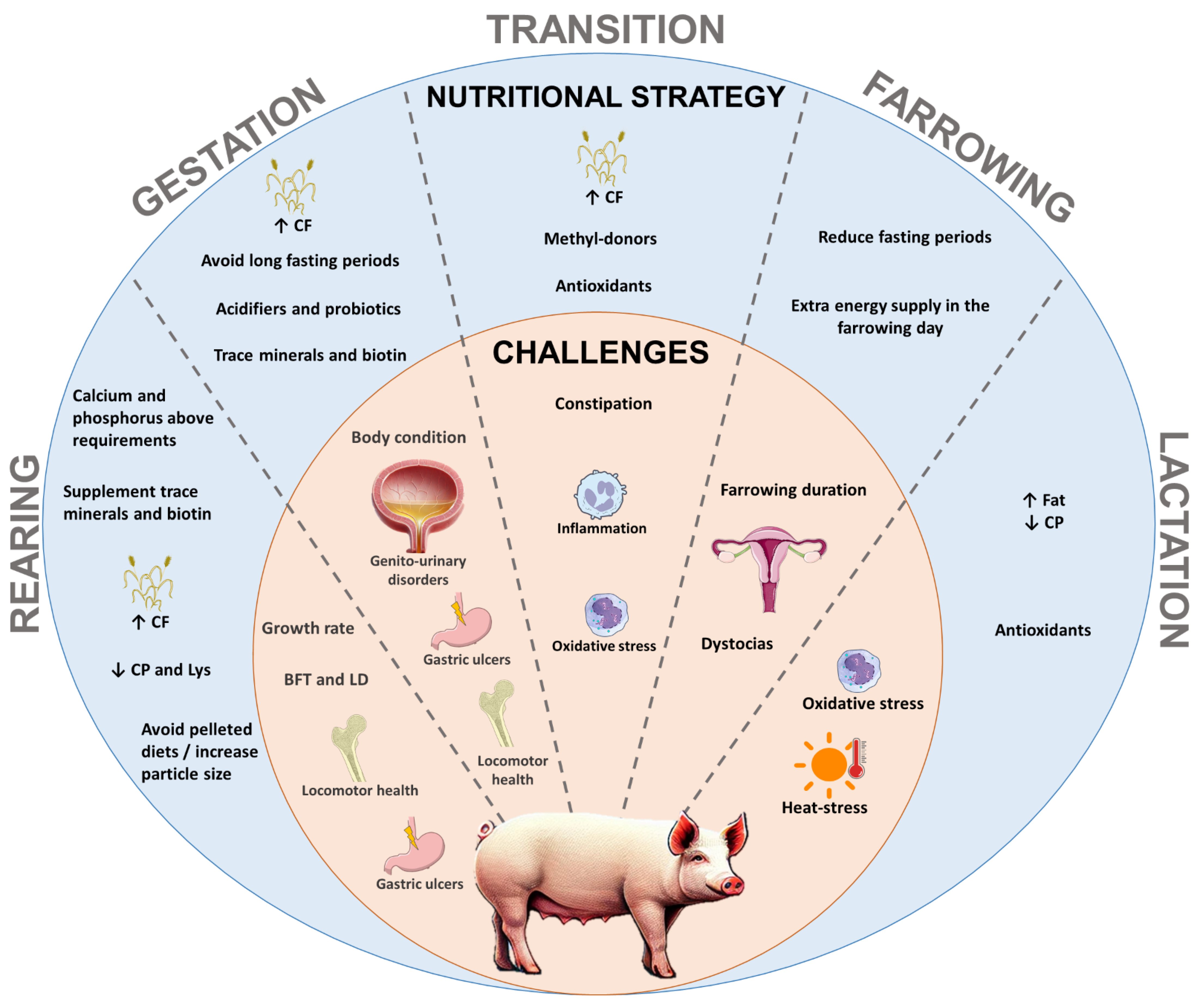
| Period | Recommendation | Target Aim or Nutritional Intervention | References |
|---|---|---|---|
| Rearing to mating |
| At mating:
| [11,24,31,34,36,84,85] |
| |||
| |||
|
| [24,86] | |
|
| [11,54] | |
|
| [58,64,65] | |
|
| [75,79,80,82,86] |
| Period | Recommendation | Target Aim or Nutritional Intervention | References |
|---|---|---|---|
| Gestation |
|
| [24,92] |
|
| [24,31,85,95,98,198] | |
|
| [79,80,82,83,86] | |
|
| [88,111,115,121,122,184] | |
|
| [24,97,195] | |
|
| [18,132,133,134,135,140,142,160] | |
|
| [58,64,65] |
| Period | Recommendation | Target Aim or Nutritional Intervention | References |
|---|---|---|---|
| Transition |
|
| [18,24,123,125,132,133,134,135,140,142,160,218,232,234,235,236,237,239] |
|
| [161,203,212] | |
|
| [242,247,250,256,260,262,264] |
| Period | Recommendation | Target Aim or Nutritional Intervention | References |
|---|---|---|---|
| Farrowing |
|
| [24,109,199,200,272,275] |
| Period | Recommendation | Target Aim or Nutritional Intervention | References |
|---|---|---|---|
| Lactation | Optimize milk yield |
| [18,24,123,125,132,133,134,135,140,142,160,218,232,234,235,236,237] |
| Period | Age | Recommendations |
|---|---|---|
| Rearing | Gilts (63–90 d and 27–50 kg) | EM: 4250–4730 kcal/day CP: 230–260 g CF: 70–140 g SID Lys: 12.5–16 g Total Ca: 10.30 g STTD P: 5.10 g Feed intake estimate: 1400–2000 g ADG estimate: 700–774 g |
| Gilts (91–119 d and 50–75 kg) | EM: 7300–8300 kcal/day CP: 290–350 g CF: 100–250 g SID Lys: 15–21 g Total Ca: 13.41 g STTD P: 6.60 g Feed intake estimate: 2000–2500 g ADG estimate: 900–1000 g | |
| Gilts (120–150 d and 75–100 kg) | EM: 7950–10,500 kcal/day CP: 310–350 g CF: 100–290 g SID Lys: 18–20.5 g Total Ca: 15.2 g STTD P: 7.45 g Feed intake estimate: 2500–2900 g ADG estimate: 900–1100 g | |
| Gilts (150–200 d and 100–150 kg) | EM: 10,500–11,100 kcal/day CP: 340–400 g CF: 175–315 g SID Lys: 17.5–20 g Total Ca: 15.2 g STTD P: 7.45 g Feed intake estimate: 3300–3700 g ADG estimate: 850–1050 g | |
| Early and mid-gestation (0–90 days) | Gilts | EM: 6670–6800 kcal/day CP: 230–270 g CF: 200–400 g SID Lys: 10.5–13 g SID Arg: 7.5–22 g Total Ca: 12.5–17 g STTD P: 5.40–8 g |
| Multiparous | EM: 6500–7500 kcal/day CP: 185–230 g CF: 200–400 g SID Lys: 6.5–11 g SID Arg: 4.5–20 g Total Ca: 9.10–18 g STTD P: 4.00–8.5 g | |
| Late gestation (90–114 d) Transition | Gilts | EM: 7500–8500 kcal/day CP: 350–380 g CF: 200–400 g SID Lys: 17.5– 21 g SID Arg: 17.5–32 g Total Ca: 18.5–20 g STTD P: 8.7 g |
| Multiparous | EM: 7700–8700 kcal/day CP: 380–400 g CF: 200–400 g SID Lys: 16.5–19.2 g SID Arg: 16.5–32 g Total Ca: 19–20 g STTD P: 9–10 g | |
| Lactation | First parity | EM: 20,500–21,500 kcal/day CP: 1175–1250 g/day CF: 40–80 g/day SID Lys: 60–70 g/day SID Arg: 60–70 g/day Total Ca: 43–53 g/day STTD P: 20–26 g/day |
| Multiparous sows | EM: 21,500–22,500 kcal/day CP: 1200–1275 g/day SID Lys: 74–84 g/day SID Arg: 74–84 g/day Total Ca: 45–55 g/day STTD P: 23–27 g/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.S.; Carnevale, R.F.; Muro, B.B.D.; Mezzina, A.L.B.; Carnino, B.B.; Poor, A.P.; Matajira, C.E.C.; Garbossa, C.A.P. The Role of Nutrition Across Production Stages to Improve Sow Longevity. Animals 2025, 15, 189. https://doi.org/10.3390/ani15020189
Monteiro MS, Carnevale RF, Muro BBD, Mezzina ALB, Carnino BB, Poor AP, Matajira CEC, Garbossa CAP. The Role of Nutrition Across Production Stages to Improve Sow Longevity. Animals. 2025; 15(2):189. https://doi.org/10.3390/ani15020189
Chicago/Turabian StyleMonteiro, Matheus Saliba, Rafaella Fernandes Carnevale, Bruno Bracco Donatelli Muro, Ana Lígia Braga Mezzina, Bruno Braga Carnino, André Pegoraro Poor, Carlos Emilio Cabrera Matajira, and Cesar Augusto Pospissil Garbossa. 2025. "The Role of Nutrition Across Production Stages to Improve Sow Longevity" Animals 15, no. 2: 189. https://doi.org/10.3390/ani15020189
APA StyleMonteiro, M. S., Carnevale, R. F., Muro, B. B. D., Mezzina, A. L. B., Carnino, B. B., Poor, A. P., Matajira, C. E. C., & Garbossa, C. A. P. (2025). The Role of Nutrition Across Production Stages to Improve Sow Longevity. Animals, 15(2), 189. https://doi.org/10.3390/ani15020189










