Saving the Mahachai Betta: Genetic Erosion and Conservation Priorities Under Urbanization Pressure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
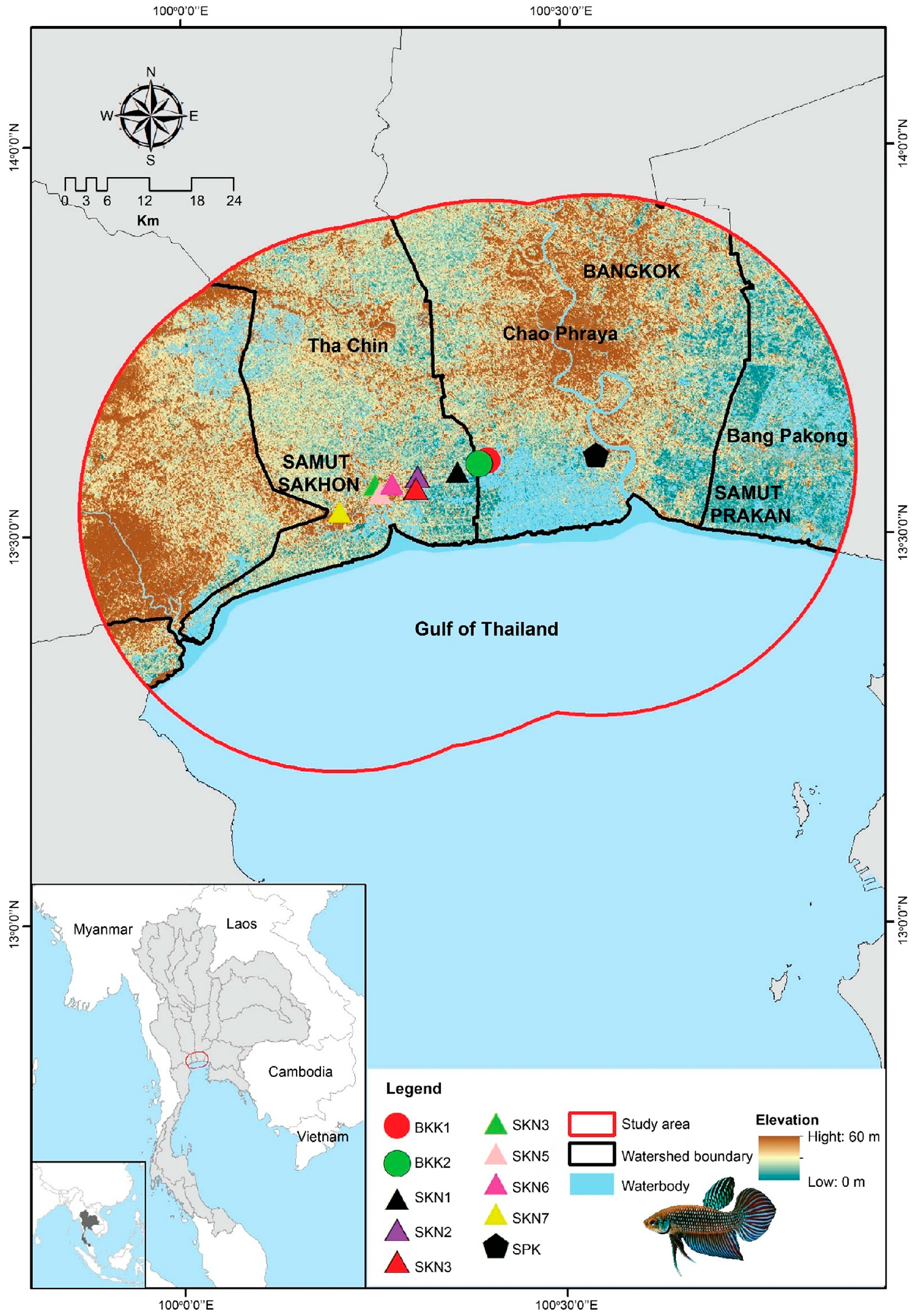
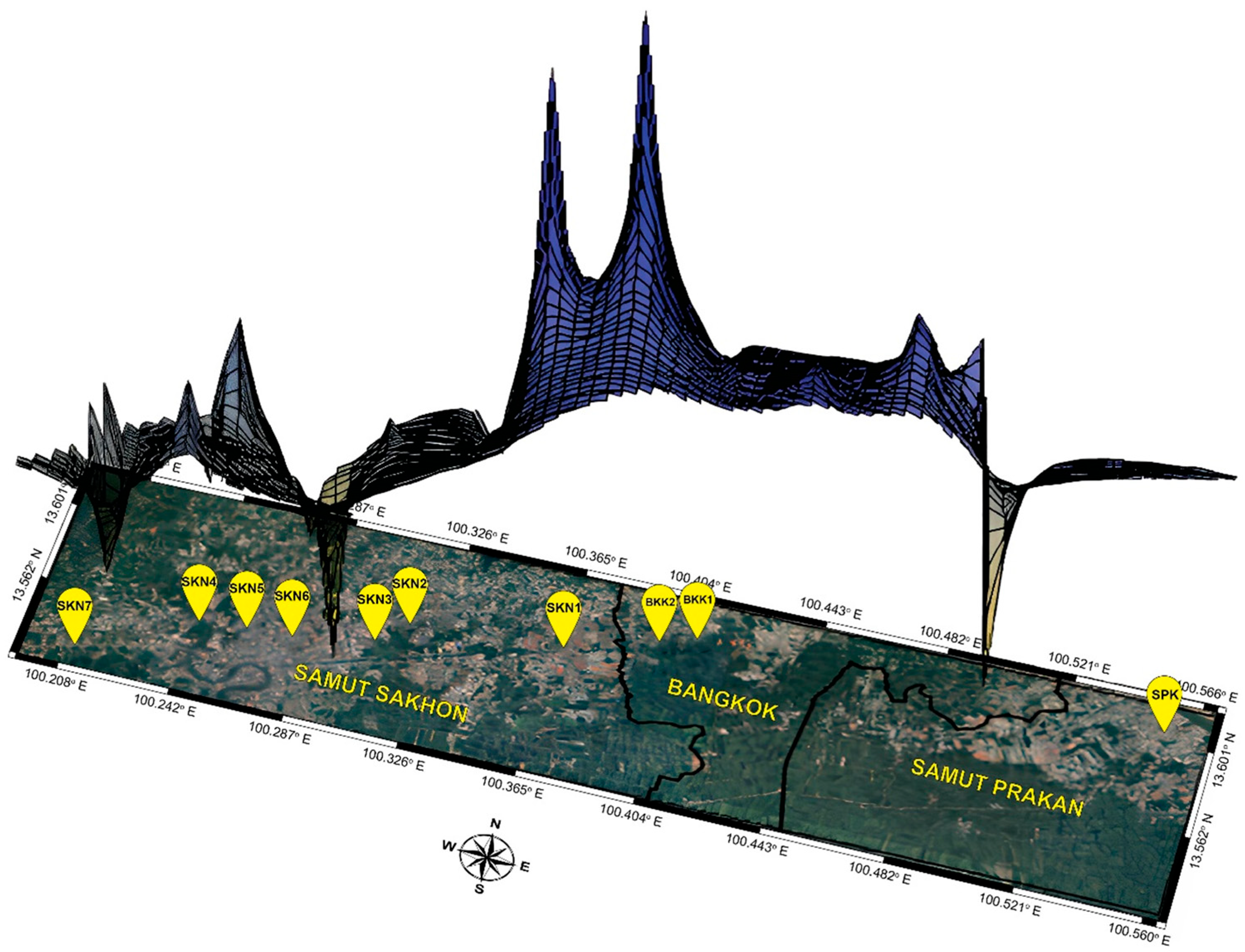
2.1. Specimen Collection and DNA Extraction
2.2. Microsatellite Genotyping and Data Analysis
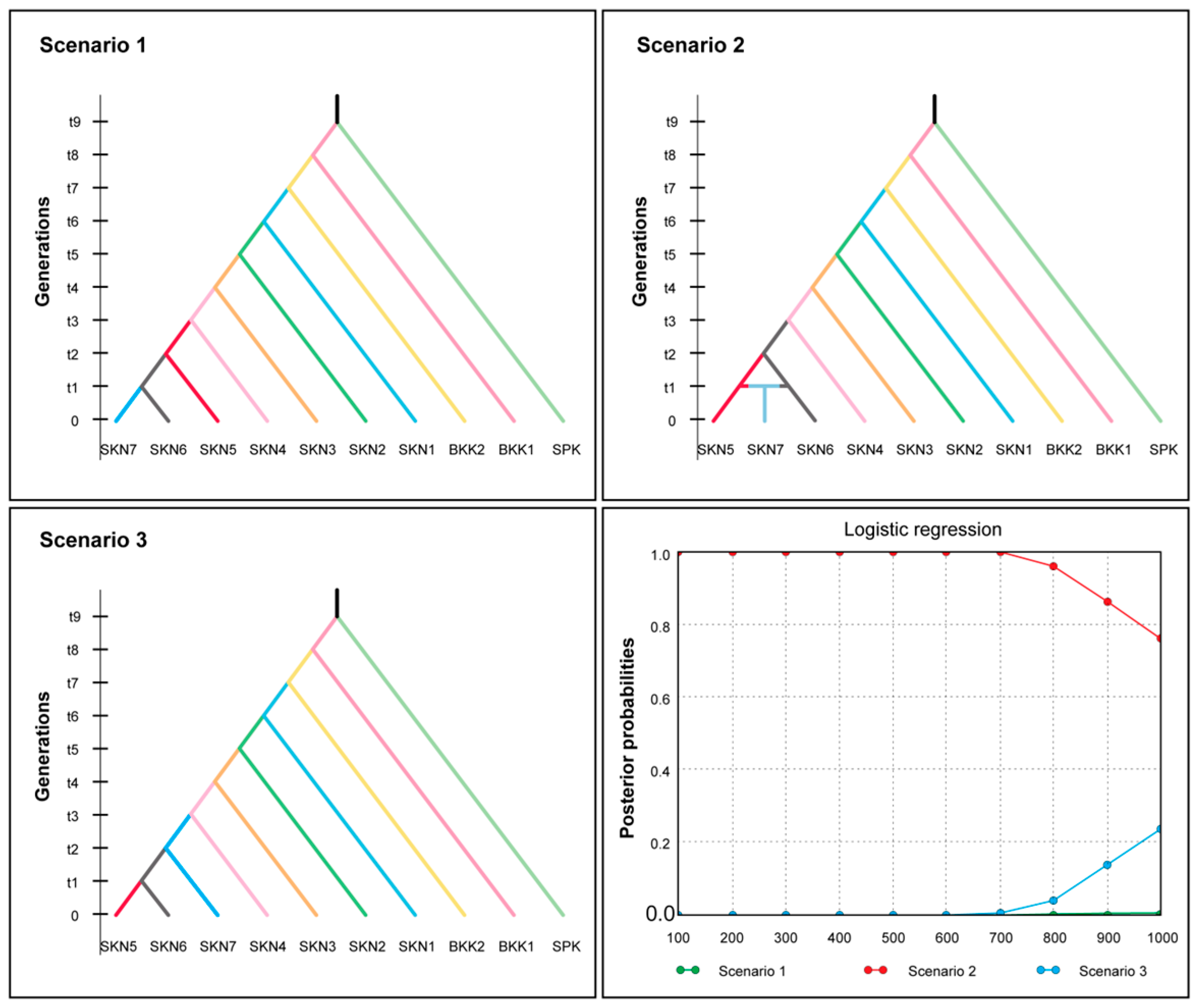
2.3. Population Genetic Structure and Demography Analyses
2.4. Analysis of Landscape Using Occurrence and Climate Data
2.5. Model Calibration Area
2.6. Ecological Niche Model
2.7. Investigating the Relationship Between Environmental Influences and Genetic Diversity
3. Results
3.1. Water Quality of Mahachai Betta Population Habitats
3.2. Assessment of the Genetic Variability of Mahachai Betta Populations
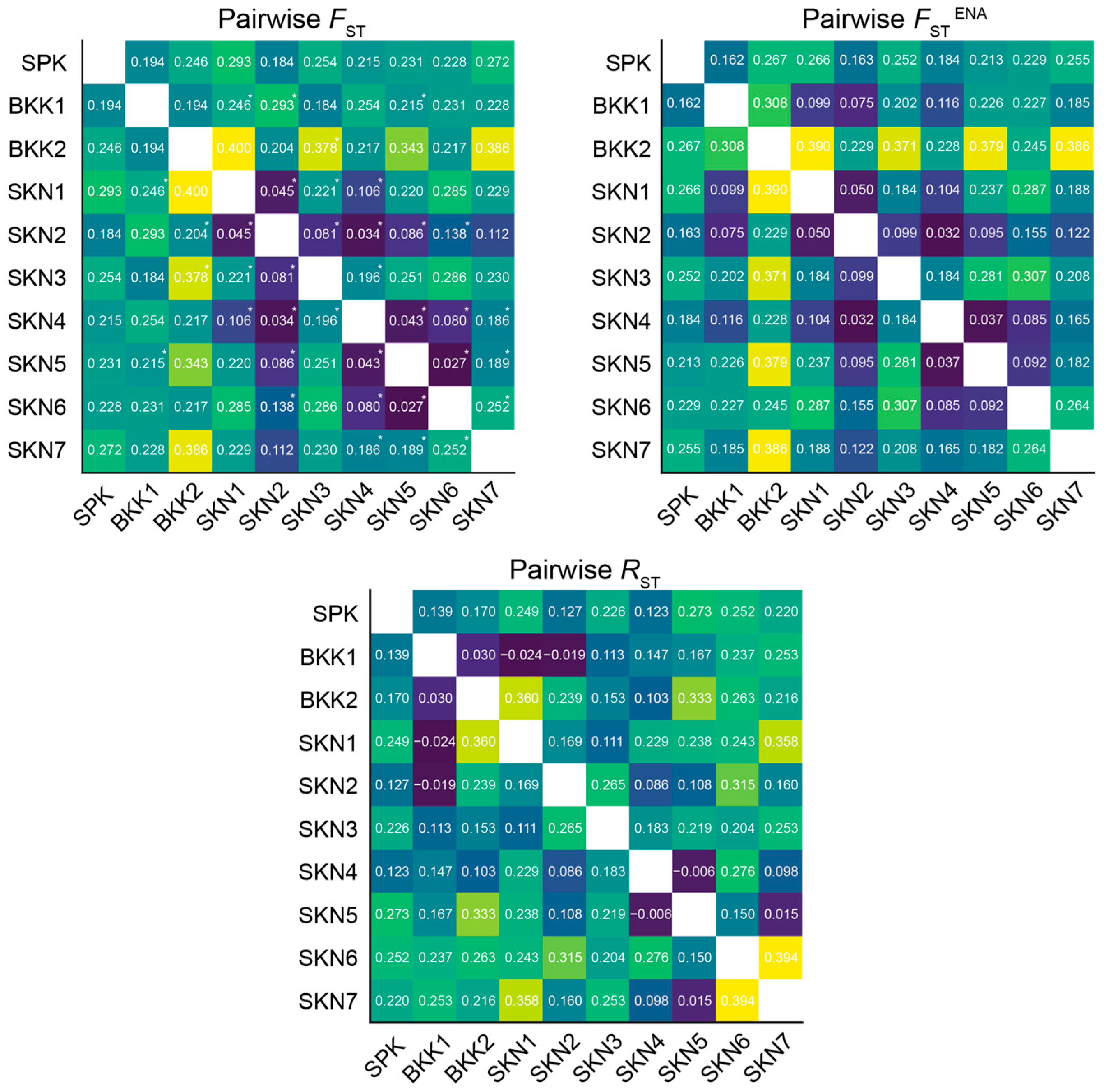
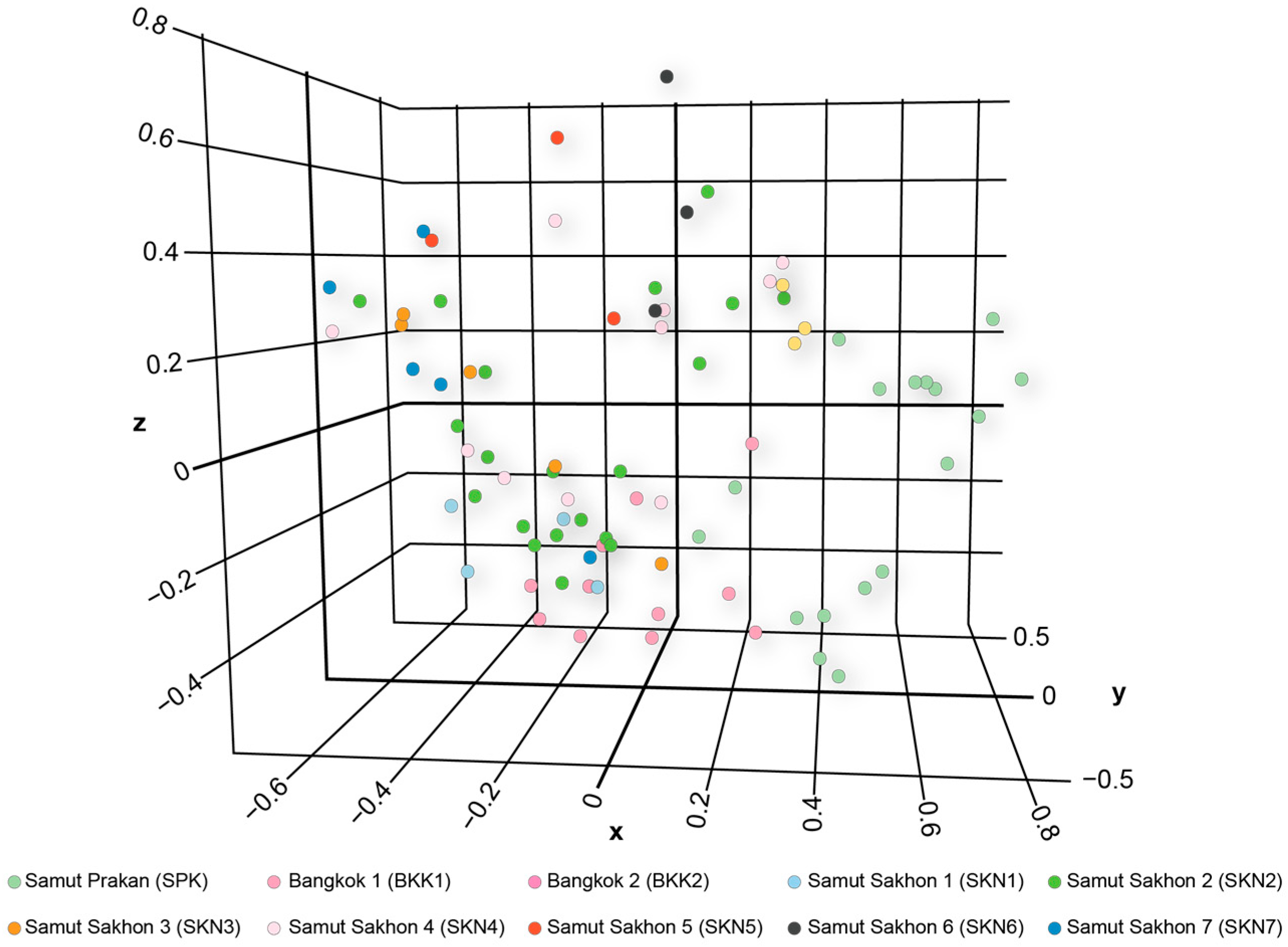
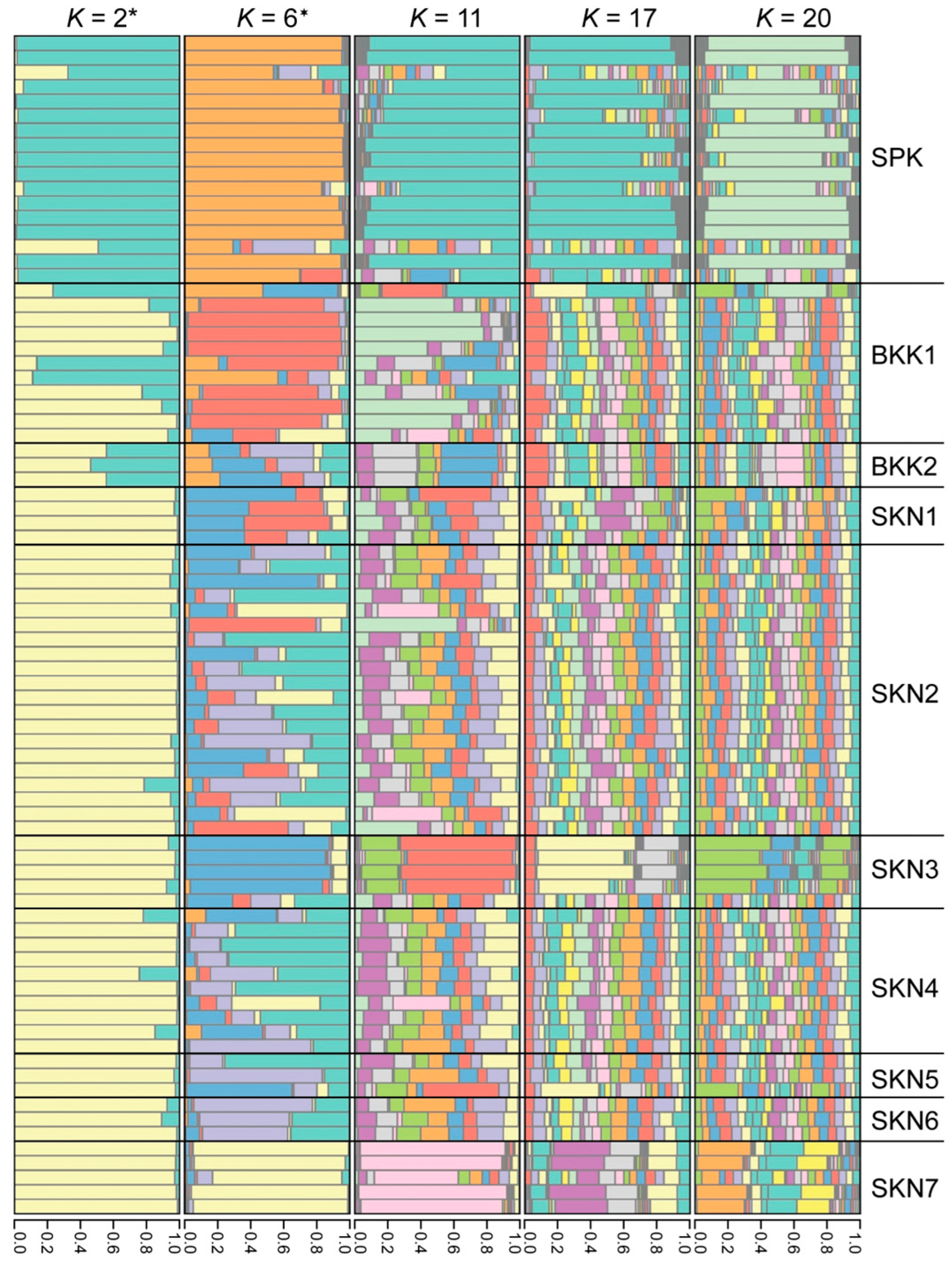
3.3. Clustering, Gene Pool Profiling, and Gene Flow
3.4. Habitat Suitability of Mahachai Betta
3.5. Genetic Diversity and Habitat Suitability of Mahachai Betta and Landscape-Level Variables
4. Discussion
4.1. Bottlenecks and Low Genetic Diversity in Mahachai Betta Populations
4.2. Urbanization and Environmental Factors Drive Genetic Differentiation in Mahachai Betta Populations
4.3. Isolated Environmental Patches: Shaping Distinct Gene Pool Patterns
4.4. Using Information to Enhance Conservation Efforts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Approximate Bayesian Computation |
| AMOVA | Analysis of Molecular Variance |
| AR | Allelic richness |
| BAM | Biotic–Abiotic–Movement framework |
| DAPC | Discriminant Analysis of Principal Components |
| DO | Dissolved Oxygen |
| ESUs | Evolutionarily Significant Units |
| F | Fixation index |
| IBD | Isolation by Distance |
| LSI | Landscape Shape Interpolation |
| MUs | Management Units |
| PCoA | Principal Coordinate Analysis |
| PIC | Polymorphic information content |
| PSU | Practical Salinity |
References
- Kowasupat, C.; Panijpan, B.; Ruenwongsa, P.; Sriwattanarothai, N. Betta mahachaiensis, a new species of bubble-nesting fighting fish (Teleostei: Osphronemidae) from Samut Sakhon Province, Thailand. Zootaxa 2012, 3522, 49–60. [Google Scholar] [CrossRef]
- Jaroensutasinee, M.; Jaroensutansinee, K. Bubble nest habitat characteristics of wild Siamese fighting fish. J. Fish. Biol. 2001, 58, 1311–1319. [Google Scholar] [CrossRef]
- Håstein, T.; Scarfe, A.D.; Lund, V.L. Science-based assessment of welfare: Aquatic animals. Rev. Sci. Tech. Off. Int. Epiz. 2005, 24, 529. [Google Scholar]
- Panijpan, B.; Sriwattanarothai, N.; Laosinchai, P. Wild Betta fighting fish species in Thailand and other Southeast Asian countries. ScienceAsia 2020, 46, 282–391. [Google Scholar] [CrossRef]
- Srikulnath, K.; Singchat, W.; Laopichienpong, N.; Ahmad, S.F.; Jehangir, M.; Subpayakom, N.; Suntronpong, A.; Jangtarwan, K.; Pongsanarm, T.; Panthum, T.; et al. Overview of the betta fish genome regarding species radiation, parental care, behavioral aggression, and pigmentation model relevant to humans. Genes Genom. 2021, 43, 91–104. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The integration of conservation, biodiversity, and sustainability. Sustainability 2019, 11, 4676. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Zohner, C.M.; Crowther, T.W.; Li, M.; Shen, F.; Guo, M.; Qin, J.; Yao, L.; Zhou, C. Direct and indirect impacts of urbanization on vegetation growth across the world’s cities. Sci. Adv. 2022, 8, eabo0095. [Google Scholar] [CrossRef]
- Baidoo, R.; Arko-Adjei, A.; Poku-Boansi, M.; Quaye-Ballard, J.A.; Somuah, D.P. Land use and land cover changes implications on biodiversity in the Owabi catchment of Atwima Nwabiagya North District, Ghana. Heliyon 2023, 9, e15238. [Google Scholar] [CrossRef]
- UN. United Nations—World Population Prospects. Department of Economic and Social Affairs Population Division. 2024. Available online: https://population.un.org/wpp/ (accessed on 30 October 2024).
- Dhakal, S.; Shrestha, A. Bangkok, Thailand. In Cities on a Finite Planet; Routledge: Abingdon, UK, 2016; pp. 63–80. [Google Scholar]
- Ministry of Interior. Statistical Profile of Bangkok Metropolis 2011; Bureau of Registration Administration, Department of Provincial Administration: Bangkok, Thailand, 2011.
- NSO. Population and Housing Census 2010. 2010. Available online: http://www.nso.go.th/ (accessed on 30 October 2024).
- Lorphensri, O.; Nettasana, T.; Ladawadee, A. Groundwater environment in Bangkok and the surrounding vicinity, Thailand. In Groundwater Environment in Asian Cities; Elsevier: Oxforf, UK, 2016; pp. 229–262. [Google Scholar]
- Schmidt, C.; Domaratzki, M.; Kinnunen, R.P.; Bowman, J.; Garroway, C.J. Continent-wide effects of urbanization on bird and mammal genetic diversity. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192497. [Google Scholar] [CrossRef]
- Buczkowski, G.; Richmond, D.S. The effect of urbanization on ant abundance and diversity: A temporal examination of factors affecting biodiversity. PLoS ONE 2012, 7, e41729. [Google Scholar] [CrossRef] [PubMed]
- del Puerto, J.M.P.; Paracampo, A.H.; García, I.D.; Maiztegui, T.; de Souza, J.R.G.; Maroñas, M.E.; Colautti, D.C. Fish assemblages and water quality in pampean streams (Argentina) along an urbanization gradient. Hydrobiologia 2021, 848, 4493–4510. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Inbreeding and extinction: A threshold effect. Conserv. Biol. 1995, 9, 792–799. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Supikamolseni, A.; Ngaoburanawit, N.; Sumontha, M.; Chanhome, L.; Suntrarachun, S.; Peyachoknagul, S.; Srikulnath, K. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet. Mol. Res. 2015, 14, 13981–13997. [Google Scholar] [CrossRef] [PubMed]
- Wattanadilokchatkun, P.; Chaiyes, A.; Ariyaraphong, N.; Wongloet, W.; Suksavate, W.; Thatukan, C.; Kumnan, N.; Panthum, T.; Thong, T.; Singchat, W.; et al. Integrative approach for landscape demography analysis of Plakad-Pa Pak-Tawan-Ok (Betta siamorientalis): Deciphering genetic and environmental factors in Eastern Thailand’s conservation efforts. Glob. Ecol. Conserv. 2024, 51, e02870. [Google Scholar] [CrossRef]
- Chailertrit, V.; Swatdipong, A.; Peyachoknagul, S.; Salaenoi, J.; Srikulnath, K. Isolation and characterization of novel microsatellite markers from Siamese fighting fish (Betta splendens, Osphronemidae, Anabantoidei) and their transferability to related species, B. smaragdina and B. imbellis. Genet. Mol. Res. 2014, 13, 7157–7162. [Google Scholar] [CrossRef]
- Wattanadilokchatkun, P.; Panthum, T.; Jaisamut, K.; Ahmad, S.F.; Dokkaew, S.; Muangmai, N.; Duengkae, P.; Singchat, W.; Srikulnath, K. Characterization of microsatellite distribution in Siamese fighting fish genome to promote conservation and genetic diversity. Fishes 2022, 7, 251. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Budi, T.; Singchat, W.; Tanglertpaibul, N.; Wongloet, W.; Chaiyes, A.; Ariyaraphong, N.; Thienpreecha, W.; Wannakan, W.; Mungmee, A.; Thong, T.; et al. Thai local chicken breeds, Chee Fah and Fah Luang, originated from Chinese black-boned chicken with introgression of red junglefowl and domestic chicken breeds. Sustainability 2023, 15, 6878. [Google Scholar] [CrossRef]
- Reddy, U.K.; Abburi, L.; Abburi, V.L.; Saminathan, T.; Cantrell, R.; Vajja, V.G.; Reddy, R.; Tomason, Y.R.; Levi, A.; Wehner, T.C.; et al. A genome-wide scan of selective sweeps and association mapping of fruit traits using microsatellite markers in watermelon. J. Hered. 2015, 106, 166–176. [Google Scholar] [CrossRef]
- Foll, M.; Gaggiotti, O.E. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Wongloet, W.; Kongthong, P.; Chaiyes, A.; Singchat, W.; Suksavate, W.; Ariyaraphong, N.; Panthum, T.; Lisachov, A.; Jaisamut, K.; Sonongbua, J.; et al. Genetic monitoring of the last captive population of greater mouse-deer on the Thai mainland and prediction of habitat suitability before reintroduction. Sustainability 2023, 15, 3112. [Google Scholar] [CrossRef]
- Kulemzina, A.I.; Yang, F.; Trifonov, V.A.; Ryder, O.A.; Ferguson-Smith, M.A.; Graphodatsky, A.S. Ferguson-Smith; and A.S. Graphodatsky. Chromosome painting in Tragulidae facilitates the reconstruction of Ruminantia ancestral karyotype. Chromosome Res. 2011, 19, 531–539. [Google Scholar] [CrossRef]
- A Wilson, G.; Rannala, B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 2003, 163, 1177–1191. [Google Scholar] [CrossRef]
- Beerli, P. Comparison of Bayesian and maximum-likelihood inference of population genetic parameters. Bioinformatics 2006, 22, 341–345. [Google Scholar] [CrossRef]
- Patta, C.; Panthum, T.; Thatukan, C.; Wongloet, W.; Chalermwong, P.; Wattanadilokchatkun, P.; Thong, T.; Srikampa, P.; Singchat, W.; Ahmad, S.F.; et al. Ahmad. Questioning inbreeding: Could outbreeding affect productivity in the North African catfish in Thailand? PLoS ONE 2024, 19, e0302584. [Google Scholar] [CrossRef]
- Miller, M. Alleles In Space (AIS): Computer software for the joint analysis of interindividual spatial and genetic information. J. Hered. 2005, 96, 722–724. [Google Scholar] [CrossRef]
- Cornuet, J.-M.; Pudlo, P.; Veyssier, J.; Dehne-Garcia, A.; Gautier, M.; Leblois, R.; Marin, J.-M.; Estoup, A. DIYABC v2. 0: A software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics 2014, 30, 1187–1189. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological niches and geographic distributions. In Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and NicheA to assure strict model transference. Geospat. Health 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Brauner, N.; Shacham, M. Role of range and precision of the independent variable in regression of data. AIChE 1998, 44, 603–611. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T., Jr. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Osorio-Olvera, L.; Jiménez-García, D. An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecol. Inform. 2019, 53, 100983. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiver. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Townsend Peterson, A.; Soberon, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Akaike, H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973, 60, 255–265. [Google Scholar] [CrossRef]
- Garza, J.C.; Williamson, E.G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 2001, 10, 305–318. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (version 1.2): A computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Tockner, K. Freshwaters: Global distribution, biodiversity, ecosystem services, and human pressures. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer: Cham, Switzerland, 2021; pp. 489–501. [Google Scholar]
- Gido, K.B.; Whitney, J.E.; Perkin, J.S.; Turner, T.F. Fragmentation, connectivity and fish species persistence in freshwater ecosystems 2. In Conservation of Freshwater Fishes; Cambridge University Press: Cambridge, UK, 2016; pp. 292–323. [Google Scholar]
- Pinto, A.V.; Hansson, B.; Patramanis, I.; Morales, H.E.; van Oosterhout, C. The impact of habitat loss and population fragmentation on genomic erosion. Conserv. Genet. 2024, 25, 49–57. [Google Scholar] [CrossRef]
- Nordstrom, S.W.; Hufbauer, R.A.; Olazcuaga, L.; Durkee, L.F.; Melbourne, B.A. How density dependence, genetic erosion and the extinction vortex impact evolutionary rescue. Proc. R. Soc. B 2023, 290, 20231228. [Google Scholar] [CrossRef]
- Bradke, D.R.; Altobelli, J.T.; Russell, A.L.; Jaeger, C.P.; Moore, J.A. Low bottleneck detection in long-lived species despite lost genetic diversity: A case study of tuatara and Eastern massasauga rattlesnakes. J. Hered. 2021, 112, 346–356. [Google Scholar] [CrossRef]
- Lichak, M.R.; Barber, J.R.; Kwon, Y.M.; Francis, K.X.; Bendesky, A. Care and use of Siamese fighting fish (Betta splendens) for research. Comp. Med. 2022, 72, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.E.; Watts, P.C.; Uusi-Heikkilä, S. The riddle of how fisheries influence genetic diversity. Fishes 2023, 8, 510. [Google Scholar] [CrossRef]
- García-Castro, K.L.; Márquez, E.J. Temporal-scale assessment of population genetics of the freshwater fish Prochilodus magdalenae in an area impacted by construction of a dam. Hydrobiologia 2024, 851, 1513–1531. [Google Scholar] [CrossRef]
- Calafell, F.; Shuster, A.; Speed, W.; Kidd, J.; Kidd, K. Short tandem repeat polymorphism evolution in humans. Eur. J. Hum. Genet. 1998, 6, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, C.; Ouyang, L.; Chen, W.; Zhao, M.; Zhang, F.; Fu, Y.; Jiang, K.; Liu, Z.; Zhang, H.; et al. Genetic diversity and population structure analysis of Lateolabrax maculatus from Chinese coastal waters using polymorphic microsatellite markers. Sci. Rep. 2021, 11, 15260. [Google Scholar] [CrossRef]
- Li, A.; Ma, M.; Li, H.; He, S.; Wang, S. Genetic Diversity and Population Differentiation of a Chinese Endangered Plant Ammopiptanthus nanus (M. Pop.) Cheng f. Genes 2023, 14, 1020. [Google Scholar] [CrossRef]
- Chinnarasri, C.; Phothiwijit, K. Appropriate engineering measures with participation of community for flood disaster reduction: Case of the Tha Chin Basin, Thailand. Arab. J. Sci. Eng. 2016, 41, 4879–4892. [Google Scholar] [CrossRef]
- Loc, H.H.; Park, E.; Chitwatkulsiri, D.; Lim, J.; Yun, S.-H.; Maneechot, L.; Phuong, D.M. Local rainfall or river overflow? Re-evaluating the cause of the Great 2011 Thailand flood. J. Hydrol. 2020, 589, 125368. [Google Scholar] [CrossRef]
- E Aguirre, W.; Reid, K.; Rivera, J.; Heins, D.C.; Veeramah, K.R.; Bell, M.A. Freshwater colonization, adaptation, and genomic divergence in threespine stickleback. Integr. Comp. Biol. 2022, 62, 388–405. [Google Scholar] [CrossRef]
- Young, S.-S.; Lin, S.-C.; Liu, M.-Y. Genetic diversity and population structure of two freshwater copepods (Copepoda: Diaptomidae), Neodiaptomus schmackeri (Poppe and Richard, 1892) and Mongolodiaptomus birulai (Rylov, 1922) from Taiwan. Diversity 2013, 5, 796–810. [Google Scholar] [CrossRef]
- Funk, W.C.; Zamudio, K.R.; Crawford, A.J. Advancing understanding of amphibian evolution, ecology, behavior, and conservation with massively parallel sequencing. In Population Genomics: Wildlife; Springer: Cham, Switzerland, 2018; pp. 211–254. [Google Scholar]
- Snead, A.A.; Tatarenkov, A.; Avise, J.C.; Taylor, D.S.; Turner, B.J.; Marson, K.; Earley, R.L. Out to sea: Ocean currents and patterns of asymmetric gene flow in an intertidal fish species. Front. Genet. 2023, 14, 1206543. [Google Scholar] [CrossRef]
- Shogren, E.H.; Sardell, J.M.; Muirhead, C.A.; Martí, E.; Cooper, E.A.; Moyle, R.G.; Presgraves, D.C.; Uy, J.A.C. Recent secondary contact, genome-wide admixture, and asymmetric introgression of neo-sex chromosomes between two Pacific island bird species. PLoS Genet. 2024, 20, e1011360. [Google Scholar] [CrossRef]
- Chankarn, A.; Boonya-aroonnet, S.; Meesuk, V.; Chitradon, R. Flow Circulation Analysis in The Mahachai and Laung Canal, Samut Sakhon Province. In Proceedings of the 14th National Convention on Civil Engineering, Nakhon Ratchasima, Thailand, 13–15 May 2009. (In Thailand). [Google Scholar]
- Mozzaquattro, L.B.; Dala-Corte, R.B.; Becker, F.G.; Melo, A.S. Effects of spatial distance, physical barriers, and habitat on a stream fish metacommunity. Hydrobiologia 2020, 847, 3039–3054. [Google Scholar] [CrossRef]
- Vega, E.; Martínez-Ramos, M.; García-Oliva, F.; Oyama, K. Influence of environmental heterogeneity and geographic distance on beta-diversity of woody communities. Plant Ecol. 2020, 221, 595–614. [Google Scholar] [CrossRef]
- Guilherme, D.R.; Pequeno, P.A.C.L.; Baccaro, F.B.; Franklin, E.; Neto, C.R.d.S.; Souza, J.L.P. Direct and indirect effects of geographic and environmental factors on ant beta diversity across Amazon basin. Oecologia 2022, 198, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Manseau, M.; Wilson, P.J. Delineating conservation units should be independent of effective population size. Trends Ecol. Evol. 2024, 39, 121–122. [Google Scholar] [CrossRef]
- Coates, D.J.; Byrne, M.; Moritz, C. Genetic diversity and conservation units: Dealing with the species-population continuum in the age of genomics. Front. Ecol. Evol. 2018, 6, 165. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Miller, A.D.; Weeks, A.R. Genetic mixing for population management: From genetic rescue to provenancing. Evol. Appl. 2021, 14, 634–652. [Google Scholar] [CrossRef] [PubMed]

| Population | N | Na | AR | Ne | I | Ho | He | F | M Ratio | PIC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPK 1 | Mean | 17.000 | 3.222 | 3.308 | 1.881 | 0.697 | 0.314 | 0.381 | 0.201 | 0.322 | 0.425 |
| SE | 0.166 | 1.575 | 0.472 | 0.223 | 0.128 | 0.091 | 0.066 | 0.134 | 0.277 | 0.217 | |
| BKK1 2 | Mean | 11.000 | 2.733 | 3.154 | 1.972 | 0.711 | 0.315 | 0.380 | 0.175 | 0.318 | 0.434 |
| SE | 0.385 | 1.340 | 0.492 | 0.281 | 0.144 | 0.094 | 0.072 | 0.154 | 0.285 | 0.245 | |
| BKK2 3 | Mean | 3.000 | 1.769 | 1.769 | 1.491 | 0.346 | 0.308 | 0.201 | −0.507 | 0.157 | 0.522 |
| SE | 0.000 | 1.120 | 0.323 | 0.212 | 0.138 | 0.122 | 0.078 | 0.084 | 0.112 | 0.281 | |
| SKN1 4 | Mean | 4.000 | 1.505 | 1.769 | 1.562 | 0.411 | 0.295 | 0.261 | −0.030 | 0.320 | 0.564 |
| SE | 0.122 | 0.693 | 0.231 | 0.179 | 0.119 | 0.113 | 0.074 | 0.202 | 0.311 | 0.131 | |
| SKN2 5 | Mean | 20.000 | 4.219 | 4.462 | 2.239 | 0.841 | 0.374 | 0.425 | 0.079 | 0.350 | 0.473 |
| SE | 0.368 | 2.514 | 0.781 | 0.445 | 0.155 | 0.092 | 0.066 | 0.147 | 0.264 | 0.207 | |
| SKN3 6 | Mean | 5.000 | 1.696 | 1.923 | 1.483 | 0.398 | 0.342 | 0.240 | −0.374 | 0.243 | 0.434 |
| SE | 0.166 | 0.718 | 0.288 | 0.159 | 0.116 | 0.116 | 0.069 | 0.152 | 0.234 | 0.207 | |
| SKN4 7 | Mean | 10.000 | 3.122 | 3.308 | 2.010 | 0.812 | 0.439 | 0.464 | 0.091 | 0.387 | 0.530 |
| SE | 0.166 | 1.319 | 0.444 | 0.147 | 0.098 | 0.097 | 0.047 | 0.165 | 0.298 | 0.102 | |
| SKN5 8 | Mean | 3.000 | 1.502 | 2.308 | 1.951 | 0.647 | 0.359 | 0.393 | 0.043 | 0.379 | 0.653 |
| SE | 0.166 | 0.331 | 0.286 | 0.224 | 0.127 | 0.116 | 0.072 | 0.205 | 0.261 | 0.210 | |
| SKN6 9 | Mean | 3.000 | 2.092 | 2.462 | 2.051 | 0.703 | 0.462 | 0.423 | −0.108 | 0.301 | 0.606 |
| SE | 0.077 | 0.710 | 0.312 | 0.267 | 0.123 | 0.110 | 0.065 | 0.176 | 0.212 | 0.179 | |
| SKN7 10 | Mean | 5.000 | 1.288 | 2.000 | 1.530 | 0.429 | 0.238 | 0.258 | 0.072 | 0.345 | 0.534 |
| SE | 0.312 | 0.277 | 0.340 | 0.161 | 0.124 | 0.076 | 0.072 | 0.109 | 0.147 | 0.103 | |
| All Population | Mean | 7.800 | 3.308 | 2.646 | 2.120 | 0.773 | 0.422 | 0.421 | 0.010 | 0.312 | 0.517 |
| SE | 0.513 | 0.190 | 0.149 | 0.092 | 0.045 | 0.034 | 0.022 | 0.051 | 0.240 | 0.188 |
| Population | Ho | He | df | t-Test | p-Value |
|---|---|---|---|---|---|
| SPK 1 | 0.314 ± 0.091 | 0.381 ± 0.066 | −0.067 | −0.596 | 0.556 |
| BKK1 2 | 0.315 ± 0.094 | 0.380 ± 0.072 | −0.065 | −0.549 | 0.590 |
| BKK2 3 | 0.308 ± 0.122 | 0.201 ± 0.078 | 0.107 | 0.739 | 0.508 |
| SKN1 4 | 0.295 ± 0.113 | 0.261 ± 0.074 | 0.034 | 0.252 | 0.811 |
| SKN2 5 | 0.374 ± 0.092 | 0.425 ± 0.066 | −0.051 | −0.450 | 0.655 |
| SKN3 6 | 0.342 ± 0.116 | 0.240 ± 0.069 | 0.102 | 0.756 | 0.476 |
| SKN4 7 | 0.439 ± 0.097 | 0.464 ± 0.047 | −0.025 | −0.232 | 0.820 |
| SKN5 8 | 0.359 ± 0.116 | 0.393 ± 0.072 | −0.034 | −0.249 | 0.818 |
| SKN6 9 | 0.462 ± 0.110 | 0.423 ± 0.065 | 0.039 | 0.305 | 0.779 |
| SKN7 10 | 0.238 ± 0.076 | 0.258 ± 0.072 | −0.020 | −0.191 | 0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.H.D.; Budi, T.; Pongsanarm, T.; Panthum, T.; Singchat, W.; Muangmai, N.; Chaiyes, A.; Suksavate, W.; Dokkaew, S.; Griffin, D.K.; et al. Saving the Mahachai Betta: Genetic Erosion and Conservation Priorities Under Urbanization Pressure. Animals 2025, 15, 2820. https://doi.org/10.3390/ani15192820
Nguyen THD, Budi T, Pongsanarm T, Panthum T, Singchat W, Muangmai N, Chaiyes A, Suksavate W, Dokkaew S, Griffin DK, et al. Saving the Mahachai Betta: Genetic Erosion and Conservation Priorities Under Urbanization Pressure. Animals. 2025; 15(19):2820. https://doi.org/10.3390/ani15192820
Chicago/Turabian StyleNguyen, Ton Huu Duc, Trifan Budi, Tavun Pongsanarm, Thitipong Panthum, Worapong Singchat, Narongrit Muangmai, Aingorn Chaiyes, Warong Suksavate, Sahabhop Dokkaew, Darren K. Griffin, and et al. 2025. "Saving the Mahachai Betta: Genetic Erosion and Conservation Priorities Under Urbanization Pressure" Animals 15, no. 19: 2820. https://doi.org/10.3390/ani15192820
APA StyleNguyen, T. H. D., Budi, T., Pongsanarm, T., Panthum, T., Singchat, W., Muangmai, N., Chaiyes, A., Suksavate, W., Dokkaew, S., Griffin, D. K., Duengkae, P., & Srikulnath, K. (2025). Saving the Mahachai Betta: Genetic Erosion and Conservation Priorities Under Urbanization Pressure. Animals, 15(19), 2820. https://doi.org/10.3390/ani15192820









