The Influence of the Dietary Lipid Level on Growth Performance, Lipid Metabolism, Oxidative Response and Hepatopancreatic Health in Macrobrachium rosenbergii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets of the Experimental Groups
2.2. Experimental Design and Management of Animals
2.3. Specimen Collection and Examination
2.4. Histological Evaluation
2.5. Stress Tests
2.6. Statistical Analysis and Calculation Formula
3. Results
3.1. Growth Performance, Feed Utilization, and the Hepatopancreas Index of M. rosenbergii
3.2. Nutritional Composition of Muscles
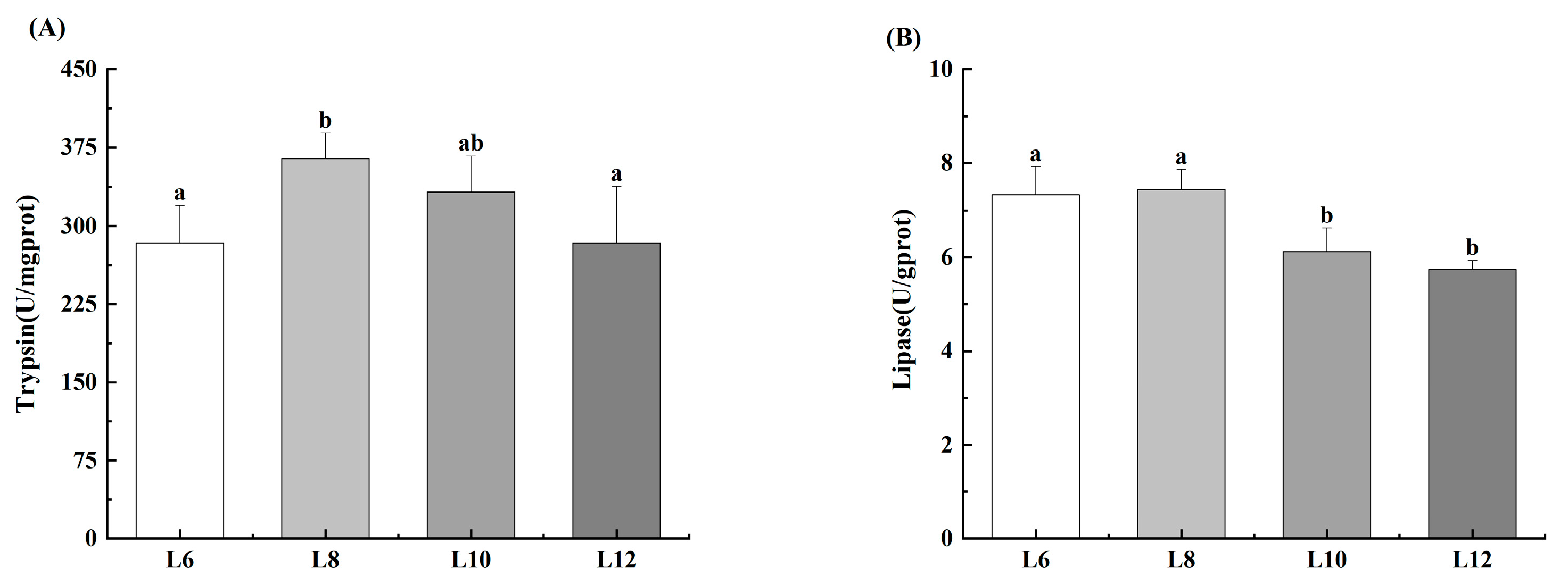
3.3. Activity of Hepatopancreatic Digestive Enzymes
3.4. Haemolymphatic Biochemical Indices
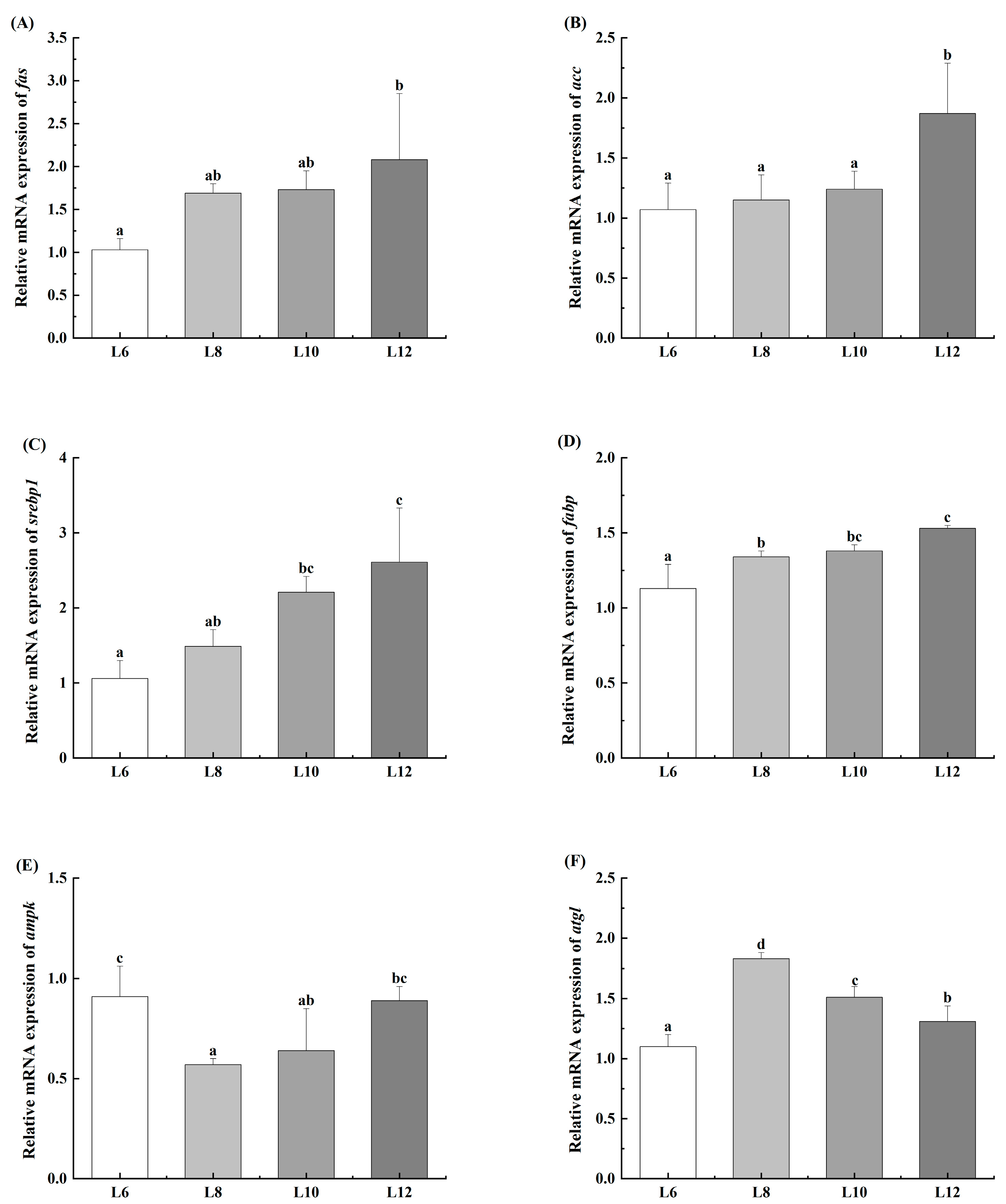
3.5. Expression of Lipid Metabolism-Associated Genes
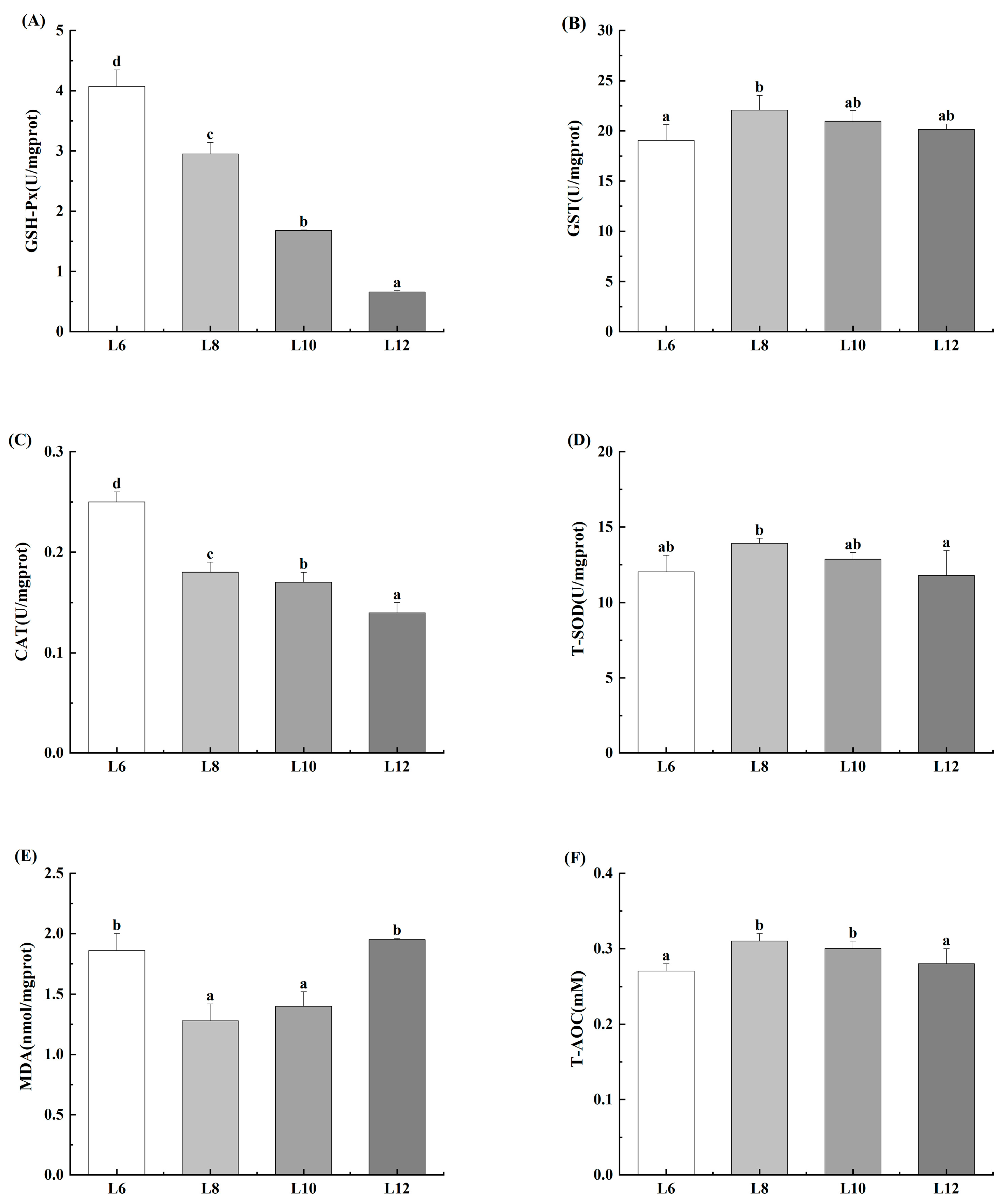
3.6. Antioxidant Capacity
3.7. Histological Examination
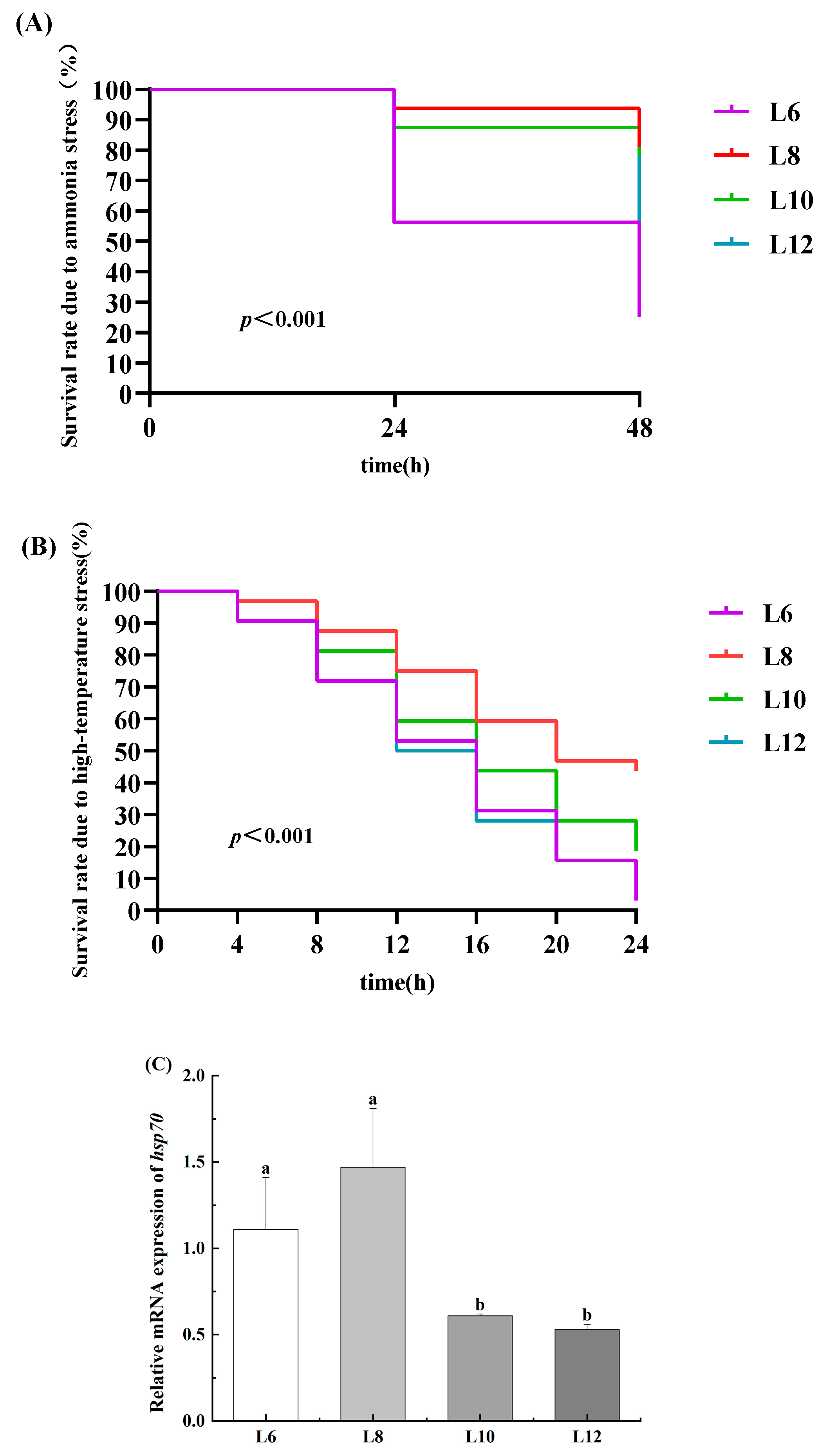
3.8. Cumulative Survival Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, S.Y.; Han, B.; Zhao, C.Z.; Hu, J.T.; Zhu, J.Y.; Zhang, X.; Sun, L.S. Effects of dietary Pediococcus acidilactici GY2 single or combined with Saccharomyces cerevisiae or/and β-glucan on the growth, innate immunity response and disease resistance of Macrobrachium rosenbergii. Fish Shellfish Immunol. 2020, 98, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Debnath, P.P.; Stentiford, G.D.; Bateman, K.S.; Salin, K.R.; Bass, D. Diseases of the giant river prawn Macrobrachium rosenbergii: A review for a growing industry. Rev. Aquac. 2023, 15, 738–758. [Google Scholar] [CrossRef]
- Ma, H.W.; Lv, M.; Lin, Y.; Chen, X.L.; Wang, D.P.; Du, X.S.; Li, J.B. Prawn (Macrobrachium rosenbergii)-plant (Hydrilla verticillata) co-culture system improves water quality, prawn production and economic benefit through stocking density and feeding regime manage. Aquac. Res. 2020, 51, 2169–2178. [Google Scholar] [CrossRef]
- Chen, C.; Tian, H.Y.; Liu, X.B.; Dai, Y.S.; Wen, X.B.; Zhao, H.H.; Wu, K. Effects of dietary lipid levels on growth, lipid metabolism, fatty acid composition and antioxidant capacity of juvenile greasyback shrimp (Metapenaeus ensis). Aquac. Rep. 2024, 36, 102146. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.B.; Lin, Y.Y.; Chen, J.M.; Karrow, N.; Ren, X.; Wang, Y. Dietary Protein and Lipid Requirements for Juvenile Largemouth Bass, Micropterus salmoides. J. World Aquacu. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Li, S.L.; Yin, J.; Zhang, H.T.; Liu, Z.K.; Chen, N.S. Effects of dietary carbohydrate and lipid levels on growth performance, feed utilization, body composition and non-specific immunity of large yellow croaker (Larimichthys crocea). Aquac. Nutr. 2019, 25, 995–1005. [Google Scholar] [CrossRef]
- Elvy, J.E.; Symonds, J.E.; Hilton, Z.; Walker, S.P.; Tremblay, L.A.; Casanovas, P.; Herbert, N.A. The relationship of feed intake, growth, nutrient retention, and oxygen consumption to feed conversion ratio of farmed saltwater Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 2022, 554, 738184. [Google Scholar] [CrossRef]
- Felix, N.; Jeyaseelan, M.J.P. Influence of dietary lipid to carbohydrate ratios on the growth and food conversion of postlarvae of Macrobrachium rosenbergii (de Man). Indian J. Fish. 2005, 52, 55–60. [Google Scholar]
- Goda, A. Effect of dietary protein and lipid levels and protein-energy ratio on growth indices, feed utilization and body composition of freshwater prawn, Macrobrachium rosenbergii (de Man 1879) post larvae. Aquac. Res. 2008, 39, 891–901. [Google Scholar] [CrossRef]
- Sun, L.S.; Wang, H.; Wei, K.; An, Z.H.; Wang, Y.X. Effects of different dietary fat levels on growth and tissue composition of Macrobrachium rosenbergii. Feed Ind. 2012, 33, 28–32. [Google Scholar]
- Hari, B.; Kurup, B.M. The Effect of Dietary Lipid Levels on the Nutrition and Growth of Juveniles of Macrobmchium rosenbergii (de Man). Fish. Technol. 2012, 43, 65–72. [Google Scholar]
- Uthairat, N.; Orapint, J. Current status & prospects of farming the giant river prawn (Macrobrachium rosenbergii de Man 1879) in Thailand. Aquac. Res. 2012, 43, 959–1063. [Google Scholar] [CrossRef]
- Zheng, X.C.; Xu, X.D.; Liu, M.Y.; Yang, J.; Yuan, M.; Sun, C.X.; Zhou, Q.L.; Chen, J.M.; Liu, B. Bile acid and short chain fatty acid metabolism of gut microbiota mediate high-fat diet induced intestinal barrier damage in Macrobrachium rosenbergii. Fish Shellfish Immunol. 2024, 146, 109376. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.M.; Lu, J.J.; Tao, X.Y.; Zhang, X.; Li, M.; Jin, M.; Sun, P.; Liu, W.J.; Jiao, L.F.; Zhou, Q.C. Vitamin D3 alleviates high-fat induced hepatopancreas lipid accumulation and inflammation by activating AMPKka/PINK1/Parkin-mediated mitophagy in Litopenaeus vannamei. Aquac. Rep. 2022, 25, 101272. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zhu, J.Y.; Hu, J.T.; Dong, X.J.; Sun, L.S.; Zhang, X.J.; Miao, S.Y. Effects of dietary Bacillus pumilus on growth performance, innate immunity and digestive enzymes of giant freshwater prawns (Macrobrachium rosenbergii). Aquac. Nutr. 2019, 25, 712–720. [Google Scholar] [CrossRef]
- Xue, H.B.; Liu, C.; Liu, Y.; Wang, W.N.; Xu, B. Roles of surface layer proteins in the regulation of Pediococcus pentosaceus on growth performance, intestinal microbiota, and resistance to Aeromonas hydrophila in the freshwater prawn Macrobrachium rosenbergii. Aquac. Int. 2021, 29, 1373–1391. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Miao, S.Y.; Hu, J.T.; Wan, W.L.; Han, B.; Zhou, Y.C.; Xin, Z.M.; Sun, L.S. Biofloc technology with addition of different carbon sources altered the antibacterial and antioxidant response in Macrobrachium rosenbergii to acute stress. Aquaculture 2020, 525, 735280. [Google Scholar] [CrossRef]
- Naksing, T.; Rattanavichai, W.; Teeka, J.; Kaewpa, D.; Borthong, J.; Areesirisuk, A. Biological activities and potential of organic banana (Musa acuminata) peel extract in enhancing the immunity of giant freshwater prawn (Macrobrachium rosenbergii). Aquac. Res. 2022, 53, 2645–2656. [Google Scholar] [CrossRef]
- Xu, J.; Deng, K.Y.; Chang, E.H.; Zhang, X.; Fu, Y.; Guo, H.Y.; Wu, Y.H.; Wang, A.R.; Deng, D.; Miao, S.Y. Effects of freshness of poultry by-product meal on the growth performance, immune response, and hepatopancreatic health of Macrobrachium rosenbergii. Aquac. Rep. 2024, 39, 102443. [Google Scholar] [CrossRef]
- Liu, X.R.; Wan, W.L.; Li, M.G.; Shi, J.Y.; Xu, J.; Zhou, Z.H.; Wang, A.R.; Miao, S.Y. Dietary Niacin Requirement of Juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquac. Nutr. 2022, 1, 8348000. [Google Scholar] [CrossRef]
- Dong, X.J.; Xue, W.; Hua, J.; Hang, Y.; Sun, L.S.; Miao, S.Y.; Wei, W.Z.; Wu, X.S.; Du, X.D. Effects of dietary betaine in allogynogenetic gibel carp (Carassius auratus gibelio): Enhanced growth, reduced lipid deposition and depressed lipogenic gene expression. Aquac. Res. 2018, 49, 1967–1972. [Google Scholar] [CrossRef]
- Bu, X.Y.; Li, Y.R.; Lai, W.C.; Yao, C.W.; Liu, Y.T.; Wang, Z.; Zhao, Z.Q.; Han, S.Z.; Du, J.L.; Yao, X.; et al. Innovation and development of the aquaculture nutrition research and feed industry in China. Rev. Aquac. 2024, 16, 759–774. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Amri, A.; Jebali, J.; Gharred, T.; Guerbej, H. Effects of dietary lipid reduction on lipid composition, fatty acid profile, plasma lipoproteins and antioxidant status in gilthead seabream (Sparus aurata). J. Appl. Aquac. 2023, 35, 878–895. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Pan, P.K.; Wu, Y.S.; Nan, F.H. Transcriptome analysis reveal the effect of freshwater sediments containing 2,3,7,8-tetrachlorodibenzo-p-dioxin on the Macrobrachium rosenbergii hepatopancreas, intestine, and muscle. Fish Shellfish Immunol. 2024, 144, 109297. [Google Scholar]
- Peng, X.; Zhong, Z.X.; Zhong, H.; Gong, J.L.; Du, T.T.; Ding, L.; Lan, X.; Tu, H.H.; Tang, Q.Y.; Xia, Z.L.; et al. Histopathological observation and comparative transcriptome analysis reveal immune response mechanisms to Aeromonas dhakensis infection in Macrobrachium rosenbergii. Fish Shellfish Immunol. 2023, 142, 109151. [Google Scholar] [CrossRef]
- Cao, H.Q.; Luo, Q.J.; Xie, S.C.; Cui, Y.H.; Zhan, W.H.; Deng, Y.; Peng, H.Y.; Tang, Z.; Tian, Y.Q.; Jin, M.; et al. Vitamin D3 alleviates the damage caused by high-fat and maintains lipid metabolism homeostasis in hepatopancreas by promoting fatty acid β-oxidation of Portunus trituberculatus. Aquac. Rep. 2024, 38, 102320. [Google Scholar] [CrossRef]
- Gou, N.N.; Jin, T.Z.; Yang, B.; Wang, K.F. Influences of dietary sodium butyrate on growth, digestion, antioxidant capacity and health in juvenile Onychostoma macrolepis fed on high-fat diet. Aquac. Rep. 2023, 33, 101808. [Google Scholar] [CrossRef]
- Liu, G.H.; Yu, H.B.; Wang, C.; Li, P.J.; Liu, S.; Zhang, X.T.; Zhang, C.; Qi, M.; Ji, H. Nano-selenium supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon Idella by reducing lipid deposition. Aquaculture 2021, 538, 736580. [Google Scholar] [CrossRef]
- Song, Y.T.; Song, L.F.; Soomro, M.A.; Dong, X.Z.; Hu, G.B. Transcriptome analysis reveals immune-related differentially expressed genes in the hepatopancreas and gills of Penaeus vannamei after Vibrio anguillarum infection. Aquac. Res. 2021, 52, 4303–4316. [Google Scholar] [CrossRef]
- Li, Y.M.; Ye, Y.C.; Yuan, H.J.; Li, S.W.; Rihan, N.; Liu, X.G.; Zhao, Y.L.; Che, X. Dietary lipid supplementation alleviated the impacts of polystyrene nanoplastic exposure in Litopenaeus vannamei. Aquat. Toxicol. 2024, 272, 106974. [Google Scholar] [CrossRef]
- Fang, H.H.; Zhuang, Z.X.; Huang, L.D.; Zhao, W.; Niu, J. Dietary Klebsormidium sp. Supplementation Improves Growth Performance, Antioxidant and Anti-Inflammatory Status, Metabolism, and Mid-Intestine Morphology of Litopenaeus Vannamei. Front. Nutr. 2022, 9, 857351. [Google Scholar] [CrossRef]
- Hou, M.N.; Pang, Y.Y.; Niu, C.; Zhang, D.X.; Zhang, Y.; Liu, Z.Q.; Song, Y.M.; Shi, A.Y.; Chen, Q.; Zhang, J.Y.; et al. Effects of Dietary L-TRP on Immunity, Antioxidant Capacity and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis) in Pond Culture. Metabolites 2023, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chu, L.L.; Du, J.L.; Nie, Z.J.; Cao, L.P.; Gao, J.C.; Xu, G.C. Oxidative stress and immune response of hepatopancreas in Chinese mitten crab Eriocheir sinensis under lipopolysaccharide challenge. Comp. Biochem. Phys. C 2023, 263, 109495. [Google Scholar] [CrossRef]
- Liu, J.; Du, H.; Liu, T.; Chen, C.; Yan, Y.; Liu, T.Q.; Liu, L.; Wang, E.R. Accurate reflection of hepatopancreas antioxidation and detoxification in Procambarus clarkii during virus infection and drug treatment: Reference gene selection, evaluation and expression analysis. Aquaculture 2022, 556, 738283. [Google Scholar] [CrossRef]
- Estrada-Cárdenas, P.; Peregrino-Uriarte, A.B.; Yepiz-Plascencia, G. Glutathione peroxidase 4 knock-down triggers ferroptosis in Penaeus vannamei hepatopancreas during hypoxia and reoxygenation. Fish Shellfish Immunol. 2023, 143, 109201. [Google Scholar] [CrossRef]
- Cai, M.L.; Qiu, X.Y.; Zhang, H.J.; Wang, A.M.; Xu, W.C.; Chen, K.J.; He, Z.G.; Hu, Y. Effects of replacing fishmeal with soybean meal on the immune and antioxidant capacity, and intestinal metabolic functions of red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2024, 149, 109600. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xie, S.C.; Cui, Y.H.; Zhan, W.H.; Deng, Y.; Peng, H.Y.; Cao, H.Q.; Tian, Y.Q.; Jin, M.; Sun, P.; et al. Vitamin C as a functional enhancer in the non-specific immune defense, antioxidant capacity and resistance to low-temperature stress of juvenile mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2024, 153, 109834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Shi, X.Y.; Liu, J.S.; Jiang, Y.; Wu, Y.P.; Xu, Y.L.; Yang, C.M. The effects of bamboo leaf flavonoids on growth performance, immunity, antioxidant status, and intestinal microflora of Chinese mitten crabs (Eriocheir sinensis). Anim. Feed Sci. Tech. 2022, 288, 115297. [Google Scholar] [CrossRef]
- Ortiz, C.; Müller, L.; Borges, L.; Pinto, L.A.D.; Cadaval, T.R.S., Jr.; Tesser, M.B.; Pedrosa, V.F.; Romano, L.A.; Wasielesky, W.; Ventura-Lima, J. The use of chitosan as an antioxidant in the feed of cultivated P. vannamei shrimp against oxidative stress induced by exposure to microplastics. Mar. Environ. Res. 2024, 202, 106747. [Google Scholar] [CrossRef]
- Lu, Y.P.; Zhang, X.X.; Zheng, P.H.; Zhang, Z.L.; Li, J.T.; Wang, D.M.; Xian, J.A.; Wang, A.L.; Wang, L. Effects of air exposure on survival, histological structure, non-specific immunity and gene expression of red claw crayfish (Cherax quadricarinatus). Aquac. Rep. 2021, 21, 100898. [Google Scholar] [CrossRef]
- Xie, X.D.; Cao, M.X.; Chen, Q.; Yu, M.L.; Liu, Q.Y.; Zhao, Y.Z.; Zhang, L.; Hu, T.J. Effect of medical herbs in Tian-Dong-Tang-Gan powder on the oxidative stress induced by ammonia and nitrite in Litopenaeus vannamei. Aquaculture 2022, 548, 737584. [Google Scholar] [CrossRef]
- Zhuo, H.B.; Lin, L.T.; Zhang, Y.; Fu, S.; Li, J.Y.; Zhou, X.X.; Wu, G.B.; Guo, C.A.; Liu, J.Y. The shrimp NF-κB pathway is activated to mediate the antioxidant defense and apoptosis under ammonia-N stress. Fish Shellfish Immunol. 2025, 161, 110295. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Ding, Z.L.; Kong, Y.Q.; Zhang, R.F.; Zhang, Y.X.; Wu, C.L.; Jiang, Z.Q.; Ye, J.Y. An evaluation of increasing linolenic acid level in the diet of Macrobrachium nipponense: Lipid deposition, fatty acid composition and expression of lipid metabolism-related genes. Aquac. Nutr. 2018, 24, 758–767. [Google Scholar] [CrossRef]
- Araújo, B.C.; Flores-Galvez, K.; Honji, R.M.; Barbosa, V.M.; Viana, M.T.; Tinajero, A.; Mata-Sotres, J.A. Arachidonic acid effects on the overall performance, fatty acid profile, hepatopancreas morphology and lipid-relevant genes in Litopenaeus vannamei juveniles. Aquaculture 2020, 523, 735207. [Google Scholar] [CrossRef]
- Liu, H.L.; Feng, Y.; Yang, M.; Huang, Y.; Li, M.H.; Geng, Y.; Ouyang, P.; Chen, D.F.; Yang, S.Y.; Yin, L.Z.; et al. Starvation induces hepatopancreas atrophy in Chinese mitten crab (Eriocheir sinensis) by inhibiting angiogenesis. Bmc Genom. 2023, 24, 612. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Carmona, R.; Merino, R.; García-Gallego, M.; Furné, M.; Domezain, A.; Sanz, A. Effect of dietary lipid content and stocking density on digestive enzymes profile and intestinal histology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 10–16. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Mir, I.N.; Srivastava, P.P.; Bhat, I.A.; Dar, J.Y.; Agarwal, D. Expression profiling of lipolytic and long-chain fatty acid biosynthesis genes in catfish larvae fed with graded lipid levels. Front. Mar. Sci. 2020, 7, 561402. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Zeng, L.W.; Cai, Y.N.; Zhu, Y.; Ma, Q.Y.; Shen, O.X.; Song, X.Y.; Zhang, J. Carbon dots induce endoplasmic reticulum stress-mediated lipid dysregulation and embryonic developmental toxicity in zebrafish. Ecotoxicol. Environ. Saf. 2024, 288, 117361. [Google Scholar] [CrossRef]
- Michael, S.M.; Jimena, R.; Jennifer, L.W. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Liu, Y.; Han, S.L.; Luo, Y.; Li, L.Y.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Impaired peroxisomal fat oxidation induces hepatic lipid accumulation and oxidative damage in Nile tilapia. Fish Physiol. Biochem. 2020, 46, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Jessie, L.B.; Manabu, T.N.; David, W.L.M. Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostag. Leukotr. Ess. 2018, 135, 1–4. [Google Scholar]
- Ma, D.F.; Li, Q.W.; Xie, Y.Y.; Kong, Y.Q.; Ding, Z.L.; Ye, J.Y.; Wu, C.L.; Liu, Y. Dietary Erucic Acid Induces Fat Accumulation, Hepatic Oxidative Damage, and Abnormal Lipid Metabolism in Nile Tilapia (Oreochromis niloticus). Aquac. Nutr. 2024, 1, 6670740. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, M.; Gong, M.; Zheng, X.; Zheng, Q.; Li, X.Y.; Fu, F.D.; Chen, Y.Y.; Cheng, J.Q.; Rao, Z.Y.; et al. Comparison of the Effects of Monounsaturated Fatty Acids and Polyunsaturated Fatty Acids on Liver Lipid Disorders in Obese Mice. Nutrients 2023, 15, 3200. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (g/100 g) | Groups | |||
|---|---|---|---|---|
| L6 (6%) | L8 (8%) | L10 (10%) | L12 (12%) | |
| Soybean meal-43% | 23.00 | 23.00 | 23.00 | 23.00 |
| Fish meal-Imported Japanese grade-70% | 16.00 | 16.00 | 16.00 | 16.00 |
| Fish meal-Domestic semi-skimmed-65% | 12.00 | 12.00 | 12.00 | 12.00 |
| Shrimp meal-61% | 7.50 | 7.50 | 7.50 | 7.50 |
| Peanut meal-53% | 5.00 | 5.00 | 5.00 | 5.00 |
| Poultry by-product meal-65% | 2.00 | 2.00 | 2.00 | 2.00 |
| Squid paste | 2.00 | 2.00 | 2.00 | 2.00 |
| Wheat flour | 19.00 | 19.00 | 19.00 | 19.00 |
| Shrimp paste | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean lecithin | 2.50 | 2.50 | 2.50 | 2.50 |
| Ca(H2PO4)2 | 1.50 | 1.50 | 1.50 | 1.50 |
| Soybean oil | 0.00 | 1.00 | 2.00 | 3.00 |
| Fish oil | 0.00 | 1.00 | 2.00 | 3.00 |
| Choline chloride-60% | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin premix 1 | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix 2 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin C—phosphatidic | 0.10 | 0.10 | 0.10 | 0.10 |
| Organic acid | 0.05 | 0.05 | 0.05 | 0.05 |
| Bentonite | 6.50 | 4.50 | 2.50 | 0.50 |
| Guar gum | 0.20 | 0.20 | 0.20 | 0.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (% dry matter) | ||||
| Moisture | 9.73 | 9.29 | 9.44 | 9.59 |
| Crude protein | 42.81 | 43.19 | 43.06 | 43.00 |
| Crude lipid | 5.94 | 8.06 | 9.97 | 12.14 |
| Ash content | 16.00 | 14.14 | 12.24 | 10.52 |
| Content (g/kg) | L6 | L8 | L10 | L12 |
|---|---|---|---|---|
| C14:0 | 2.25 | 2.98 | 3.71 | 4.44 |
| C16:0 | 10.05 | 12.88 | 15.71 | 18.54 |
| C16:1 | 2.41 | 3.41 | 4.41 | 5.41 |
| C18:0 | 2.41 | 3.18 | 3.95 | 4.72 |
| C18:1 | 12.02 | 15.39 | 18.76 | 22.13 |
| C18:2 | 18.39 | 23.81 | 29.23 | 34.65 |
| C18:3 | 3.06 | 3.88 | 4.70 | 5.52 |
| C20:1 | 1.73 | 3.26 | 4.79 | 6.32 |
| C20:4 | 0.77 | 0.80 | 0.83 | 0.86 |
| C22:1 | 1.95 | 4.18 | 6.41 | 8.64 |
| C22:5 | 0.67 | 0.75 | 0.83 | 0.91 |
| C22:6 | 2.47 | 3.32 | 4.17 | 5.02 |
| Sum | 58.17 | 77.83 | 97.49 | 117.15 |
| Primer Name | Position | Primer Sequence | Length |
|---|---|---|---|
| fas | F (5′-3′) | TCACTTCTCAACACCCAATCCA | 22 |
| R (5′-3′) | TTGCAGACCGAAGAAGGACG | 20 | |
| acc | F (5′-3′) | GATGAGGGATTCAAGCCCAGTT | 22 |
| R (5′-3′) | TCCCTGTCTTCACCCCACGA | 20 | |
| srebp-1 | F (5′-3′) | CAGACACTGGCCGAGATGTG | 20 |
| R (5′-3′) | GAGCTGGAGCATGTCTTCGAT | 21 | |
| fabp | F (5′-3′) | AACGACGAATGGACGCTGAA | 20 |
| R (5′-3′) | TTCCCTTAGTGGCGTTCTGG | 20 | |
| ampk | F (5′-3′) | TGGAAAGTGAGCATTGACGAAG | 22 |
| R (5′-3′) | CATTGGGGTCACGCAACAGA | 20 | |
| atgl | F (5′-3′) | TTGTATCGCTTTGCCCGTAT | 20 |
| R (5′-3′) | CAAAGGGTAAGACATAAGGCAAT | 23 | |
| hsp70 | F (5′-3′) | TGACAAGGGTCGCCTCAGTA | 20 |
| R (5′-3′) | CATTATCTTGTTGCGATCCTC | 21 | |
| β-actin | F (5′-3′) | TCCGTAAGGACCTGTATGCC | 20 |
| R (5′-3′) | TCGGGAGGTGCGATGATTTT | 20 |
| Treatment | Diets (Lipid Level %) | |||
|---|---|---|---|---|
| L6 (6%) | L8 (8%) | L10 (10%) | L12 (12%) | |
| IW (g) | 0.85 ± 0.01 | 0.85 ± 0.01 | 0.86 ± 0.01 | 0.86 ± 0.01 |
| FW (g) | 11.31 ± 0.40 a | 12.67 ± 0.32 b | 12.42 ± 0.17 b | 12.16 ± 0.49 b |
| WGR (%) | 1230.63 ± 48.75 a | 1386.03 ± 30.61 c | 1340.59 ± 19.62 bc | 1306.79 ± 56.87 b |
| SGR (%/d) | 4.17 ± 0.06 a | 4.35 ± 0.03 c | 4.30 ± 0.02 bc | 4.26 ± 0.06 b |
| HSI (%) | 3.46 ± 0.23 | 2.92 ± 0.36 | 2.93 ± 0.58 | 3.29 ± 0.45 |
| FCR | 2.82 ± 0.15 b | 2.55 ± 0.11 a | 2.58 ± 0.07 a | 2.59 ± 0.17 a |
| CF (%) | 11.45 ± 0.34 a | 12.29 ± 0.24 b | 12.14 ± 0.19 b | 11.96 ± 0.39 b |
| L6 (6%) | L8 (8%) | L10 (10%) | L12 (12%) | |
|---|---|---|---|---|
| Moisture (%) | 77.45 ± 0.36 | 77.53 ± 0.24 | 77.46 ± 0.74 | 77.36 ± 0.55 |
| Crude protein (%) | 21.04 ± 0.06 | 21.30 ± 0.24 | 21.38 ± 0.49 | 21.24 ± 0.27 |
| Crude lipid (%) | 0.83 ± 0.01 | 0.87 ± 0.04 | 0.85 ± 0.03 | 0.84 ± 0.03 |
| Ash (%) | 1.38 ± 0.06 | 1.43 ± 0.08 | 1.42 ± 0.05 | 1.41 ± 0.07 |
| L6 (6%) | L8 (8%) | L10 (10%) | L12 (12%) | |
|---|---|---|---|---|
| 24 h mortality rate (%) | 43.75 ± 7.22 b | 6.25 ± 7.22 a | 12.50 ± 0.00 a | 6.25 ± 7.22 a |
| 48 h mortality rate (%) | 75.00 ± 10.21 c | 18.75 ± 7.22 a | 21.88 ± 6.25 a | 50.00 ± 17.68 b |
| L6 (6%) | L8 (8%) | L10 (10%) | L12 (12%) | |
|---|---|---|---|---|
| 4 h mortality rate (%) | 9.38 ± 6.25 | 3.13 ± 6.25 | 3.13 ± 6.25 | 9.38 ± 6.25 |
| 8 h mortality rate (%) | 28.13 ± 6.25 b | 12.50 ± 0.00 a | 18.75 ± 7.22 a | 28.13 ± 6.25 b |
| 12 h mortality rate (%) | 46.88 ± 6.25 b | 25.00 ± 10.21 a | 40.63 ± 6.25 b | 50.00 ± 10.21 b |
| 16 h mortality rate (%) | 68.75 ± 7.22 b | 40.63 ± 11.97 a | 56.25 ± 7.22 b | 71.88 ± 6.25 c |
| 20 h mortality rate (%) | 84.38 ± 6.25 c | 53.13 ± 6.25 a | 71.88 ± 6.25 b | 84.38 ± 6.25 c |
| 24 h mortality rate (%) | 96.88 ± 6.25 c | 56.25 ± 7.22 a | 81.25 ± 7.22 b | 93.75 ± 7.22 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Xu, J.; Deng, K.; Wang, A.; Huang, C.; Zhang, M.; Deng, D.; Chen, H.; Miao, S. The Influence of the Dietary Lipid Level on Growth Performance, Lipid Metabolism, Oxidative Response and Hepatopancreatic Health in Macrobrachium rosenbergii. Animals 2025, 15, 2818. https://doi.org/10.3390/ani15192818
Guo H, Xu J, Deng K, Wang A, Huang C, Zhang M, Deng D, Chen H, Miao S. The Influence of the Dietary Lipid Level on Growth Performance, Lipid Metabolism, Oxidative Response and Hepatopancreatic Health in Macrobrachium rosenbergii. Animals. 2025; 15(19):2818. https://doi.org/10.3390/ani15192818
Chicago/Turabian StyleGuo, Haoyue, Jie Xu, Kangyu Deng, Anran Wang, Chungui Huang, Min Zhang, Deng Deng, Huangen Chen, and Shuyan Miao. 2025. "The Influence of the Dietary Lipid Level on Growth Performance, Lipid Metabolism, Oxidative Response and Hepatopancreatic Health in Macrobrachium rosenbergii" Animals 15, no. 19: 2818. https://doi.org/10.3390/ani15192818
APA StyleGuo, H., Xu, J., Deng, K., Wang, A., Huang, C., Zhang, M., Deng, D., Chen, H., & Miao, S. (2025). The Influence of the Dietary Lipid Level on Growth Performance, Lipid Metabolism, Oxidative Response and Hepatopancreatic Health in Macrobrachium rosenbergii. Animals, 15(19), 2818. https://doi.org/10.3390/ani15192818





