Mitoquinone Can Effectively Improve the Quality of Thawed Boar Sperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Preparation of Reagents and Extenders
2.3. Semen Collection and Processing
2.3.1. Sperm Collection
2.3.2. Sperm Cryopreservation and Thawing Process
2.4. Sperm Quality Test
2.4.1. Detection of Sperm Quality and Kinetic Parameters
2.4.2. Detection of Sperm Acrosome Integrity and Plasma Membrane Integrity
2.4.3. Detection of Sperm Mitochondrial Activity and DNA Integrity
2.5. Detection of Sperm Antioxidant Capacity
2.6. Detection of Apoptotic Proteins in Thawed Sperm by Western Blotting
2.7. Sperm Penetration Experiment
2.8. Statistical Analysis
3. Results
3.1. The Influence of MitoQ on the Post-Thawing Kinetic Parameters
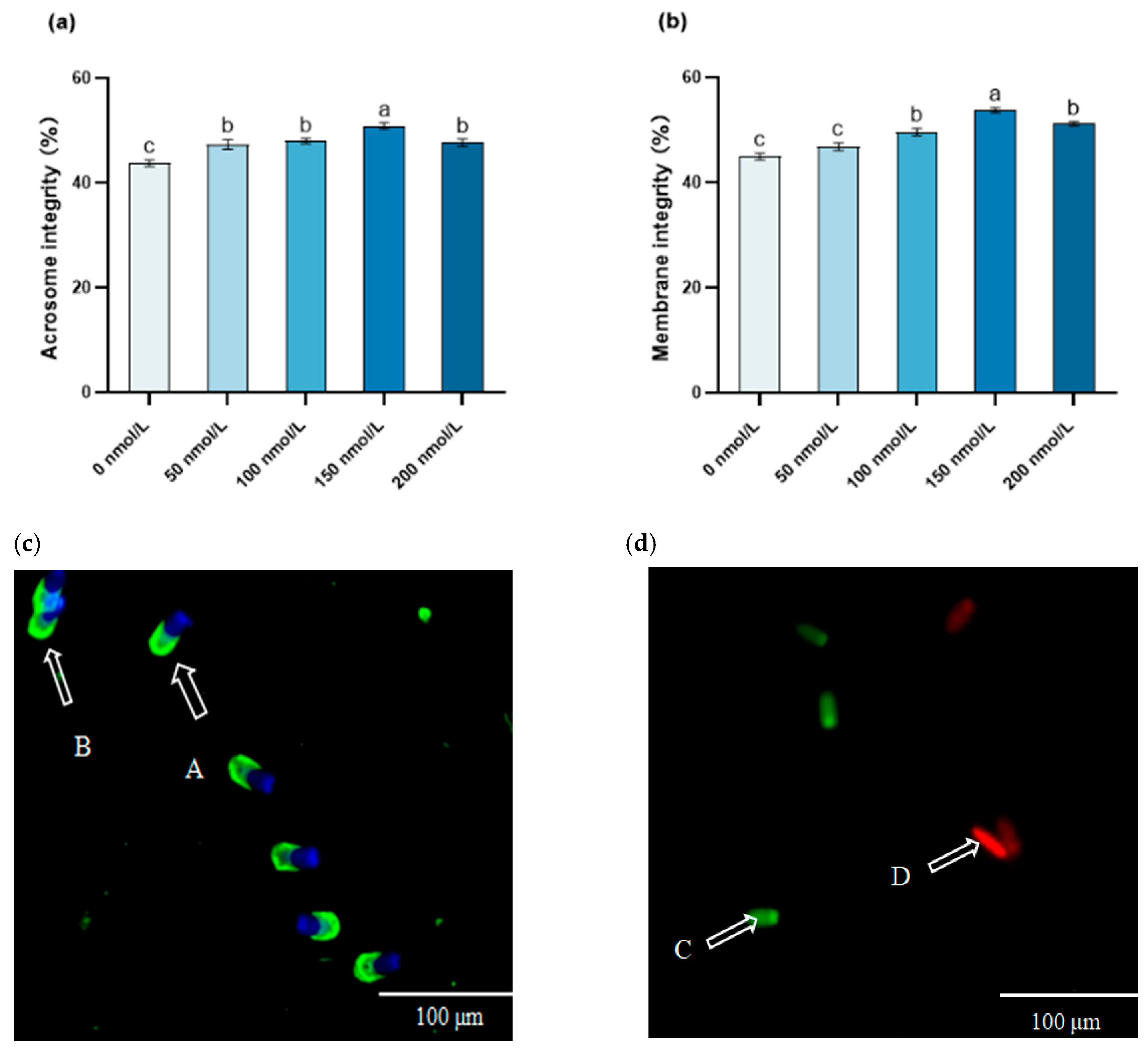
3.2. The Influence of MitoQ on the Post-Thawing Acrosome Integrity and Membrane Integrity
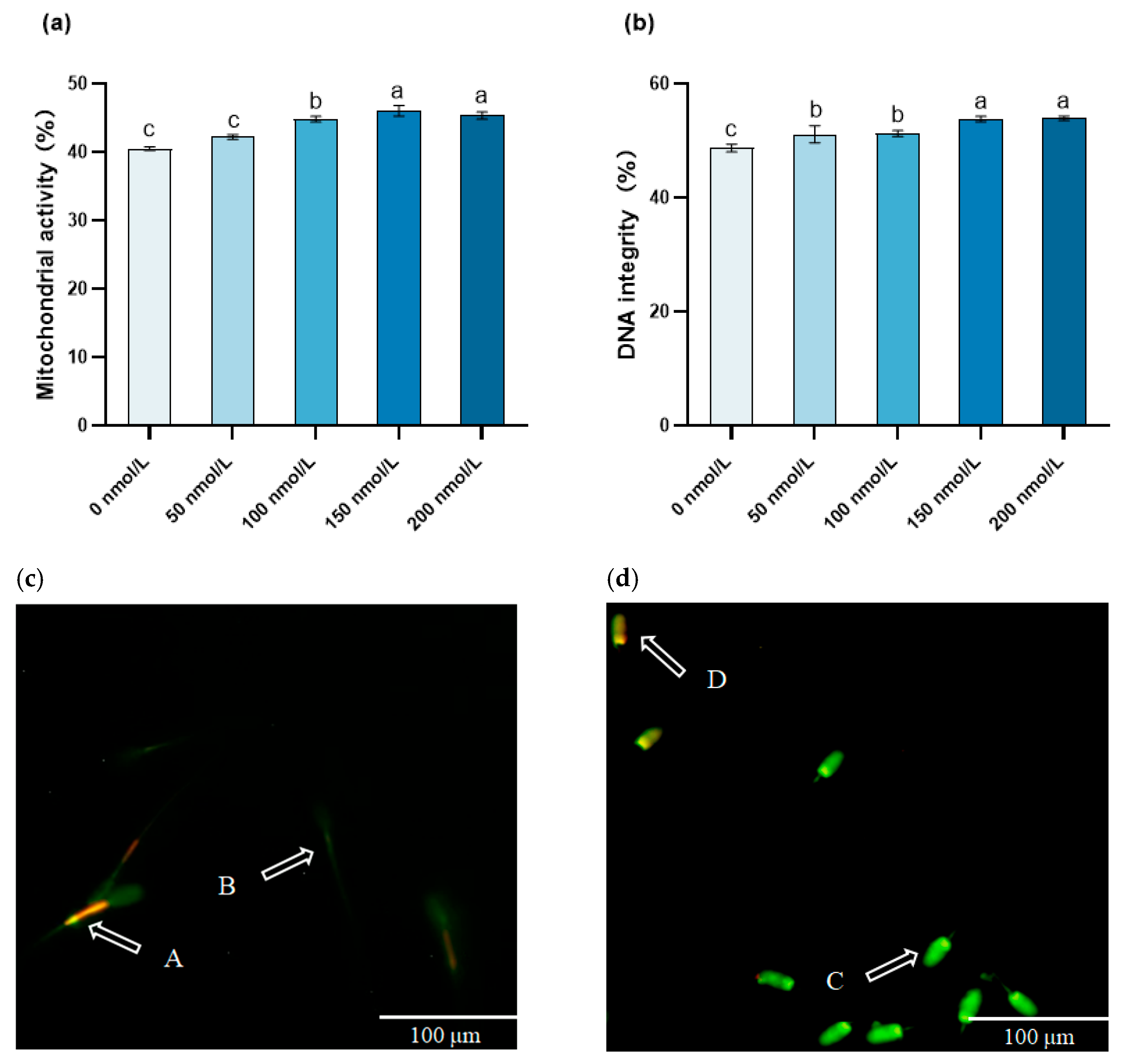
3.3. The Influence of MitoQ on the Post-Thawing Mitochondrial Activity and DNA Integrity
3.4. The Influence of MitoQ on the Post-Thawing Antioxidant Enzyme Activity
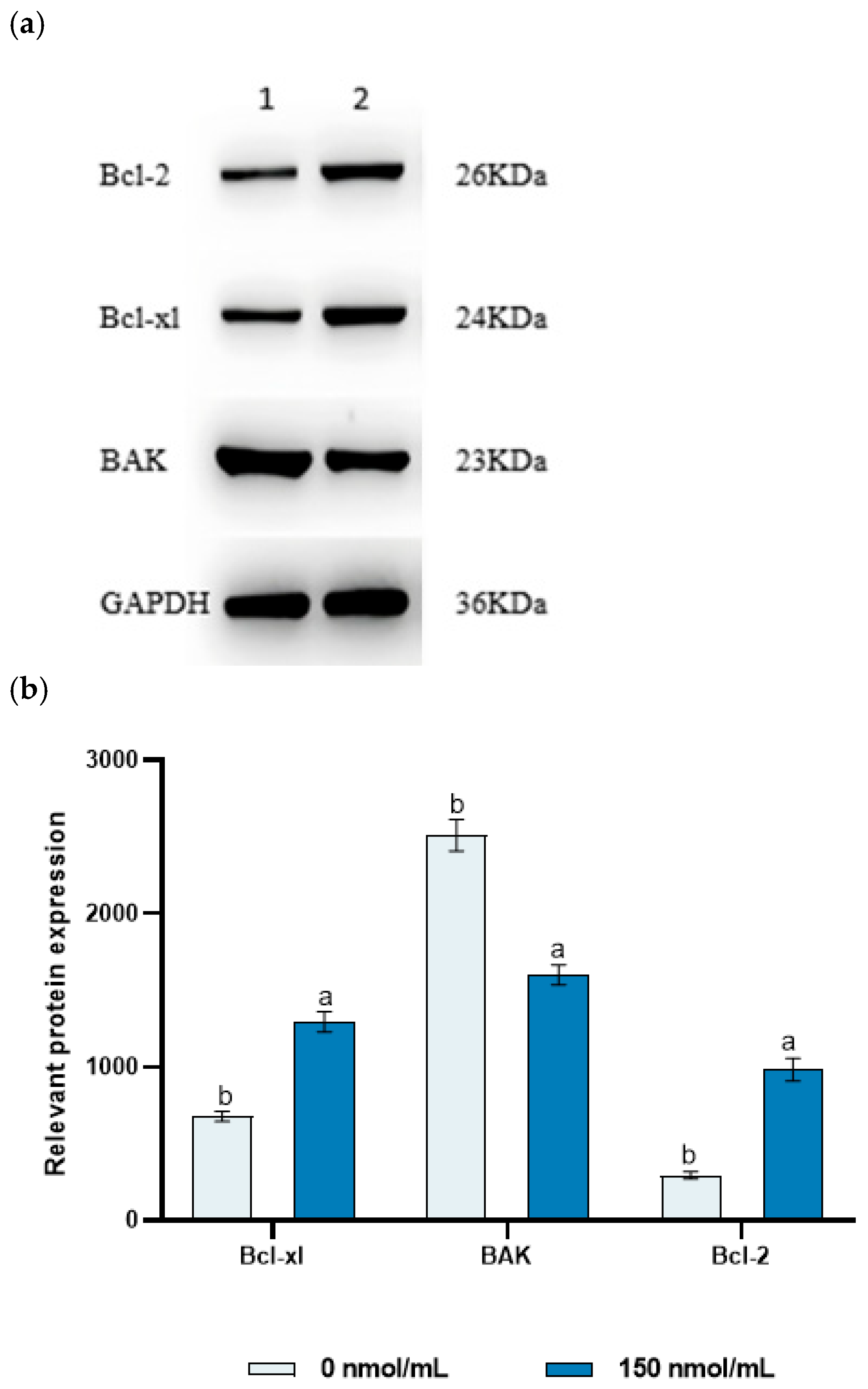
3.5. The Effect of MitoQ on Sperm Protein Expression
3.6. The Effect of MitoQ on the Sperm Adhesion Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, M.L.; Van Eenennaam, A.L. Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agric. Biosci. 2022, 3, 13. [Google Scholar] [CrossRef]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserves sperm quality during cryopreservation. Theriogenology 2022, 179, 22–319. [Google Scholar] [CrossRef] [PubMed]
- Petrone, O.; Serafini, S.; Yu, B.Y.K.; Filonenko, V.; Gout, I.; O’Flaherty, C. Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa. Int. J. Mol. Sci. 2023, 24, 12526. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Devenish, R.J.; Prescott, M.; Rodgers, A.J. The structure and function of mitochondrial F1F0-ATP synthases. Int. Rev. Cell Mol. Biol. 2008, 267, 1–58. [Google Scholar]
- Lee, S.; Tak, E.; Lee, J.; Rashid, M.A.; Murphy, M.P.; Ha, J.; Kim, S.S. Mitochondrial H2O2 generated from electron transport chain complex I stimulate muscle differentiation. Cell Res. 2011, 21, 817–834. [Google Scholar] [CrossRef]
- Gasparre, G.; Porcelli, A.M.; Lenaz, G.; Romeo, G. Relevance of mitochondrial genetics and metabolism in cancer development. Cold Spring Harb. Perspect. Biol. 2013, 5, a011411. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Mateo-Otero, Y.; Delgado-Bermúdez, A.; Bucci, D.; Tamanini, C.; Yeste, M.; Barranco, I. Role of exogenous antioxidants on the performance and function of pig sperm after preservation in liquid and frozen states: A systematic review. Theriogenology 2021, 173, 279–294. [Google Scholar] [CrossRef]
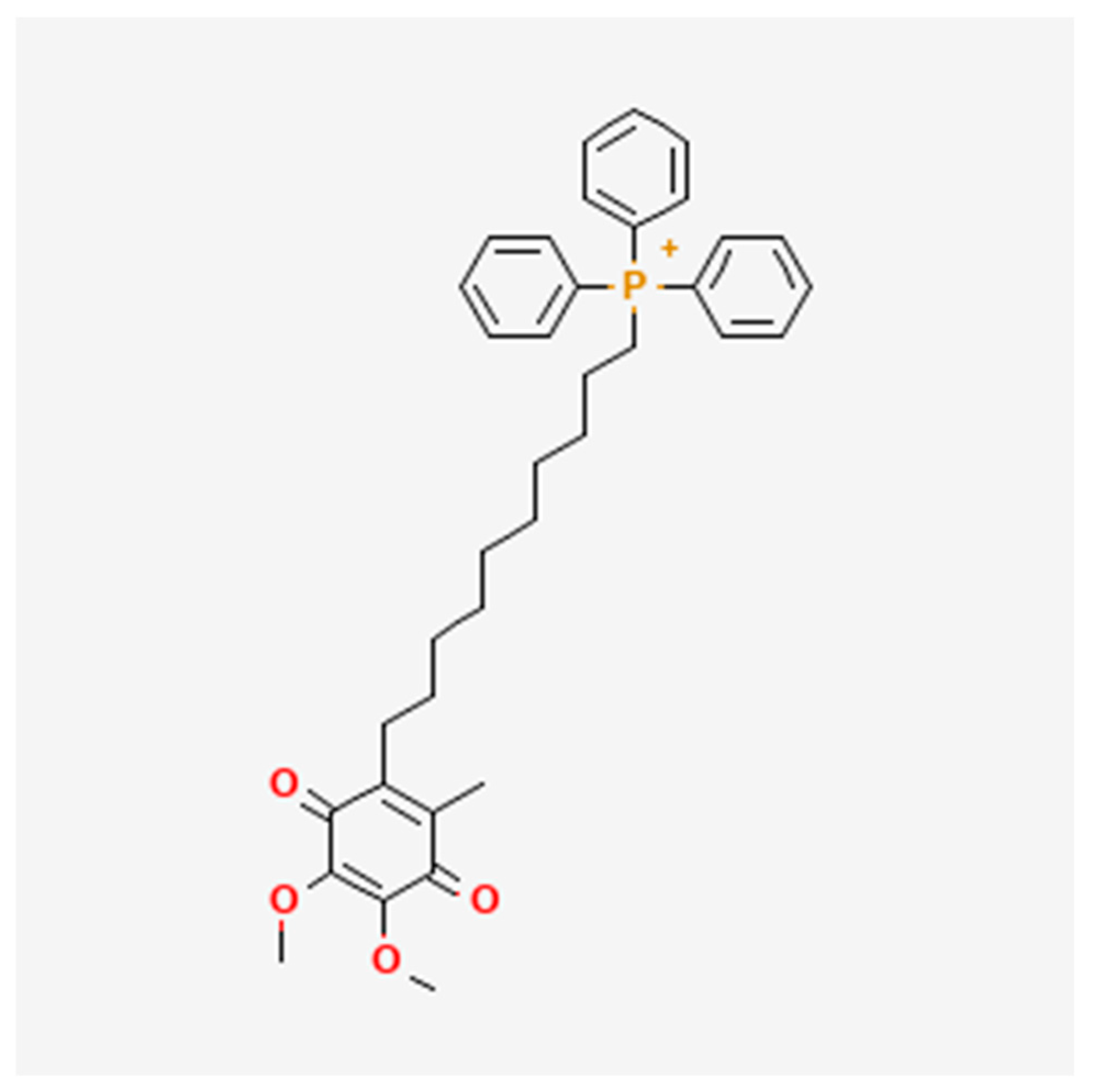
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11388332, Mitoquinone; National Center for Biotechnology Information: Bethesda, MD, USA, 2025. [Google Scholar]
- Kageyama, M.; Ito, J.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Mitochondrial reactive oxygen species regulate mitochondrial biogenesis in porcine embryos. J. Reprod. Dev. 2021, 67, 141–147. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Kotamraju, S.; Kalivendi, S.V.; Matsunaga, T.; Shang, T.; Keszler, A.; Joseph, J.; Kalyanaraman, B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J. Biol. Chem. 2004, 279, 37575–37587. [Google Scholar] [CrossRef]
- Saretzki, G.; Murphy, M.P.; von Zglinicki, T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003, 2, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Masoudi, R.; Hatefi, A.; Alipour-Jenaghard, P.; Esmaeili, V. The effects of MitoQ as a mitochondrial-targeted antioxidant in a plant-based extender on buck sperm quality parameters during cryopreservation. Nim Reprod Sci. 2024, 266, 107517. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Widén, C.; Gellert, N.; Swettenham, E.; Zobalova, R.; Dong, L.F.; Wang, X.F.; Lidebjer, C.; Dalen, H.; Headrick, J.P.; et al. Mitochondria transmit apoptosis signalling in cardiomyocyte-like cells and isolated hearts exposed to experimental ischemia-reperfusion injury. Redox Rep. 2007, 12, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Câmara, D.R.; Ibanescu, I.; Siuda, M.; Bollwein, H. Mitoquinone does not improve sperm cryo-resistance in bulls. Reprod. Domest. Anim. 2022, 57, 10–18. [Google Scholar] [CrossRef]
- Javaheri Barfourooshi, H.; Asadzadeh, N.; Masoudi, R. The mitochondria-targeted antioxidant “MitoQ” preserves quality and reproductive performance of ram spermatozoa cryopreserved in soybean lecithin-based extender. Theriogenology 2023, 208, 71–76. [Google Scholar] [CrossRef]
- Elkhawagah, A.R.; Donato, G.G.; Poletto, M.; Martino, N.A.; Vincenti, L.; Conti, L.; Necchi, D.; Nervo, T. Effect of Mitoquinone on sperm quality of cryopreserved stallion semen. J. Equine Vet. Sci. 2024, 141, 105168. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Van den Bosch, L.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J.L.M.R. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef]
- Zhou, D.; Zhuan, Q.; Luo, Y.; Liu, H.; Meng, L.; Du, X.; Wu, G.; Hou, Y.; Li, J.; Fu, X. Mito-Q promotes porcine oocytes maturation by maintaining mitochondrial thermogenesis via UCP2 downregulation. Theriogenology 2022, 187, 205–214. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, K.; Xu, X.; Qin, Y.; Xiao, L.; Zhou, C.; Wu, J.; Liu, Y.; Bai, J. Effect of cholesterol-loaded cyclodextrin treatment on boar sperm cryopreservation. Anim. Biosci. 2024, 37, 1558–1567. [Google Scholar] [CrossRef]
- Baishya, S.K.; Biswas, R.K.; Kadirvel, G.; Deka, B.C.; Kumar, S. A comparative study of effects of different freezing methods on sperm quality, DNA integrity and membrane protein of cryopreserved boar semen. Indian J. Anim. Sci. 2018, 88, 285–289. [Google Scholar] [CrossRef]
- Spinaci, M.; Vallorani, C.; Bucci, D.; Bernardini, C.; Tamanini, C.; Seren, E.; Galeati, G. Effect of liquid storage on sorted boar spermatozoa. Theriogenology 2010, 74, 741–748. [Google Scholar] [CrossRef]
- Gliozzi, T.M.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Spermatozoa DNA and plasma membrane integrity after pellet optimized processing for cryopreservation in meat type chicken breeders. Br. Poult. Sci. 2017, 58, 578–584. [Google Scholar] [CrossRef]
- Nesci, S.; Spinaci, M.; Galeati, G.; Nerozzi, C.; Pagliarani, A.; Algieri, C.; Tamanini, C.; Bucci, D. Sperm function and mitochondrial activity: An insight on boar sperm metabolism. Theriogenology 2020, 144, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nur Karakus, F.; Bulgurcuoglu Kuran, S.; Solakoglu, S. Effect of curcumin on sperm parameters after the cryopreservation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, Y.; Wang, H.; Zhang, Q.; Guo, Q.; Li, Y. The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen-Thawed Boar Sperm. Animals 2025, 15, 843. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Yanagimachi, H.; Rogers, B.J. The use of zona-free animal ova as a test system for the assessment of the fertilizing capacity of human spermatozoa. Biol. Reprod. 1976, 15, 471–476. [Google Scholar] [CrossRef]
- Kim, E.; Yu, I.J.; Lee, J.; Jeon, Y. Effects of MnTBAP on Porcine Semen Cryopreservation and Capacitation. Antioxidants 2024, 13, 672. [Google Scholar] [CrossRef]
- Shepherd, M.J.; Herickhoff, L.A. A novel experimental design for boar sperm cryopreservation. J. Anim. Sci. 2022, 100, skac169. [Google Scholar] [CrossRef]
- Sharma, M.; Punetha, M.; Saini, S.; Chaudhary, S.; Jinagal, S.; Thakur, S.; Kumar, P.; Kumar, R.; Sharma, R.K.; Yadav, P.S.; et al. Mito-Q supplementation of in vitro maturation or in vitro culture medium improves maturation of buffalo oocytes and developmental competence of cloned embryos by reducing ROS production. Anim. Reprod. Sci. 2024, 260, 107382. [Google Scholar] [CrossRef]
- Jia, M.; Xiao, Y.; Zhang, C.; Jiang, T.; Huang, Y.; Gao, J.; Li, Y.; Zhou, L. Mitoxantrone alleviates hepatic steatosis induced by high-fat diet in broilers. Biochem. Biophys. Res. Commun. 2022, 627, 52–59. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Setiawan, R.; Christi, R.F.; Alhuur, K.R.G.; Widyastuti, R.; Solihati, N.; Rasad, S.D.; Hidajat, K.; Do, D.N. Impact of glucose and pyruvate on adenosine triphosphate production and sperm motility in goats. Anim. Biosci. 2024, 37, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Mol. Biol. 2012, 810, 183–205. [Google Scholar]
- Zheng, J.; Lu, Y.; Qu, X.; Wang, P.; Zhao, L.; Gao, M.; Shi, H.; Jin, X. Correction: Decreased Sperm Motility Retarded ICSI Fertilization Rate in Severe Oligozoospermia but Good-Quality Embryo Transfer Had Achieved the Prospective Clinical Outcoms. PLoS ONE 2016, 11, e0165684. [Google Scholar] [CrossRef]
- Atmoko, W.; Savira, M.; Shah, R.; Chung, E.; Agarwal, A. Isolated teratozoospermia: Revisiting its relevance in male infertility: A narrative review. Transl. Androl. Urol. 2024, 13, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarayra, N.; Al-Alami, Z.M.; Battah, A.; Muhaidat, N. Addition of Mitoquinone (MitoQ) to Fresh Human Sperm Enhances Sperm Motility without Attenuating Viability. Biology 2024, 13, 653. [Google Scholar] [CrossRef]
- Del Prete, C.; Blanco Prieto, O.; Mislei, B.; Iacono, E.; Mari, G.; Cocchia, N.; Gasparrini, B.; Merlo, B.; Bucci, D. Assessment of an open-access CASA software for bovine and buffalo sperm motility analysis. Anim. Reprod. Sci. 2022, 247, 107089. [Google Scholar] [CrossRef]
- Masoudi, R.; Dadashpour-Davachi, N.; Asadzadeh, N.; Hatefi, A.; Alipour-Jenaghard, P. MitoQ preserves the quality and fertility of liquid-preserved ram sperm. Theriogenology 2024, 216, 8–11. [Google Scholar] [CrossRef]
- Guo, H.T.; Wang, J.R.; Sun, L.Z.; Jin, X.H.; Shi, X.Y.; Lin, J.Y.; Yue, S.L.; Zhou, J.B. Effects of astaxanthin on plasma membrane function and fertility of boar sperm during cryopreservation. Theriogenology 2021, 164, 58–64. [Google Scholar] [CrossRef]
- Szlasa, W.; Kiełbik, A.; Szewczyk, A.; Rembiałkowska, N.; Novickij, V.; Tarek, M.; Saczko, J.; Kulbacka, J. Oxidative Effects during Irreversible Electroporation of Melanoma Cells-In Vitro Study. Molecules 2020, 26, 154. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Maldjian, A.; Pizzi, F.; Gliozzi, T.; Cerolini, S.; Penny, P.; Noble, R. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology 2005, 63, 411–421. [Google Scholar] [CrossRef]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Narasimman, M.; Khodamoradi, K.; Khosravizadeh, Z.; Ramasamy, R. How defective spermatogenesis affects sperm DNA integrity. Andrologia 2021, 53, e13615. [Google Scholar] [PubMed]
- Fatehi, A.N.; Bevers, M.M.; Schoevers, E.; Roelen, B.A.; Colenbrander, B.; Gadella, B.M. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J. Androl. 2006, 27, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.F.; Prime, T.A.; Abakumova, I.; James, A.M.; Porteous, C.M.; Smith, R.A.; Murphy, M.P. Rapid and extensive uptake and activation of hydrophobic triphenylphosphonium cations within cells. Biochem. J. 2008, 411, 633–645. [Google Scholar]
- Ernster, L.; Forsmark, P.; Nordenbrand, K. The mode of action of lipid-soluble antioxidants in biological membranes. Relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. J. Nutr. Sci. Vitaminol. 1992, 38, 548–551. [Google Scholar] [CrossRef]
- Rezaei, A.; Bahmani, H.R.; Mafakheri, S.; Farshad, A.; Nazari, P.; Masoudi, R. Protective effects of different doses of MitoQ separately and combined with trehalose on oxidative stress and sperm function of cryopreserved Markhoz goat semen. Cryobiology 2023, 110, 36–43. [Google Scholar]
- Tiwari, S.; Mohanty, T.K.; Bhakat, M.; Kumar, N.; Baithalu, R.K.; Nath, S.; Yadav, H.P.; Dewry, R.K. Comparative evidence support better antioxidant efficacy of mitochondrial-targeted (Mitoquinone) than cytosolic (Resveratrol) antioxidant in improving in-vitro sperm functions of cryopreserved buffalo (Bubalus bubalis) semen. Cryobiology 2021, 101, 125–134. [Google Scholar]
- Moradi Gardeshi, T.; Shahandeh, E.; Tavakolpoor Saleh, N.; Karami, S.; Mirzaei Azandaryani, Z.; Mazaheri, F.; Mohammadi, H. Evaluation of the effect of mitoquinone on functional parameters, DNA structure, and genes expression related to the apoptotic and antioxidants of human sperm after freezing-thawing. Mol. Biol. Rep. 2024, 51, 183. [Google Scholar] [CrossRef]
- Bartosz, G. Total antioxidant capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar]
- Murawski, M.; Saczko, J.; Marcinkowska, A.; Chwiłkowska, A.; Gryboś, M.; Banaś, T. Evaluation of superoxide dismutase activity and its impact on semen quality parameters of infertile men. Folia Histochem. Cytobiol. 2007, 45 (Suppl. S1), S123–S126. [Google Scholar]
- Bagchi, D.; Moser, J.; Stohs, S.J. Quantitative determination of urinary lipid metabolites by high pressure liquid chromatography as indicators of menadione-induced in vivo lipid peroxidation. Arch. Environ. Contam. Toxicol. 1994, 26, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, M.; Xu, J.; Wu, C.; Zhang, S.; Zhang, D.; Dai, J.; Gao, J. Does Antioxidant Mitoquinone (MitoQ) Ameliorate Oxidative Stress in Frozen-Thawed Rooster Sperm. Animals 2022, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.F.; Grabow, S.; Chappaz, S.; Dewson, G.; Hockings, C.; Kluck, R.M.; Debrincat, M.A.; Gray, D.H.; Witkowski, M.T.; Evangelista, M.; et al. Physiological restraint of Bak by Bcl-xL is essential for cell survival. Genes. Dev. 2016, 30, 1240–1250. [Google Scholar] [CrossRef]
- Giriş, M.; Depboylu, B.; Doğru-Abbasoğlu, S.; Erbil, Y.; Olgaç, V.; Aliş, H.; Aykaç-Toker, G.; Uysal, M. Effect of taurine on oxidative stress and apoptosis-related protein expression in trinitrobenzene sulphonic acid-induced colitis. Clin. Exp. Immunol. 2008, 152, 102–110. [Google Scholar] [CrossRef]
- Gonzalo, Ó.; Benedi, A.; Vela, L.; Anel, A.; Naval, J.; Marzo, I. Study of the Bcl-2 Interactome by BiFC Reveals Differences in the Activation Mechanism of Bax and Bak. Cells 2023, 12, 800. [Google Scholar] [CrossRef]
- Seervi, M.; Rani, A.; Sharma, A.K.; Santhosh Kumar, T.R. ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed. Pharmacother. 2018, 106, 200–209. [Google Scholar] [CrossRef]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef]
- Wang, X.; Qian, H.; Huang, X.; Li, J.; Zhang, J.; Zhu, N.; Chen, H.; Zhu, C.; Wang, J.; Zhang, P.; et al. UCP2 Mitigates the Loss of Human Spermatozoa Motility by Promoting mROS Elimination. Cell Physiol. Biochem. 2018, 50, 952–962. [Google Scholar] [CrossRef]

| Parameters | 0 | 50 | MiotQ (nmol/mL) 100 | 150 | 200 |
|---|---|---|---|---|---|
| Viability (%) | 49.75 ± 0.06 d | 65.93 ± 0.05 c | 73.86 ± 0.13 b | 80.83 ± 0.32 a | 75.70 ± 0.50 ab |
| Motility (%) | 48.40 ± 0.50 c | 53.78 ± 1.55 c | 63.30 ± 0.37 b | 75.60 ± 0.24 a | 65.29 ± 0.20 b |
| Malformation rate (%) | 27.23 ± 0.55 d | 20.98 ± 0.05 c | 16.70 ± 0.15 bc | 11.51 ± 0.16 a | 15.07 ± 0.40 cd |
| VAP (μm/s) | 26.29 ± 0.33 d | 35.87 ± 0.67 c | 40.59 ± 0.13 b | 49.98 ± 0.74 a | 40.62 ± 1.15 b |
| VCL (μm/s) | 31.89 ± 2.04 d | 42.52 ± 1.48 d | 46.45 ± 1.17 c | 51.73 ± 2.04 a | 43.60 ± 0.93 cb |
| VSL (μm/s) | 29.66 ± 1.28 c | 39.64 ± 0.33 b | 42.72 ± 0.40 b | 50.96 ± 1.43 a | 42.45 ± 1.49 b |
| BCF (Hz) | 6.24 ± 0.16 c | 8.16 ± 0.50 bc | 8.67 ± 0.88 b | 12.68 ± 1.17 a | 8.67 ± 0.52 bc |
| Group | Number of Oocytes (n = 3) | Adhesion Index |
|---|---|---|
| 0 nmol/mL MiotQ | 54 | 2.67 ± 0.58 b |
| 150 nmol/mL MiotQ | 58 | 5.17 ± 1.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wang, Q.; Wang, H.; Guo, Q.; Li, Y.; Li, J. Mitoquinone Can Effectively Improve the Quality of Thawed Boar Sperm. Animals 2025, 15, 2808. https://doi.org/10.3390/ani15192808
Dong Y, Wang Q, Wang H, Guo Q, Li Y, Li J. Mitoquinone Can Effectively Improve the Quality of Thawed Boar Sperm. Animals. 2025; 15(19):2808. https://doi.org/10.3390/ani15192808
Chicago/Turabian StyleDong, Yingying, Qian Wang, Hechuan Wang, Qing Guo, Yanbing Li, and Jingchun Li. 2025. "Mitoquinone Can Effectively Improve the Quality of Thawed Boar Sperm" Animals 15, no. 19: 2808. https://doi.org/10.3390/ani15192808
APA StyleDong, Y., Wang, Q., Wang, H., Guo, Q., Li, Y., & Li, J. (2025). Mitoquinone Can Effectively Improve the Quality of Thawed Boar Sperm. Animals, 15(19), 2808. https://doi.org/10.3390/ani15192808






