Effect of Dietary Protein Levels on Performance and Health Status of Adult Companion Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Building, Equipment and Animals
2.2. Experimental Diets and Periods
2.3. Digestibility Trial
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Digestibility Trial
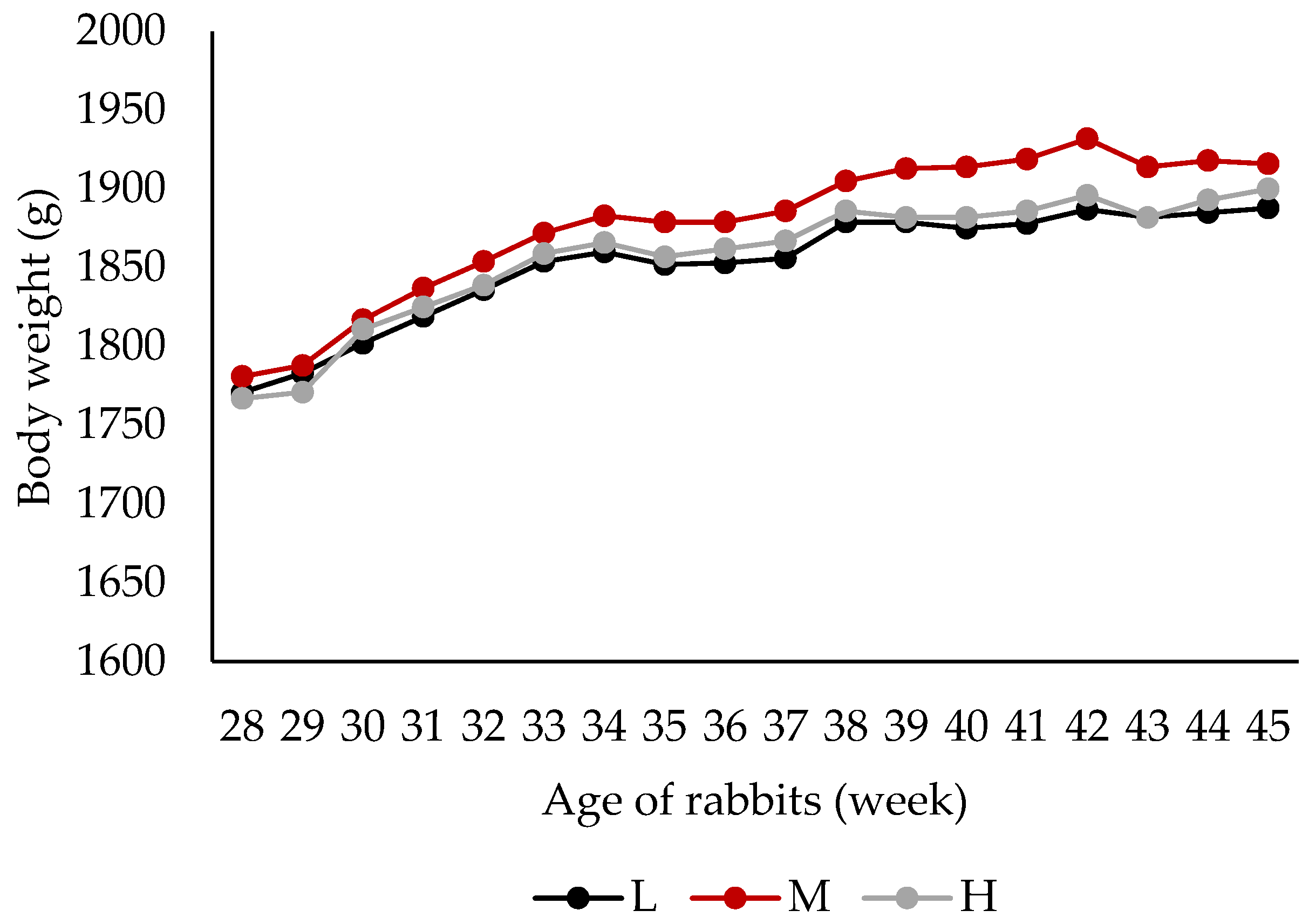
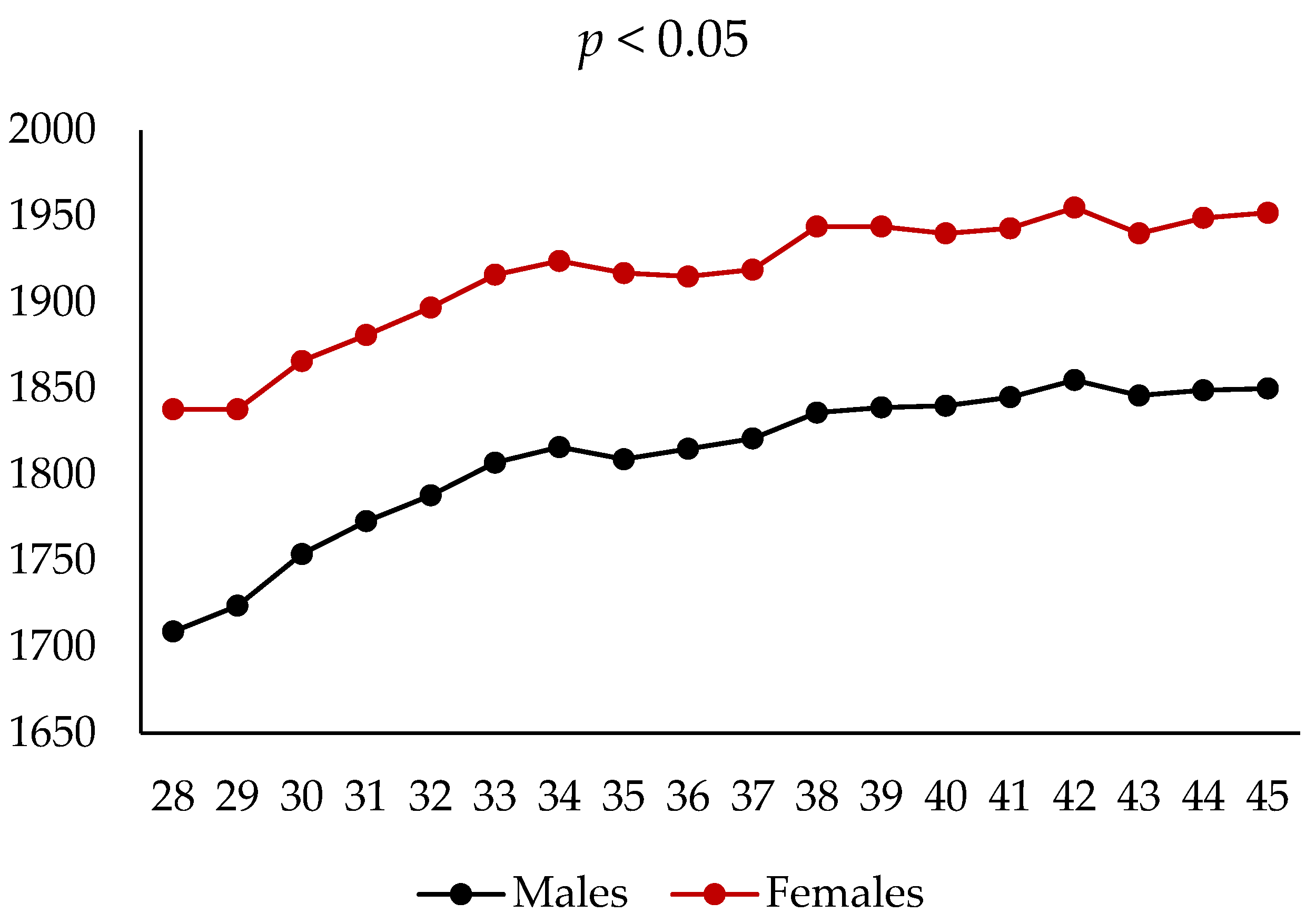
3.2. Live Performance and Health Status
3.3. Protein and Energy Diet Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Crude protein |
| FCR | Feed conversion ratios |
| LW | Live weight |
| LW0.75 | Metabolic live weight |
| DE | Digestible energy |
| DP | Digestible protein |
| L | Low crude protein diet |
| M | Medium crude protein diet |
| H | High crude protein diet |
| SBP | Sugar beet pulp |
| DM | Dry matter |
| EE | Ether extract |
| CF | Crude fiber |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| ADL | Acid detergent lignin |
| GE | Gross energy |
| ASE | Accelerated solvent extraction |
| GC | Gas chromatography |
| ANOVA | Analysis of variance |
References
- Hilger, C.; Kler, S.; Arumugam, K.; Revets, D.; Muller, C.P.; Charpen-tier, C.; Lehners, C.; Morisset, M.; Hentges, F. Identification and isolation of a Fel d 1-like molecule as a major rabbit allergen. J. Allergy Clin. Immunol. 2014, 133, 759–766. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.; Yan, X.; Hao, Y.; Zhu, J.; Weng, Q.Q.; Wu, X.S. Gene expression on profiling analysis reveals coat color formation in Rex rabbits (Oryctolagus cuniculus). In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016; pp. 873–876. [Google Scholar] [CrossRef]
- Maftoum, L.; Mayer, J. Prescription diets for rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2014, 17, 485–502. [Google Scholar] [CrossRef]
- Clauss, M.; Hatt, J.M. Evidence-based rabbit housing and nutrition. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.M. Care of the geriatric rabbit. Vet. Clin. N. Am. Exot. Anim. Pract. 2010, 13, 123–133. [Google Scholar] [CrossRef]
- Rioja-Lang, F.; Bacon, H.; Connor, M.; Dwyer, C.M. Rabbit welfare: Determining priority welfare issues for pet rabbits using a modified Delphi method. Vet. Rec. Open. 2019, 21, 6. [Google Scholar] [CrossRef]
- Carabaño, R.; Badiola, I.; Chamorro, S.; García, J.; García-Ruiz, A.I.; García-Rebollar, P.; Gómez-Conde, M.S.; Gutiérrez, I.; Nicodemus, N.; Villamide, M.J.; et al. New trends in rabbit feeding: Influence of nutrition on intestinal health. Spanish J. Agric. Res. 2008, 6, 15–25. [Google Scholar] [CrossRef]
- Carpenter, J.W.; Wolf, K.N.; Kolmstetter, C. Feeding Small Pet Mammals. In Small Animal Clinical Nutrition, 5th ed.; Hand, M.S., Thatcher, C.D., Remillard, R.L., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 1215–1236. [Google Scholar]
- Campbell-Ward, M.L. [Rabbits]. Gastrointestinal physiology and nutrition. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 183–192. [Google Scholar]
- Gidenne, T. Dietary fibres in the nutrition of the growing rabbit and recommendations to preserve digestive health: A review. Animal 2015, 9, 227–242. [Google Scholar] [CrossRef] [PubMed]
- De Blas, C.; Mateos, G.G. Feed formulation. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 222–232. [Google Scholar]
- Xiccato, G.; Trocino, A. Energy and protein metabolism and requirements. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 83–118. [Google Scholar] [CrossRef]
- Trocino, A.; Fragkiadakis, M.; Majolini, D.; Tazzoli, M.; Radaelli, G.; Xiccato, G. Soluble fibre, starch and protein level in diets for growing rabbits: Effects on digestive efficiency and productive traits. AFST 2013, 180, 73–82. [Google Scholar] [CrossRef]
- Lei, Q.X.; Li, F.C.; Jiao, H.C. Effects of Dietary Crude Protein on Growth Performance, Nutrient Utilization, Immunity Index and Protease Activity in Weaner to 2 Month-old New Zealand Rabbits. Asian Australas. J. Anim. Sci. 2004, 17, 1447–1451. [Google Scholar] [CrossRef]
- Aly, M.S.; Amber, S.G.; El Sayed, M. Production and application of Spirulina platensis rich in fatty acids and vitamins. J. Am. Sci. 2011, 7, 36–45. [Google Scholar]
- Hajati, H.; Zaghari, M.; Oliveira, H.C. Arthrospira (Spirulina) Platensis can be considered as a probiotic alternative to reduce heat stress in laying Japanese quails. Braz. J. Poult. Sci. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Michalak, I.; Mahrose, K.M. Seaweeds, intact and processed, as a valuable component of poultry feed. J. Mar. Sci. Eng. 2020, 8, 620. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Asker, M.M.S.; Ibrahim, Z.K. Functional Bioactive Compounds and Biological Activities of Spirulina platensis Lipids. Czech J. Food Sci. 2008, 26, 211–222. [Google Scholar] [CrossRef]
- Hassan, F.; Mobarez, S.; Mohamed, M.; Attia, Y.; Mekawy, A.; Mahrose, K. Zinc and/or Selenium Enriched Spirulina as Antioxidants in Growing Rabbit Diets to Alleviate the Deleterious Impacts of Heat Stress during Summer Season. Animals 2021, 11, 756. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus Hyemalis essential oils. Food Control 2008, 1, 681–687. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Thomas, D.G.; Morris, P.J.; Hawthorne, A.J. Energy requirements of adult cats. Br. J. Nutr. 2010, 103, 1083–1093. [Google Scholar] [CrossRef]
- Lowe, J.A. Pet rabbit feeding and nutrition. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 294–314. [Google Scholar]
- Xiccato, G. Nutrition of lactating does. In Proceedings of the 6th World Rabbit Congress, Toulose, France, 9–12 July 1996. [Google Scholar]
- Lebas, F. Reflections on rabbit nutrition with a special emphasis on feed ingredients utilization. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004. [Google Scholar]
- Ricci, R.; Sartori, A.; Palagiano, C.; Dalle Zotte, A. Study on the nutrient adequacy of feeds for pet rabbits available in the Italian market. World Rabbit Sci. 2010, 18, 131–137. [Google Scholar] [CrossRef]
- Pérez, J.M.; Lebas, F.; Gidenne, T.; Maertens, L.; Xiccato, G.; Parigi-Bini, R.; Dalle Zotte, A.; Cossu, M.E.; Carazzolo, A.; Villamide, M.J.; et al. European reference method for in vivo determination of diet digestibility in rabbits. World Rabbit Sci. 1995, 3, 41–43. [Google Scholar] [CrossRef]
- AOAC. Officials Methods of Analysis. In Association of Official Analytical Chemists, 17th ed.; Horwitz, W., Ed.; AOAC: Gaithersburg, MD, USA, 2000; pp. 20877–24174. [Google Scholar]
- ISO 9831:1998; Animal Feeding Stuffs, Animal Products, and Faeces or Urine. Determination of Gross Calorific Value. Bomb Calorimeter Method. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISTISAN Report 1996/34; Humidity in Spices Istisan Reports 1996 34 PAG.7 Met. B. Istituto Superiore di Sanità: Roma, Italy, 1996; pp. 161–190.
- Lee, C.; Trevino, B.; Chaiyawat, M.A. A Simple and Rapid Solvent Extraction Method for Determining Total Lipids in Fish Tissue. J. AOAC Int. 1995, 79, 487–492. [Google Scholar]
- SAS Institute. Statistical Analysis Software for Windows (SAS), Statistics version 9.1.3 ed.; SAS Institute: Cary, NC, USA, 2008.
- Chamorro, S.; Gómez-Conde, M.S.; Pérez de Rozas, A.M.; Badiola, I.; Carabaño, R.; De Blas, J.C. Effect on digestion and performance of dietary protein content and of increased substitution of lucerne hay with soya-bean protein concentrate in starter diets for young rabbits. Animal 2007, 1, 651–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsisi, G.F.; Ayyat, M.S.; Gabr, H.A.; El-Rahman, G.A.A. Effect of dietary protein levels and zinc supplementation on growth performance, digestibility, blood constituents and carcass traits of growing rabbits. J. Anim. Poult. Prod. 2017, 44, 1369–1378. [Google Scholar] [CrossRef]
- Santoma, G.; De Bias, J.C.; Caraballo, R.; Fraga, M.J. The effects of different fats and their inclusion level in diets for growing rabbits. Anim. Prod. 1987, 45, 291–300. [Google Scholar] [CrossRef]
- Fernández, C.; Cobos, A.; Fraga, M.J. The effect of fat inclusion on diet digestibility in growing rabbits. J. Anim. Sci. 1994, 72, 1508–1515. [Google Scholar] [CrossRef]
- Casado, C.; Moya, V.J.; Fernández, C.; Pascual, J.J.; Blas, E.; Cervera, C. Diet digestibility in growing rabbits: Effect of origin and oxidation level of dietary fat and vitamin E supplementation. World Rabbit Sci. 2010, 18, 57–63. [Google Scholar] [CrossRef]
- Gidenne, T.; García, J.; Lebas, F.; Licois, D. Nutrition and feeding strategy: Interactions with pathology. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 179–199. [Google Scholar]
- Younglai, E.V.; Moor, B.C.; Dimond, P. Effects of sexual activity on luteinizing hormone and testosterone levels in the adult male rabbit. J. Endocrinol. 1976, 69, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Eniwaiye, A.A.; Rani-Kamwendo, Z.T. Potential Growth and Chemical Composition Changes During the Growth of New Zealand White Rabbits. Animals 2025, 15, 1670. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.L.; Fried, S.K.; Greenberg, A.S.; Xiao, X.Q.; Clegg, D.J. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int. J. Obes. 2010, 34, 989–1000. [Google Scholar] [CrossRef]
- Ouhayoun, J. Croissance et qualités bouchères du lapin. Cuniculture 1984, 11, 181–188. [Google Scholar]
| Diets 1 | |||
|---|---|---|---|
| L | M | H | |
| Dry matter, g/kg | 882 | 887 | 886 |
| Crude protein, g/kg | 165 | 173 | 175 |
| Ether extract, g/kg | 40.0 | 44.0 | 32.0 |
| Ash, g/kg | 77.0 | 87.0 | 88.0 |
| Starch, g/kg | 69.0 | 73.0 | 73.0 |
| Crude fibre, g/kg | 173 | 176 | 173 |
| Neutral detergent fiber g/kg | 352 | 353 | 342 |
| Acid detergent fiber, g/kg | 205 | 214 | 208 |
| Acid detergent lignin, g/kg | 34.0 | 38.0 | 37.0 |
| Gross Energy, MJ/kg | 16.5 | 16.5 | 16.2 |
| Ca, g/kg | 9.70 | 12.1 | 11.0 |
| P, g/kg | 4.70 | 5.00 | 4.70 |
| K, g/kg | 15.0 | 16.2 | 15.7 |
| Na, g/kg | 1.80 | 2.20 | 3.70 |
| Ca:P | 2.06 | 2.42 | 2.34 |
| Vitamin D2, UI/kg | 1326 | 1310 | 1108 |
| Vitamin D3, UI/kg | 4594 | 8363 | 4006 |
| Lysine, mg/100 g | 1.05 | 1.10 | 1.83 |
| Tryptophane, mg/100 g | 0.27 | 0.27 | 0.29 |
| Nutritive value: | |||
| Digestible Energy (DE), MJ/kg | 10.0 | 9.70 | 9.60 |
| Digestible Protein (DP), g/kg | 120 | 124 | 128 |
| DP to DE ratio, g/MJ | 12.0 | 12.7 | 13.4 |
| Diet 1 (D) | Sex (S) | p Value 2 | RSD 3 | |||||
|---|---|---|---|---|---|---|---|---|
| L | M | H | Male | Female | D | S | ||
| No. of rabbits | 8 | 8 | 8 | 10 | 14 | |||
| Dry matter excreta, g | 90.0 | 111 | 110 | 110 | 97.8 | ns | ns | 24.0 |
| Feed intake, g | 252 | 295 | 300 | 296 | 269 | ns | ns | 56.0 |
| Feed intake, g/DM | 222 | 260 | 265 | 261 | 237 | ns | ns | 49.0 |
| Feed intake, g DM/kg LW | 122 | 141 | 145 | 146 | 126 | ns | <0.05 | 0.20 |
| Apparent faecal digestibility: | ||||||||
| Dry matter, % | 59.5 | 57.6 | 58.6 | 58.2 | 58.9 | ns | ns | 1.70 |
| Organic matter, % | 59.7 | 57.5 | 58.2 | 58.2 | 58.8 | ns | ns | 1.70 |
| Crude protein, % | 72.7 | 71.4 | 73.4 | 72.8 | 72.2 | ns | ns | 2.90 |
| Ether extract, % | 81.4 A | 80.2 A | 76.7 B | 79.0 | 79.9 | <0.01 | ns | 2.20 |
| Starch, % | 98.1 | 97.7 | 97.7 | 97.7 | 97.9 | ns | ns | 0.40 |
| Crude Fibre (Weende), % | 22.5 A | 22.4 A | 17.6 B | 20.9 | 20.7 | <0.01 | ns | 4.00 |
| Neutral-detergent fibre (NDF), % | 36.8 A | 32.9 B | 30.9 B | 33.8 | 33.3 | <0.0001 | ns | 2.80 |
| Acid-detergent fibre (ADF), % | 30.5 A | 28.0 A | 25.7 B | 28.2 | 27.9 | 0.0002 | ns | 1.70 |
| Cellulose (ADF-ADL), % | 37.3 A | 33.6 B | 32.2 B | 34.2 | 34.6 | <0.0001 | ns | 1.90 |
| Hemicelluloses (NDF-ADF), % | 45.6 A | 40.6 B | 38.9 B | 42.0 | 41.4 | 0.0001 | ns | 1.80 |
| NNCC, % 4 | 80.2 | 80.1 | 82.2 | 79.5 | 82.2 | ns | <0.05 | 2.30 |
| Gross energy, % | 60.7 | 58.8 | 59.0 | 59.3 | 59.8 | ns | ns | 1.80 |
| Ca, % | 52.9 | 53.4 | 52.9 | 51.9 | 54.2 | ns | ns | 3.70 |
| P, % | 12.1 | 11.5 | 12.2 | 12.5 | 11.4 | ns | ns | 5.30 |
| Diet 1 (D) | Sex (S) | p Value 2 | RSD 3 | |||||
|---|---|---|---|---|---|---|---|---|
| L | M | H | Male | Female | D | S | ||
| No. of rabbits | 39 | 39 | 39 | 60 | 57 | |||
| Weeks of age: | ||||||||
| 28–29 | 83.7 | 86.3 | 82.9 | 91.6 | 77.0 | ns | <0.001 | 20.5 |
| 29–30 | 87.4 | 95.0 | 100 | 95.3 | 93.1 | ns | ns | 23.6 |
| 30–31 | 86.9 | 94.1 | 92.6 | 94.0 | 88.4 | ns | ns | 20.1 |
| 31–32 | 86.3 | 91.4 | 92.7 | 91.8 | 88.5 | ns | ns | 18.9 |
| 32–33 | 87.3 | 91.8 | 94.7 | 93.2 | 89.3 | ns | ns | 18.0 |
| 33–34 | 83.9 | 91.2 | 94.3 | 91.9 | 87.7 | ns | ns | 16.1 |
| 34–35 | 81.3 | 87.9 | 89.4 | 87.3 | 85.1 | ns | ns | 15.8 |
| 35–36 | 74.4 B | 81.0 AB | 87.7 A | 82.7 | 79.5 | <0.01 | ns | 17.7 |
| 36–37 | 75.4 b | 83.3 ab | 86.1 a | 84.6 | 78.6 | <0.05 | ns | 18.4 |
| 37–38 | 78.5 b | 85.7 ab | 88.9 a | 86.3 | 82.4 | <0.05 | ns | 17.8 |
| 38–39 | 77.6 | 86.2 | 84.5 | 85.6 | 79.8 | ns | ns | 16.9 |
| 39–40 | 70.0 B | 79.8 B | 80.4 A | 78.2 | 74.3 | <0.01 | ns | 15.8 |
| 40–41 | 71.4 B | 81.2 A | 83.4 A | 82.5 | 74.8 | <0.001 | <0.01 | 14.4 |
| 41–42 | 77.6 b | 82.7 ab | 86.1 a | 85.6 | 78.7 | <0.05 | <0.05 | 14.4 |
| 42–43 | 72.8 | 75.3 | 77.0 | 78.1 | 71.9 | ns | <0.05 | 14.7 |
| 43–44 | 72.4 | 74.8 | 77.9 | 76.9 | 73.2 | ns | ns | 16.1 |
| 44–45 | 66.8 B | 72.0 AB | 78.0 A | 75.4 | 69.2 | <0.01 | <0.05 | 15.1 |
| 28–45 | 77.2 | 82.9 | 82.1 | 86.0 | 75.2 | ns | <0.05 | 14.4 |
| Diet 1 (D) | Sex (S) | p Value 2 | RSD 3 | |||||
|---|---|---|---|---|---|---|---|---|
| L | M | H | Male | Female | D | S | ||
| No. of rabbits | 39 | 39 | 39 | 60 | 57 | |||
| Weeks of age: | ||||||||
| 28–29 | 1.82 | 1.01 | 0.52 | 2.19 | 0.04 | ns | ns | 6.19 |
| 29–30 | 2.73 b | 4.06 ab | 5.76 a | 4.32 | 4.05 | <0.05 | ns | 4.83 |
| 30–31 | 2.30 | 2.85 | 2.01 | 2.69 | 2.08 | ns | ns | 3.73 |
| 31–32 | 2.45 | 2.46 | 1.84 | 2.21 | 2.28 | ns | ns | 3.85 |
| 32–33 | 2.57 | 2.54 | 2.93 | 2.62 | 2.74 | ns | ns | 3.24 |
| 33–34 | 0.92 | 1.69 | 0.97 | 1.28 | 1.11 | ns | ns | 3.15 |
| 34–35 | −1.18 | −0.57 | −1.18 | −1.03 | −0.92 | ns | ns | 3.34 |
| 35–36 | 0.17 | −0.01 | 0.67 | 0.93 | −0.38 | ns | ns | 4.49 |
| 36–37 | 0.46 | 0.97 | 0.76 | 0.84 | 0.62 | ns | ns | 4.18 |
| 37–38 | 3.30 | 2.72 | 2.68 | 2.21 | 3.59 | ns | ns | 3.84 |
| 38–39 | −0.01 | 1.04 | −0.53 | 0.31 | 0.03 | ns | ns | 3.77 |
| 39–40 | −0.68 | 0.28 | −0.10 | 0.22 | −0.55 | ns | ns | 3.80 |
| 40–41 | 0.49 | 0.65 | 0.62 | 0.77 | 0.41 | ns | ns | 3.71 |
| 41–42 | 1.33 | 1.92 | 1.35 | 1.41 | 1.66 | ns | ns | 3.85 |
| 42–43 | −0.68 b | −2.58 a | −1.97 ab | −1.37 | −2.12 | <0.05 | ns | 3.31 |
| 43–44 | 0.41 | 0.53 | 1.61 | 0.49 | 1.21 | ns | ns | 3.86 |
| 44–45 | 0.40 | −0.26 | 1.04 | 0.18 | 0.60 | ns | ns | 3.36 |
| 28–45 | 0.99 | 1.13 | 1.12 | 1.19 | 0.97 | ns | ns | 1.02 |
| Diet 1 (D) | Sex (S) | p Value | RSD 2 | |||||
|---|---|---|---|---|---|---|---|---|
| L | M | H | Male | Female | D | S | ||
| No. of rabbits | 39 | 39 | 39 | 60 | 57 | |||
| Skin fold width at 28th week, mm | 3.24 | 3.36 | 3.25 | 3.49 | 3.08 | ns | <0.001 | 0.49 |
| Skin fold width at 45th week, mm | 3.85 | 3.92 | 4.08 | 4.35 | 3.56 | ns | <0.001 | 0.63 |
| Skin fold width change, mm | 0.61 | 0.56 | 0.83 | 0.85 | 0.48 | ns | <0.05 | 0.77 |
| Skin fold width change, % | 20.9 | 19.2 | 27.6 | 26.9 | 18.3 | ns | ns | 25.1 |
| Diet 1 (D) | p Value | RSD 2 | |||
|---|---|---|---|---|---|
| L | M | H | |||
| No. of rabbits | 39 | 39 | 39 | ||
| Mortality, % | 0 | 0 | 0 | ||
| Morbidity, % | 33.9 | 35.6 | 32.4 | ns | 5.81 |
| Diet 1 (D) | Sex (S) | p Value 2 | RSD 3 | |||||
|---|---|---|---|---|---|---|---|---|
| L | M | H | Male | Female | D | S | ||
| No. of rabbits | 39 | 39 | 39 | 60 | 57 | |||
| Final live weight (45th week) | 1883 | 1916 | 1902 | 1846 | 1950 | ns | 0.03 | 0.26 |
| Metabolic live weight (kg0.75) | 1605 | 1626 | 1615 | 1581 | 1650 | ns | 0.03 | 0.17 |
| Maintenance energy requirement, kJ DE/d | 690 | 699 | 694 | 680 | 709 | ns | 0.03 | 71.7 |
| Feed intake, g/d (44th to 45th week) | 69.7 b | 71.5 ab | 77.9 a | 75.0 | 71.0 | 0.04 | ns | 14.6 |
| DE intake, kJ/d | 698 | 695 | 745 | 737 | 689 | ns | ns | 142 |
| DE > maintenance energy, kJ/d | 7.53 | −3.69 | 52.5 | 57.9 | −20.3 | ns | 0.0006 | 119 |
| DP intake, g/d | 8.36 C | 8.84 B | 10.0 A | 9.37 | 8.77 | 0.0005 | ns | 1.81 |
| Maintenance DP requirement, g/d | 6.10 | 6.18 | 6.14 | 6.00 | 6.27 | ns | 0.03 | 0.63 |
| DP > maintenance DP, g/d | 2.26 B | 2.66 AB | 3.86 A | 3.37 | 2.50 | <0.0001 | 0.003 | 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, B.; Dalle Zotte, A. Effect of Dietary Protein Levels on Performance and Health Status of Adult Companion Rabbits. Animals 2025, 15, 2784. https://doi.org/10.3390/ani15192784
Palumbo B, Dalle Zotte A. Effect of Dietary Protein Levels on Performance and Health Status of Adult Companion Rabbits. Animals. 2025; 15(19):2784. https://doi.org/10.3390/ani15192784
Chicago/Turabian StylePalumbo, Bianca, and Antonella Dalle Zotte. 2025. "Effect of Dietary Protein Levels on Performance and Health Status of Adult Companion Rabbits" Animals 15, no. 19: 2784. https://doi.org/10.3390/ani15192784
APA StylePalumbo, B., & Dalle Zotte, A. (2025). Effect of Dietary Protein Levels on Performance and Health Status of Adult Companion Rabbits. Animals, 15(19), 2784. https://doi.org/10.3390/ani15192784





