Evaluation of the Effects of Enlarged Housing on Social Play and Reward Seeking in Rats
Simple Summary
Abstract
1. Introduction
- 1.
- Social play behaviour:
- 2.
- Reward sensitivity and motivation
2. Materials and Methods
2.1. Housing, Animals, Husbandry and Enrichment
- Standard housing (Makrolon® IV, Tecniplast, Varese, Italy) with standard (mandatory) enrichment (Perspex shelter (Techniplast, Varese Italy), Bulkysoft tissues (Lyreco, Marly, France) and an Aspen gnawing stick (Bioservices, Schaijk, Netherlands), N = 16.
- EC4Rats (Scanbur, Karlslunde, Denmark) with standard (mandatory) enrichment (elevated platform, Perspex shelter (Techniplast, Varese Italy), Bulkysoft tissues (Lyreco, Marly, France) and an Aspen gnawing stick (Bioservices, Schaijk, Netherlands)), N = 16.
- EC4Rats with EC4Rats enrichment (Elevated platform, ladder, hammock, tube and hanging wooden block for gnawing, Scanbur, Karlslunde, Denmark), N = 16.
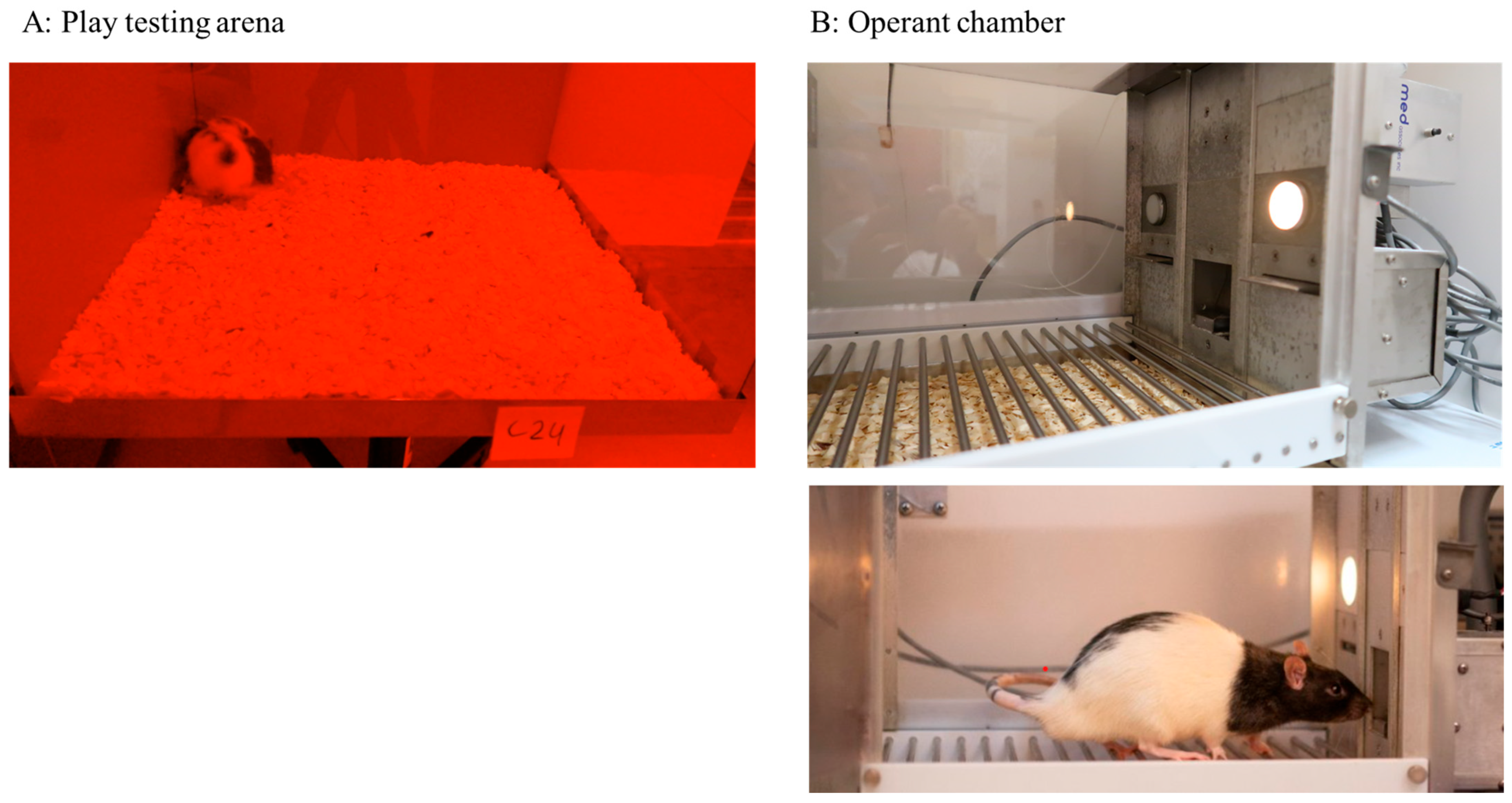
2.2. Assessment of Social Play Behaviour
2.3. Behavioural Assessment of Operant Conditioning for Sucrose
2.4. Statistical Analysis
3. Results
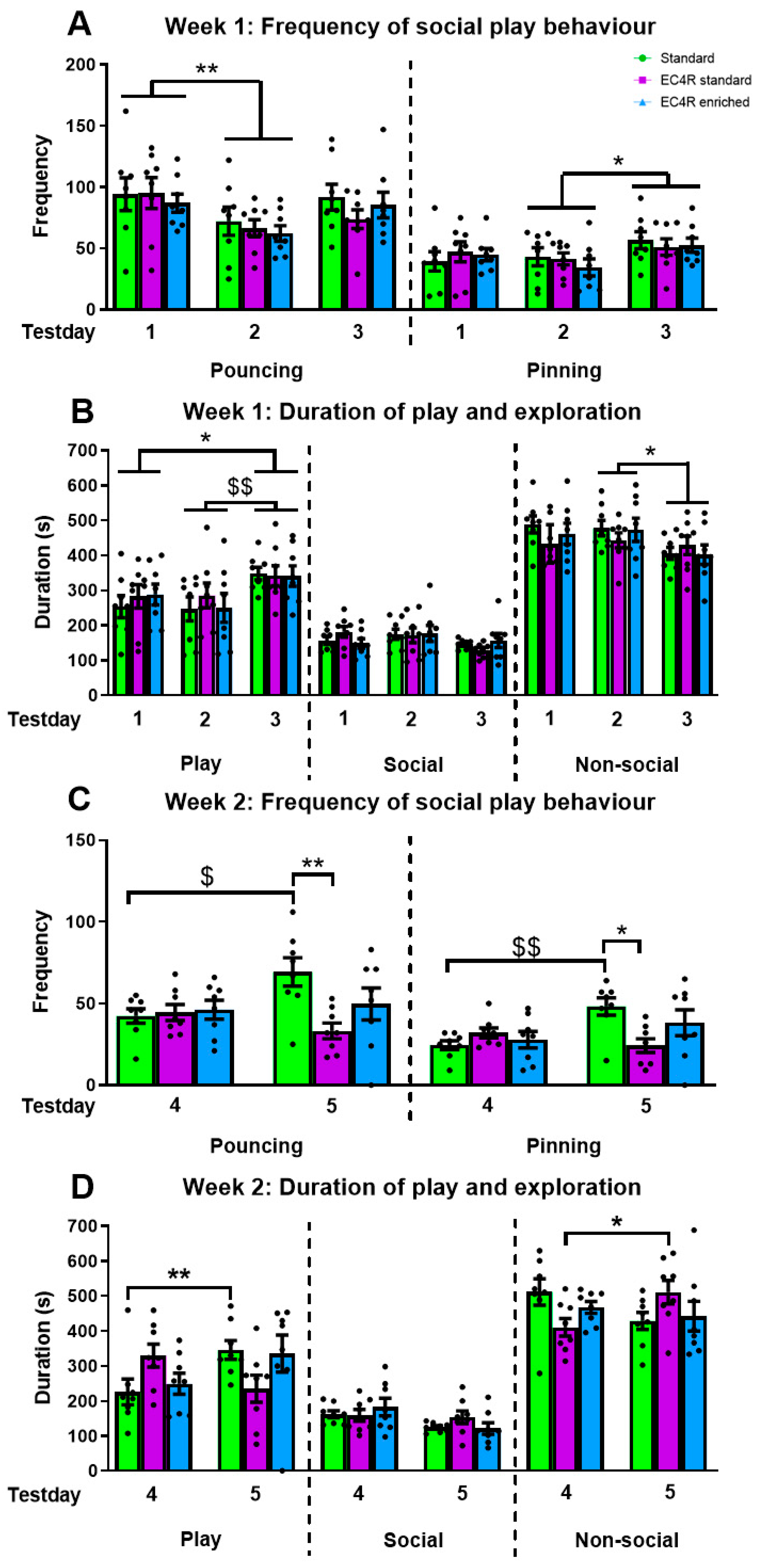
3.1. Play Peak Week 1
3.1.1. Play Frequency and Latency
3.1.2. Duration of Play and Exploration
3.2. Play Peak Week 2
3.2.1. Play Frequency and Latency
3.2.2. Duration of Play and Exploration
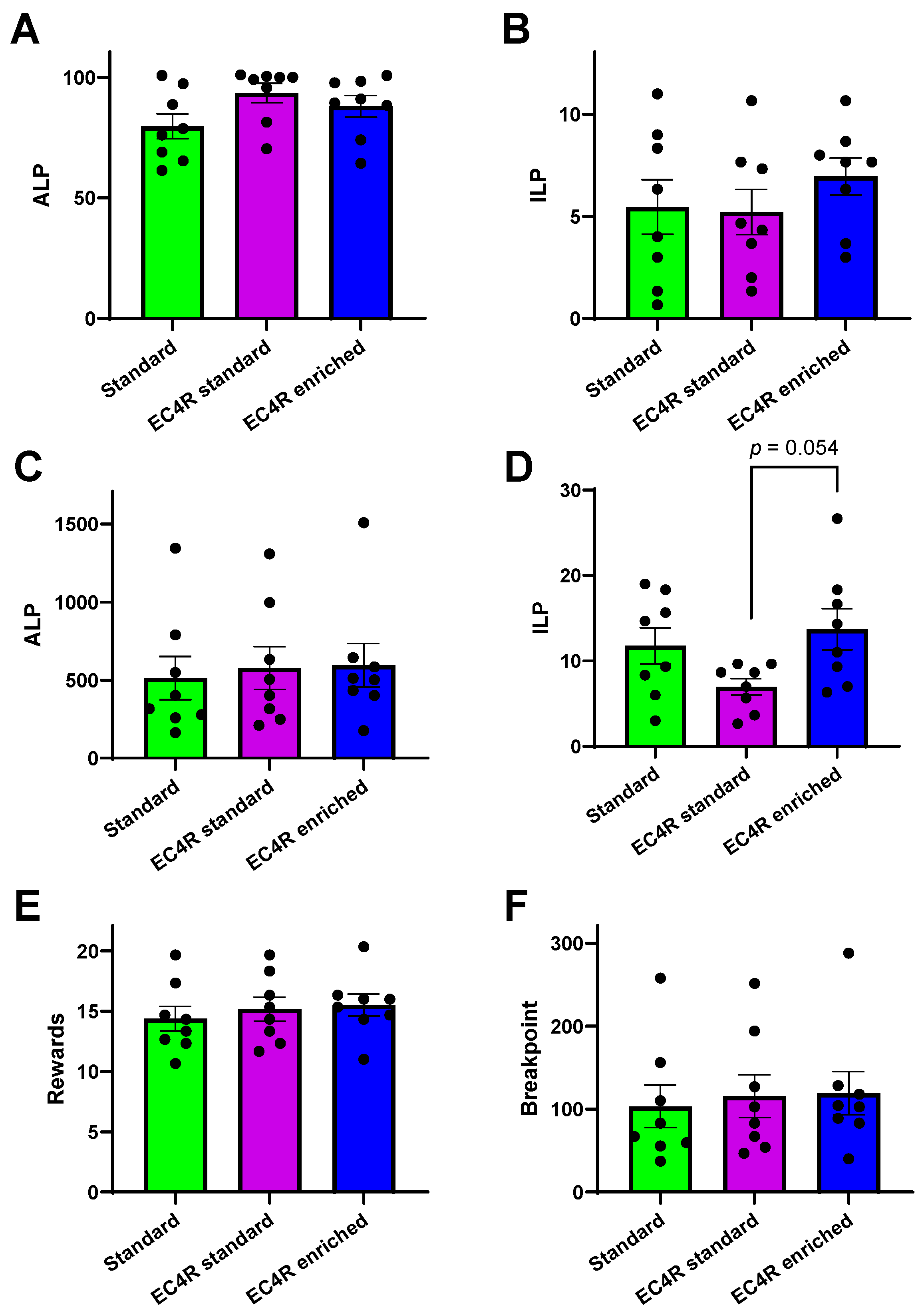
3.3. Operant Conditioning for Sucrose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawkins, M.S. Behaviour as a tool in the assessment of animal welfare. Zoology 2003, 106, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Yeates, J. Naturalness and Animal Welfare. Animals 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. 2010. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 11 September 2025).
- Mazhary, H.; Hawkins, P. Applying the 3Rs: A Case Study on Evidence and Perceptions Relating to Rat Cage Height in the UK. Animals 2019, 9, 1104. [Google Scholar] [CrossRef]
- Van Loo, P.L.P.; Allaart, J.; Oosterveld-Romijn, T.; Visser, E.; De Wit-Korstanje, M. Evaluatie van omgevingsverrijking voor verschillende diersoorten. Biotechniek 2021, 60, 27–32. [Google Scholar]
- Berdoy, M. The Laboratory Rat: A Natural History. Oxford University, 2002. Available online: https://www.youtube.com/watch?v=giu5WjUt2GA (accessed on 11 September 2025).
- Makowska, I.J.; Weary, D.M. A Good Life for Laboratory Rodents? ILAR J. 2021, 60, 373–388. [Google Scholar] [CrossRef]
- Spangenberg, E.; Augustsson, H.; Dahlborn, K.; Essén-Gustavsson, B.; Cvek, K. Housing-related activity in rats: Effects on body weight, urinary corticosterone levels, muscle properties and performance. Lab. Anim. 2005, 39, 45–57. [Google Scholar] [CrossRef]
- Scariot, P.P.M.; Manchado-Gobatto, F.d.B.; Torsoni, A.S.; Torsoni, M.A.; Reis, I.G.M.d.; Beck, W.R.; Gobatto, C.A. Wide housing space and chronic exercise enhance physical fitness and adipose tissue morphology in rats. Appl. Physiol. Nutr. Metab. 2015, 40, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Roemers, P.; Hulst, Y.; van Heijningen, S.; van Dijk, G.; van Heuvelen, M.J.G.; De Deyn, P.P.; van der Zee, E.A. Inducing Physical Inactivity in Mice: Preventing Climbing and Reducing Cage Size Negatively Affect Physical Fitness and Body Composition. Front. Behav. Neurosci. 2019, 13, 221. [Google Scholar] [CrossRef]
- Caires, C.R.S.; Bossolani-Martins, A.L. Which form of environmental enrichment is most effective in rodent models of autism? Behav. Process. 2023, 211, 104915. [Google Scholar] [CrossRef]
- Grimm, J.W.; Sauter, F. Environmental enrichment reduces food seeking and taking in rats: A review. Pharmacol. Biochem. Behav. 2020, 190, 172874. [Google Scholar] [CrossRef] [PubMed]
- Ratuski, A.S.; Weary, D.M. Environmental Enrichment for Rats and Mice Housed in Laboratories: A Metareview. Animals 2022, 12, 414. [Google Scholar] [CrossRef]
- Hansen, H. A super enriched rat cage—With increased ergonomics for staff. In Proceedings of the 15th FELASA Congress, Marseille, France, 13–16 June 2022; pp. 124–125. [Google Scholar]
- van Tilborg, E.; Achterberg, E.J.M.; van Kammen, C.M.; van der Toorn, A.; Groenendaal, F.; Dijkhuizen, R.M.; Heijnen, C.J.; Vanderschuren, L.; Benders, M.; Nijboer, C.H.A. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 2018, 66, 78–93. [Google Scholar] [CrossRef]
- Vaes, J.E.G.; Kosmeijer, C.M.; Kaal, M.; van Vliet, R.; Brandt, M.J.V.; Benders, M.; Nijboer, C.H. Regenerative Therapies to Restore Interneuron Disturbances in Experimental Models of Encephalopathy of Prematurity. Int. J. Mol. Sci. 2020, 22, 211. [Google Scholar] [CrossRef]
- Bateson, P. Playfulness and creativity. Curr. Biol. 2015, 25, R12–R16. [Google Scholar] [CrossRef]
- Ginsburg, K.R. The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics 2007, 119, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Pellis, S.; Pellis, V. The Playful Brain: Venturing to the Limits of Neuroscience; Oneworld: Oxford, UK, 2009. [Google Scholar]
- Vanderschuren, L.J.M.J.; Trezza, V. What the laboratory rat has taught us about social play behavior: Role in behavioral development and neural mechanisms. Curr. Top. Behav. Neurosci. 2014, 16, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. The ontogeny of play in rats. Dev. Psychobiol. 1981, 14, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Baarendse, P.J.; Counotte, D.S.; O’Donnell, P.; Vanderschuren, L.J. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology 2013, 38, 1485–1494. [Google Scholar] [CrossRef]
- Bijlsma, A.; Omrani, A.; Spoelder, M.; Verharen, J.P.H.; Bauer, L.; Cornelis, C.; de Zwart, B.; van Dorland, R.; Vanderschuren, L.; Wierenga, C.J. Social Play Behavior Is Critical for the Development of Prefrontal Inhibitory Synapses and Cognitive Flexibility in Rats. J. Neurosci. 2022, 42, 8716–8728. [Google Scholar] [CrossRef]
- Bijlsma, A.; Vanderschuren, L.; Wierenga, C.J. Social play behavior shapes the development of prefrontal inhibition in a region-specific manner. Cereb. Cortex 2023, 33, 9399–9408. [Google Scholar] [CrossRef]
- van den Berg, C.L.; Hol, T.; Van Ree, J.M.; Spruijt, B.M.; Everts, H.; Koolhaas, J.M. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999, 34, 129–138. [Google Scholar] [CrossRef]
- Kentrop, J.; Smid, C.R.; Achterberg, E.J.M.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J.; Joëls, M.; van der Veen, R. Effects of Maternal Deprivation and Complex Housing on Rat Social Behavior in Adolescence and Adulthood. Front. Behav. Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef]
- Siviy, S.M.; Harrison, K.A. Effects of neonatal handling on play behavior and fear towards a predator odor in juvenile rats (Rattus norvegicus). J. Comp. Psychol. 2008, 122, 1. [Google Scholar] [CrossRef]
- Bijlsma, A. Opportunities for risk-taking during play alters cognitive performance and prefrontal inhibition in rats. Eur. J. Neurosci. 2024, 59, 2748–2765. [Google Scholar]
- Castelhano-Carlos, M.J.; Aslani, S.; Sousa, N. The Impact of Physical Enrichment in the Structure of the Medial Prefrontal Cortex and Nucleus Accumbens of the Adult Male Rat Brain. Neuroscience 2021, 454, 51–60. [Google Scholar] [CrossRef]
- Achterberg, E.J.M.; Damsteegt, R.; Vanderschuren, L. On the central noradrenergic mechanism underlying the social play-suppressant effect of methylphenidate in rats. Behav. Brain Res. 2018, 347, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.; Van Ree, J.M. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 1989, 28, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, L.J.M.J.; Niesink, R.J.; Spruijt, B.M.; Van Ree, J.M. Influence of environmental factors on social play behavior of juvenile rats. Physiol. Behav. 1995, 58, 119–123. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Trezza, V.; Griffioen-Roose, S.; Schiepers, O.J.; Van Leeuwen, N.; De Vries, T.J.; Schoffelmeer, A.N. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology 2008, 33, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Beatty, W.W. Social deprivation and play in rats. Behav. Neural Biol. 1980, 30, 197–206. [Google Scholar] [CrossRef]
- Pellis, S.M.; Pellis, V.C. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 1987, 13, 227–242. [Google Scholar] [CrossRef]
- Poole, T.; Fish, J. An investigation of playful behaviour in Rattus norvegicus and Mus musculus (Mammalia). J. Zool. 2009, 175, 61–71. [Google Scholar] [CrossRef]
- Trezza, V.; Baarendse, P.J.; Vanderschuren, L.J. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010, 31, 463–469. [Google Scholar] [CrossRef]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Minnaard, A.M.; Luijendijk, M.C.M.; Baars, A.M.; Drost, L.; Ramakers, G.M.J.; Adan, R.A.H.; Lesscher, H.M.B.; Vanderschuren, L.J.M.J. Increased elasticity of sucrose demand during hyperdopaminergic states in rats. Psychopharmacology 2022, 239, 773–794. [Google Scholar] [CrossRef]
- Tanaś, Ł.; Ostaszewski, P.; Iwan, A. Effects of post-weaning social isolation and environment al enrichment on exploratory behavior and ankiety in Wistar rats. Acta Neurobiol. Exp. 2015, 75, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Morley-Fletcher, S.; Rea, M.; Maccari, S.; Laviola, G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci. 2003, 18, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Fornaguera, J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: Associations with depressive-like behavior and ventral striatum dopamine. Neurosci. Lett. 2008, 436, 278–282. [Google Scholar] [CrossRef]
- Grimm, J.W.; Osincup, D.; Wells, B.; Manaois, M.; Fyall, A.; Buse, C.; Harkness, J.H. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav. Pharmacol. 2008, 19, 777–785. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achterberg, E.J.M.; Baars, A.-M.J.M.; van Hal, D.A.; Lesscher, H.M.B.; Van Loo, P.L.P. Evaluation of the Effects of Enlarged Housing on Social Play and Reward Seeking in Rats. Animals 2025, 15, 2757. https://doi.org/10.3390/ani15182757
Achterberg EJM, Baars A-MJM, van Hal DA, Lesscher HMB, Van Loo PLP. Evaluation of the Effects of Enlarged Housing on Social Play and Reward Seeking in Rats. Animals. 2025; 15(18):2757. https://doi.org/10.3390/ani15182757
Chicago/Turabian StyleAchterberg, E. J. Marijke, Anne-Marie J. M. Baars, Daphne A. van Hal, Heidi M. B. Lesscher, and Pascalle L. P. Van Loo. 2025. "Evaluation of the Effects of Enlarged Housing on Social Play and Reward Seeking in Rats" Animals 15, no. 18: 2757. https://doi.org/10.3390/ani15182757
APA StyleAchterberg, E. J. M., Baars, A.-M. J. M., van Hal, D. A., Lesscher, H. M. B., & Van Loo, P. L. P. (2025). Evaluation of the Effects of Enlarged Housing on Social Play and Reward Seeking in Rats. Animals, 15(18), 2757. https://doi.org/10.3390/ani15182757





