Effects of Chronic Thermal Stress on the Physiology, Metabolism, Histology, and Gut Microbiota of Juvenile Schizothorax grahami
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fish and Experimental Design
2.2. Sample Preparation
2.3. Serum Biochemical Indicator Measurement
2.4. Intestine Histological Analysis and Measurements
2.5. Western Blot Analysis
2.6. RT-qPCR Analysis
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
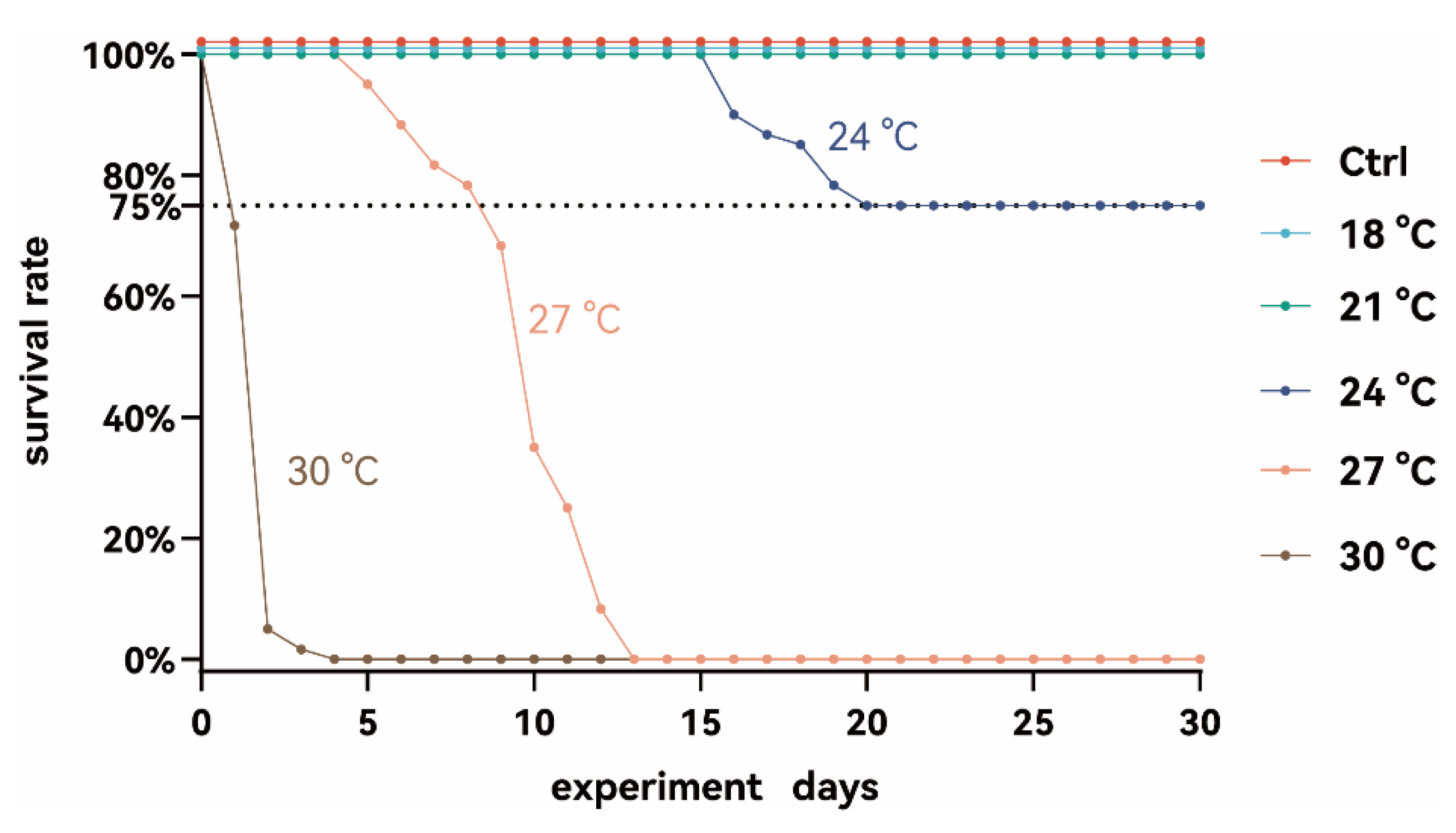
3.1. Effects of Chronic Thermal Stress on Survival of Juvenile S. grahami
3.2. Effects of Chronic Thermal Stress on Serum Basic Biochemical Parameters and Antioxidant Enzyme Activities in Juvenile S. grahami
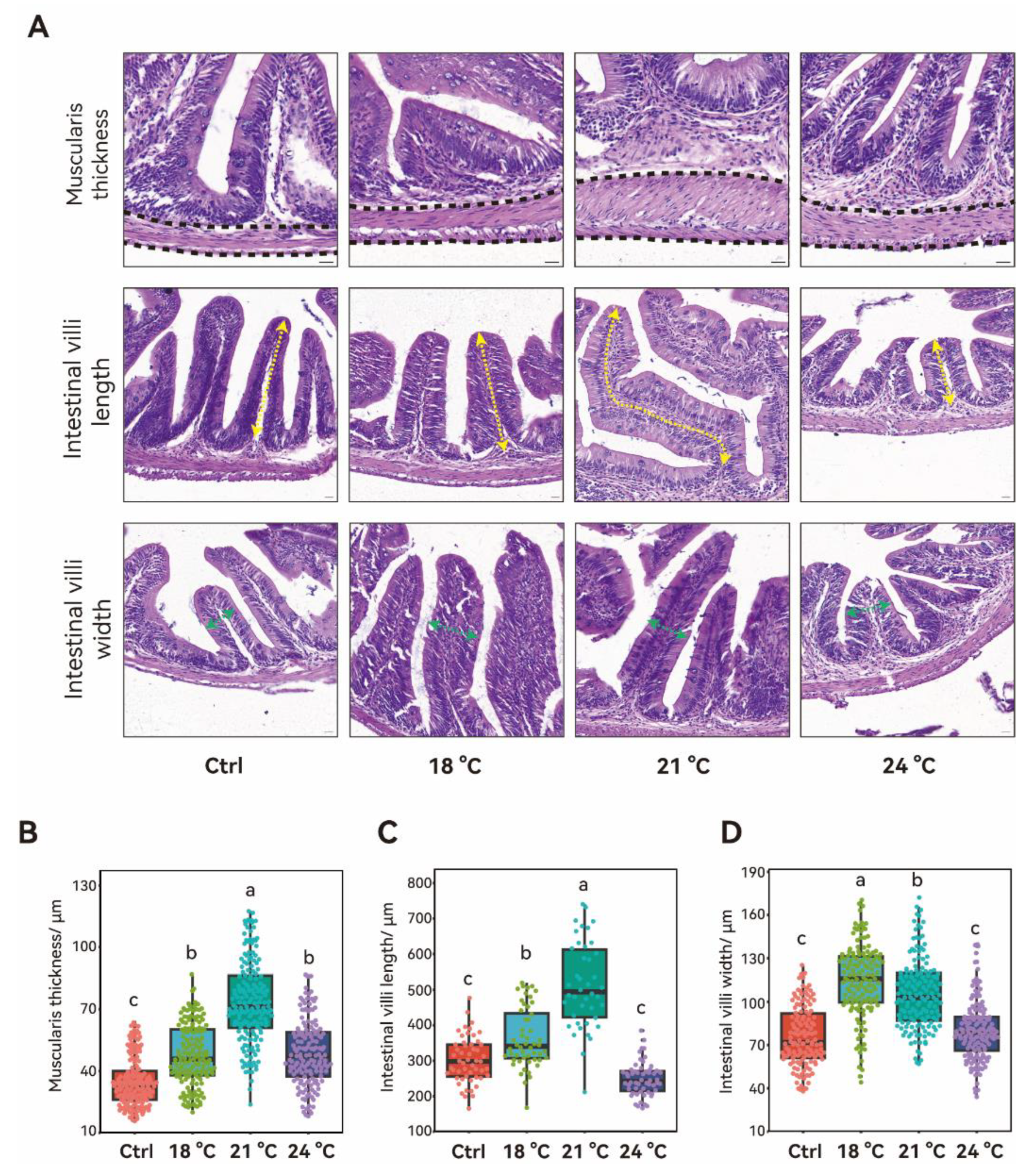
3.3. Effects of Chronic Thermal Stress on Middle Intestinal Structure in Juvenile S. grahami
3.4. Effects of Chronic Thermal Stress on Hepatic Stress- and Inflammation-Related Protein Expression in Juvenile S. grahami
3.5. Effects of Chronic Thermal Stress on Hepatic Gene Expression in Juvenile S. grahami
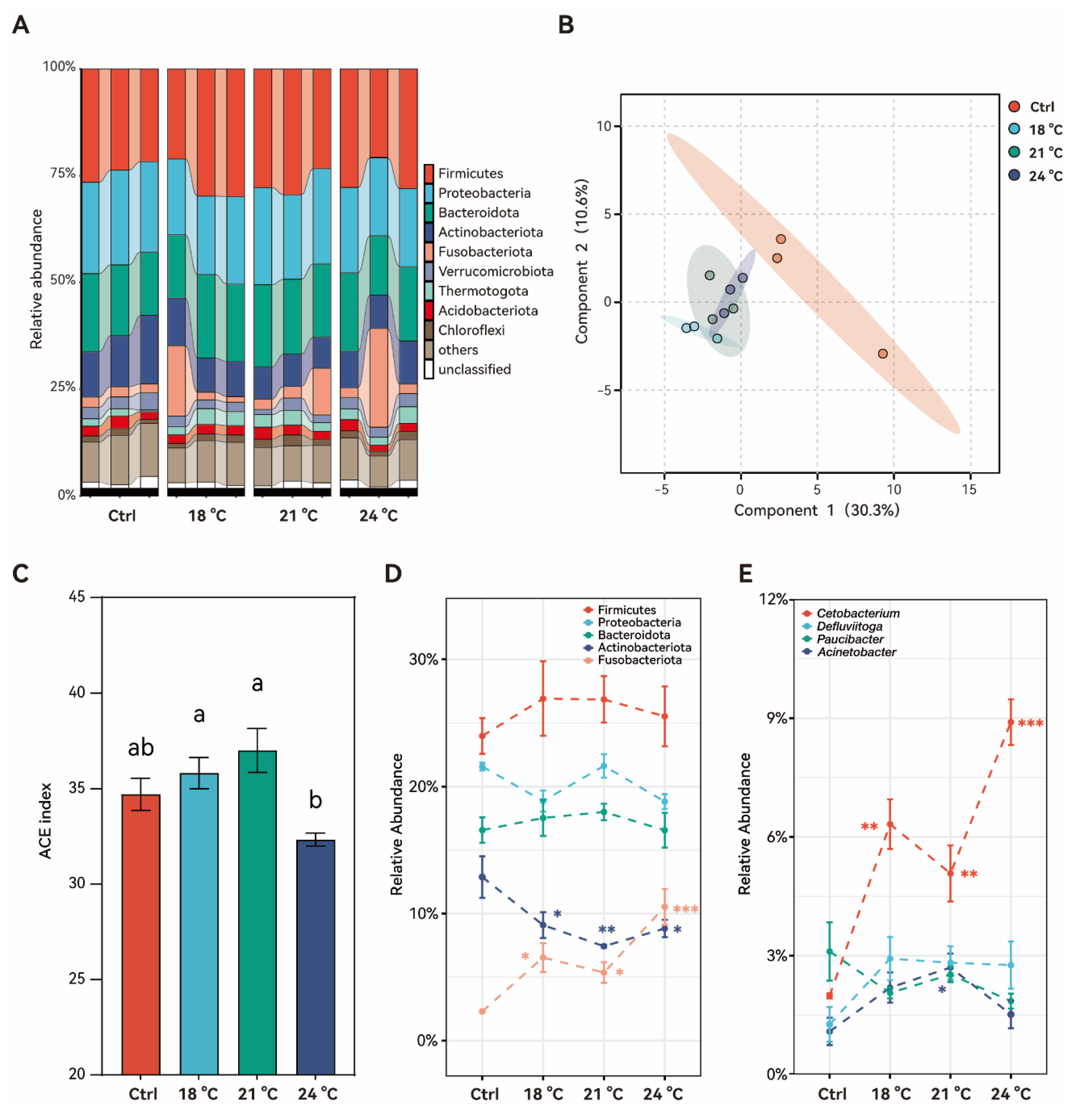
3.6. Effects of Chronic Thermal Stress on Gut Microbiota in Juvenile S. grahami
4. Discussion
4.1. Exposure to Temperatures Above 30 °C Induces Acute Lethality in Juvenile S. grahami
4.2. Chronic Exposure to Thermal Stress Leads to Rapid Energy Depletion and Disruption of the Antioxidant System in Juvenile S. grahami
4.3. Chronic Exposure to Thermal Stress Leads to Intestinal Villus Hypertrophy and Alters the Gut Microbiota Community Structure in Juvenile S. grahami
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, T.; Shelton, J.M.; Dallas, H.F. Does thermal history influence thermal tolerance of the freshwater fish Galaxias zebratus in a global biodiversity hotspot? J. Therm. Biol. 2021, 97, 102890. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.K.; Geargeoura, S.; Fraser, D.J. Effects of climate on salmonid productivity: A global meta-analysis across freshwater ecosystems. Glob. Change Biol. 2022, 28, 7250–7269. [Google Scholar] [CrossRef]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, K.M.; Connon, R.E.; Davis, B.E.; Komoroske, L.M.; Britton, M.T.; Sommer, T.; Todgham, A.E.; Fangue, N.A. Effects of high temperatures on threatened estuarine fishes during periods of extreme drought. J. Exp. Biol. 2016, 219, 1705–1716. [Google Scholar] [CrossRef]
- Sayer, C.A.; Fernando, E.; Jimenez, R.R.; Macfarlane, N.B.W.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Contreras-MacBeath, T.; Cox, N.A.; Harrison, I.; et al. One-quarter of freshwater fauna threatened with extinction. Nature 2025, 638, 138–145. [Google Scholar] [CrossRef]
- Tao, J.; He, D.; Kennard, M.J.; Ding, C.; Bunn, S.E.; Liu, C.; Jia, Y.; Che, R.; Chen, Y. Strong evidence for changing fish reproductive phenology under climate warming on the Tibetan Plateau. Glob. Change Biol. 2018, 24, 2093–2104. [Google Scholar] [CrossRef]
- Li, W.; Guo, W.J.; Liu, H.; Qiao, Q.L.; Zhao, K.F.; Ai, D.H.; Liu, C.Y.; Zhao, W.H. Spawning grounds of Schizothoracinae fish on the Tibetan Plateau. Environ. Biol. Fishes 2024, 107, 1263–1276. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 2021, 22, 261. [Google Scholar] [CrossRef]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and acute temperature rise induces crossing endocrine, metabolic, and immunological pathways in maraena whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Hassenruck, C.; Reinwald, H.; Kunzmann, A.; Tiedemann, I.; Gardes, A. Effects of thermal stress on the gut microbiome of juvenile milkfish (Chanos chanos). Microorganisms 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Moeller, A.H. The effects of temperature on animal gut microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Schnurr, M.E.; Yin, Y.; Scott, G.R. Temperature during embryonic development has persistent effects on metabolic enzymes in the muscle of zebrafish. J. Exp. Biol. 2014, 217, 1370–1380. [Google Scholar] [CrossRef]
- Bilyk, K.T.; Sformo, T.L. Varying heat tolerance among Arctic nearshore fishes. Polar Biol. 2021, 44, 607–612. [Google Scholar] [CrossRef]
- Chen, X. Checklist of Fishes of Yunnan. Zool. Res. 2013, 34, 281–343. [Google Scholar] [CrossRef]
- Li, S.; Guo, H.; Chen, Z.; Jiang, Y.; Shen, J.; Pang, X.; Li, Y. Effects of acclimation temperature regime on the thermal tolerance, growth performance and gene expression of a cold-water fish, Schizothorax prenanti. J. Therm. Biol. 2021, 98, 102918. [Google Scholar] [CrossRef]
- He, Y.; Wu, X.; Zhu, Y.; Li, H.; Li, X.; Yang, D. Effect of rearing temperature on growth and thermal tolerance of Schizothorax (Racoma) kozlovi larvae and juveniles. J. Therm. Biol. 2014, 46, 24–30. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Haung, G.; Wang, T.; Wang, Q.; Su, X.; Ren, Z.; Chotamonsak, C.; Limsakul, A.; Torsri, K. Characteristics of super drought in Southwest China and the associated compounding effect of multiscalar anomalies. Sci. China Earth Sci. 2024, 67, 2084–2102. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Zhou, X.; Feng, W.; Gao, G.; Li, M.; Zheng, G. Causes of the severe drought in Southwest China during the summer of 2022. Atmos. Res. 2024, 303, 107320. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schleger, I.C.; Pereira, D.M.C.; Resende, A.C.; Romao, S.; Herrerias, T.; Neundorf, A.K.A.; Sloty, A.M.; Guimaraes, I.M.; de Souza, M.; Carster, G.P.; et al. Cold and warm waters: Energy metabolism and antioxidant defenses of the freshwater fish Astyanax lacustris (Characiformes: Characidae) under thermal stress. J. Comp. Physiol. B 2022, 192, 77–94. [Google Scholar] [CrossRef]
- Yan, H.; Du, J.; Li, S.; Lei, C.; Zhu, T.; Han, L.; Song, H. Chronic heat stress is capable of reducing the growth performance, causing damage to the liver structure, and altering the liver glucose metabolism and lipid metabolism in largemouth bass (Micropterus salmoides L.). Fish Physiol. Biochem. 2025, 51, 24. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Cui, C.; Du, Z.J.; He, Z.; Wang, Y.; Li, X.W.; Yang, S. Influence of long-term temperature stress on respiration frequency, Na(+)/K(+)-ATPase activity, and lipid metabolism in common carp (Cyprinus carpio). J. Therm. Biol. 2019, 83, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Res. 2009, 2009, 501739. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Sanderson, L.M.; Matilainen, M.; Stienstra, R.; Carlberg, C.; de Groot, P.J.; Muller, M.; Kersten, S. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007, 2007, 26839. [Google Scholar] [CrossRef]
- Mueller, I.A.; Grim, J.M.; Beers, J.M.; Crockett, E.L.; O’Brien, K.M. Inter-relationship between mitochondrial function and susceptibility to oxidative stress in red- and white-blooded Antarctic notothenioid fishes. J. Exp. Biol. 2011, 214 Pt 22, 3732–3741. [Google Scholar] [CrossRef]
- Chang, C.H.; Zhou, X.W.; Wang, Y.C.; Lee, T.H. Differential effects of hypothermal stress on lactate metabolism in fresh water- and seawater-acclimated milkfish, Chanos chanos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 248, 110744. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Deng, W.; Zhang, D.; Gao, Y.; Yang, Z.; Shi, X.; Sun, J.; Zhou, J.; Ji, H. Antioxidant defenses of Onychostoma macrolepis in response to thermal stress: Insight from mRNA expression and activity of superoxide dismutase and catalase. Fish Shellfish Immunol. 2017, 66, 50–61. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Yang, Y.; Li, J.; Yang, X.; Li, L.; Zheng, Z.; Yang, B.; Zhang, P.; Liu, H. Effects of chronic cold stress and thermal stress on growth performance, hepatic apoptosis, oxidative stress, immune response and gut microbiota of juvenile hybrid sturgeon (Acipenser baerii ♀ x A. schrenkii ♂). Fish Shellfish Immunol. 2025, 157, 110078. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress-induced oxidative damages in the liver and associated death in fish, Labeo rohita. Fish Physiol. Biochem. 2021, 47, 21–32. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Zhao, C.; Xiao, Z.; Xu, S.; Xiao, Y.; Wang, Y.; Liu, Q.; Li, J. The expression pattern of hsp70 plays a critical role in thermal tolerance of marine demersal fish: Multilevel responses of Paralichthys olivaceus and its hybrids (P. olivaceus ♀ x P. dentatus ♂) to chronic and acute heat stress. Mar. Environ. Res. 2017, 129, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Hani, Y.M.I.; Marchand, A.; Turies, C.; Kerambrun, E.; Palluel, O.; Bado-Nilles, A.; Beaudouin, R.; Porcher, J.M.; Geffard, A.; Dedourge-Geffard, O. Digestive enzymes and gut morphometric parameters of threespine stickleback (Gasterosteus aculeatus): Influence of body size and temperature. PLoS ONE 2018, 13, e0194932. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Hossain, M.A.; Afrose, F.; Roy, N.C.; Iqbal, M.M. Effect of temperature on the growth performance, haematological properties and histomorphology of gill, intestine and liver tissues in juvenile butter catfish Ompok bimaculatus. Aquac. Fish Fish. 2022, 2, 277–286. [Google Scholar] [CrossRef]
- Zhu, T.; Li, X.; Wu, X.; Yang, D. Temperature acclimation alters the thermal tolerance and intestinal heat stress response in a Tibetan fish Oxygymnocypris stewarti. Front. Microbiol. 2022, 13, 898145. [Google Scholar] [CrossRef]
- Prabu, D.L.; Kalidas, C.; Ranjith, L.; Ebeneezar, S.; Kavitha, M.; Zacharia, P.U.; Vijayagopal, P.; Babu, A.M.; Muniswaran, B.R. Effect of water temperature on growth, blood biochemistry, digestive, metabolic enzymology, and antioxidant defences of Trachinotus blochii juveniles. Aquac. Int. 2022, 31, 1499–1522. [Google Scholar] [CrossRef]
- Huang, J.; Liao, Y.; Zhong, R.; Yang, C.; Wang, Q.; Deng, Y. Effects of temperature, salinity, and light on the growth performance, survival, final biomass, and digestive enzyme activities of juvenile Sipunculus nudus. Aquac. Rep. 2023, 33, 101772. [Google Scholar] [CrossRef]
- Kokou, F.; Sasson, G.; Nitzan, T.; Doron-Faigenboim, A.; Harpaz, S.; Cnaani, A.; Mizrahi, I. Host genetic selection for cold tolerance shapes microbiome composition and modulates its response to temperature. Elife 2018, 7, e36398. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2017, 10, 626–640. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, S.; Ka, W.; Gao, P.; Li, Y.; Long, R.; Wang, J. Association of gut microbiota with metabolism in rainbow trout under acute heat stress. Front. Microbiol. 2022, 13, 846336. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, H.; Wang, X.; Zhang, L.; Mou, C.; Huang, Z.; Ke, H.; Duan, Y.; Zhou, J.; Li, Q. Effects of different temperatures on Leiocassis longirostris gill structure and intestinal microbial composition. Sci. Rep. 2024, 14, 7150. [Google Scholar] [CrossRef]
- Steiner, K.; Laroche, O.; Walker, S.P.; Symonds, J.E. Effects of water temperature on the gut microbiome and physiology of Chinook salmon (Oncorhynchus tshawytscha) reared in a freshwater recirculating system. Aquaculture 2022, 560, 738529. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Yi, M.; Liu, Z.; Ke, X.; Gao, F.; Cao, J.; Wang, M.; Chen, G.; Lu, M. Characterization of the core gut microbiota of Nile tilapia (Oreochromis niloticus): Indication of a putative novel Cetobacterium species and analysis of its potential function on nutrition. Arch. Microbiol. 2022, 204, 690. [Google Scholar] [CrossRef]
- Ofek, T.; Lalzar, M.; Laviad-Shitrit, S.; Izhaki, I.; Halpern, M. Comparative study of intestinal microbiota composition of six edible fish species. Front. Microbiol. 2021, 12, 760266. [Google Scholar] [CrossRef]
- Xiao, F.; Liao, L.; Xu, Q.; He, Z.; Xiao, T.; Wang, J.; Huang, J.; Yu, Y.; Wu, B.; Yan, Q. Host-microbiota interactions and responses to grass carp reovirus infection in Ctenopharyngodon idellus. Environ. Microbiol. 2021, 23, 431–447. [Google Scholar] [CrossRef]
- Breen, P.; Winters, A.D.; Theis, K.R.; Withey, J.H. Vibrio cholerae infection induces strain-specific modulation of the zebrafish intestinal microbiome. Infect. Immun. 2021, 89, e0015721. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Wang, T. An empirical Bayes approach to normalization and differential abundance testing for microbiome data. BMC Bioinform. 2020, 21, 225. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Z.; Ding, Q.; Yang, Y.; Bindelle, J.; Ran, C.; Zhou, Z. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Sun, J.L.; Liang, J.; Liu, Q.; Luo, J.; Li, Z.Q.; Yan, T.M.; Zhou, J.; Yang, S. Enhancing lipid metabolism and inducing antioxidant and immune responses to adapt to acute hypoxic stress in Schizothorax prenanti. Aquaculture 2020, 519, 734933. [Google Scholar] [CrossRef]
- Zhang, R.; Mo, Q.; Wang, A.; Zhang, X.; Chen, C.; Wang, L.; Wang, Y. Axin interactor, dorsalization-associated (AIDA) protein promotes appetite and regulates hepatic glycolipid metabolism in Schizothorax prenanti. Aquaculture 2025, 595, 741549. [Google Scholar] [CrossRef]
- Silvestri, C.; Martella, A.; Poloso, N.J.; Piscitelli, F.; Capasso, R.; Izzo, A.; Woodward, D.F.; Di Marzo, V. Anandamide-derived prostamide F2α negatively regulates adipogenesis. J. Biol. Chem. 2013, 288, 23307–23321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Zheng, S.F.; Wang, S.C.; Liu, Q.Q.; Xu, S.W. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, S.; Zhang, Q.; Yang, Z.; Yin, K.; Xu, S. Atrazine hinders PMA-induced neutrophil extracellular traps in carp via the promotion of apoptosis and inhibition of ROS burst, autophagy and glycolysis. Environ. Pollut. 2018, 243, 282–291. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Li, T.; Kong, L.; Bi, B.; Hu, Q. Effects of Chronic Thermal Stress on the Physiology, Metabolism, Histology, and Gut Microbiota of Juvenile Schizothorax grahami. Animals 2025, 15, 2749. https://doi.org/10.3390/ani15182749
Bai S, Li T, Kong L, Bi B, Hu Q. Effects of Chronic Thermal Stress on the Physiology, Metabolism, Histology, and Gut Microbiota of Juvenile Schizothorax grahami. Animals. 2025; 15(18):2749. https://doi.org/10.3390/ani15182749
Chicago/Turabian StyleBai, Shuangqian, Tingyin Li, Lingfu Kong, Baoliang Bi, and Qing Hu. 2025. "Effects of Chronic Thermal Stress on the Physiology, Metabolism, Histology, and Gut Microbiota of Juvenile Schizothorax grahami" Animals 15, no. 18: 2749. https://doi.org/10.3390/ani15182749
APA StyleBai, S., Li, T., Kong, L., Bi, B., & Hu, Q. (2025). Effects of Chronic Thermal Stress on the Physiology, Metabolism, Histology, and Gut Microbiota of Juvenile Schizothorax grahami. Animals, 15(18), 2749. https://doi.org/10.3390/ani15182749




