A New Species of Boulenophrys (Megophridae) from Mt. Hengshan, Hunan Province, China, with Re-Description on B. hengshanensis †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
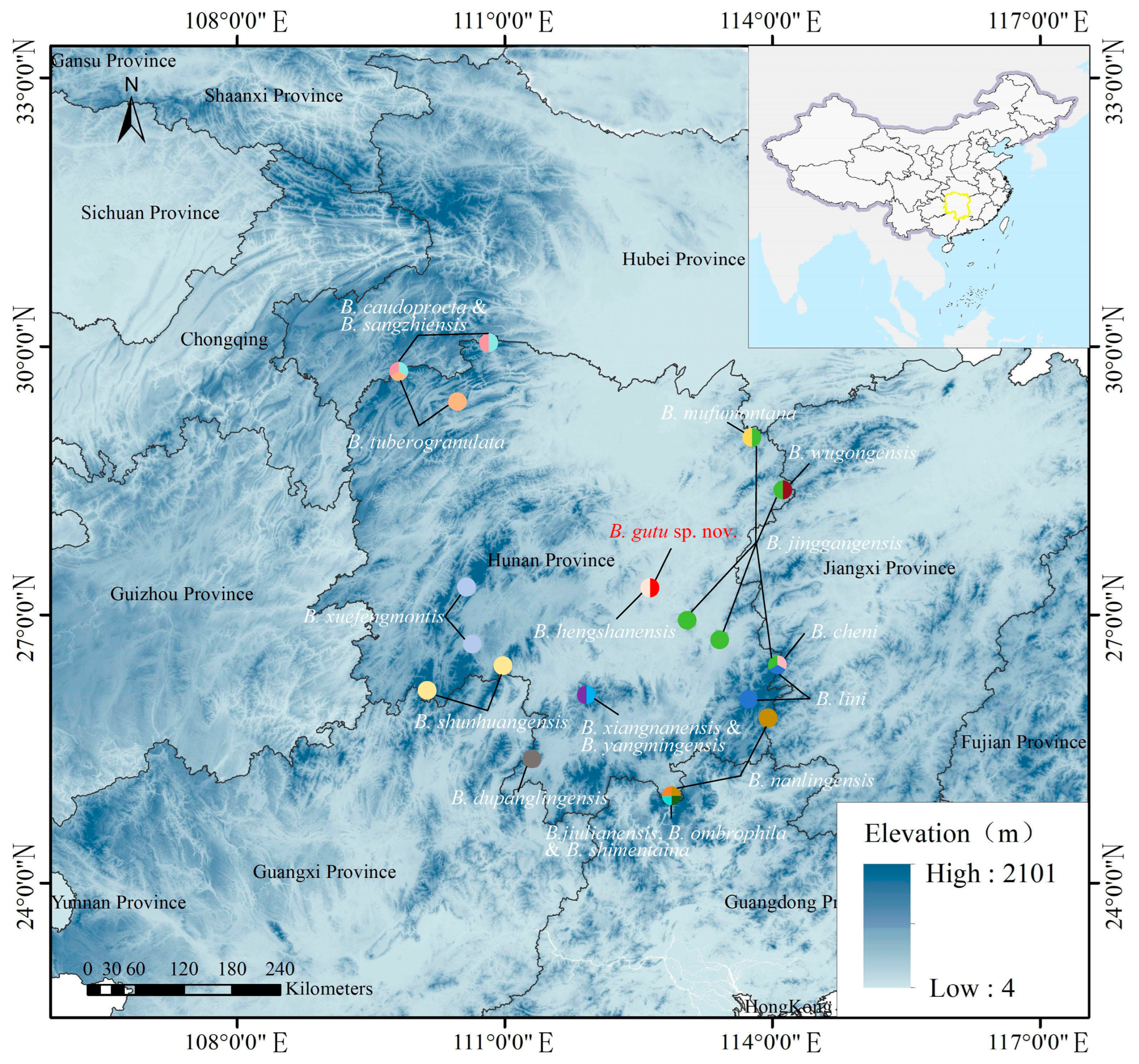
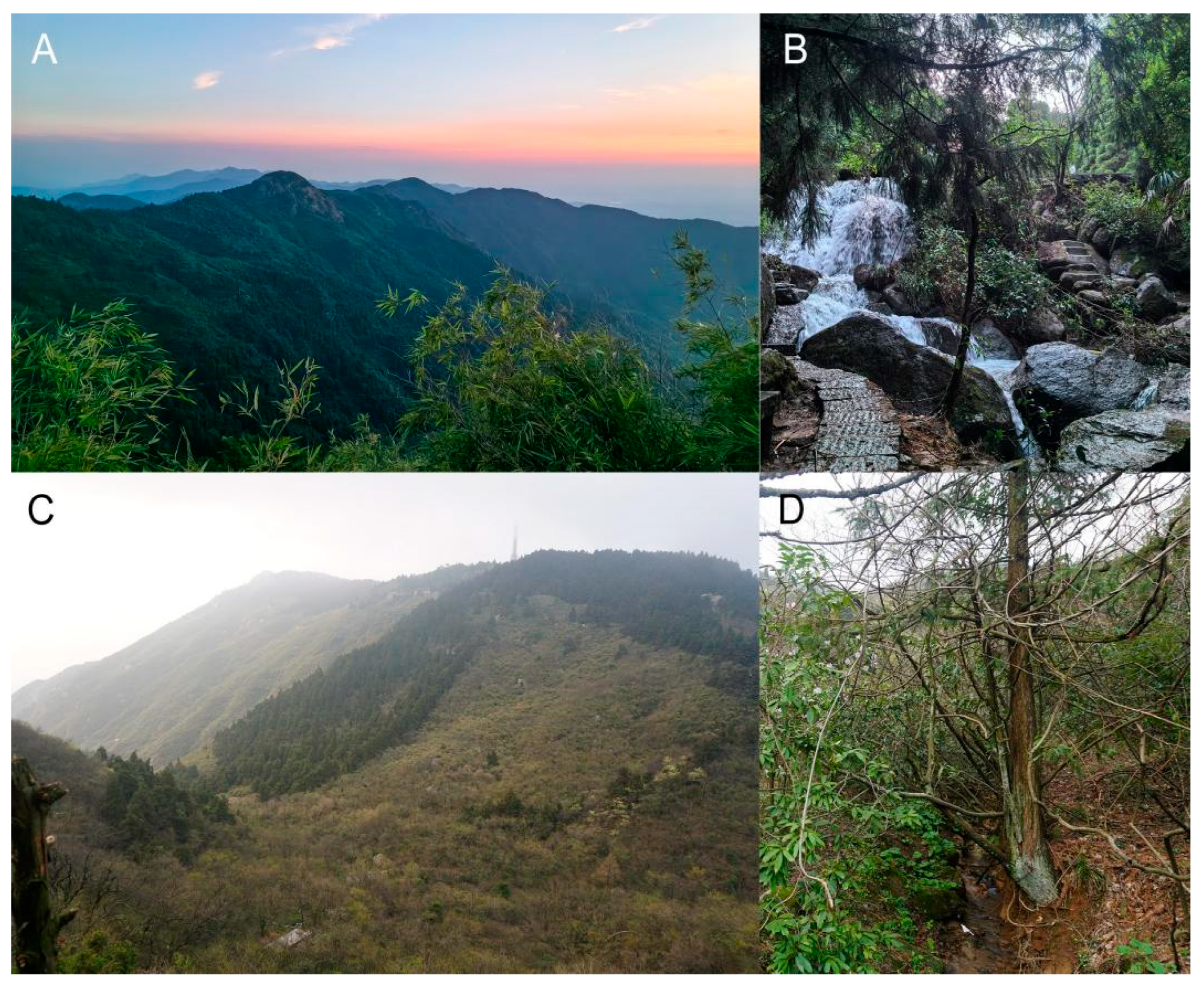
2.1. Sampling
2.2. Molecular and Phylogenetic Analyses
2.3. Morphological Analysis
3. Results
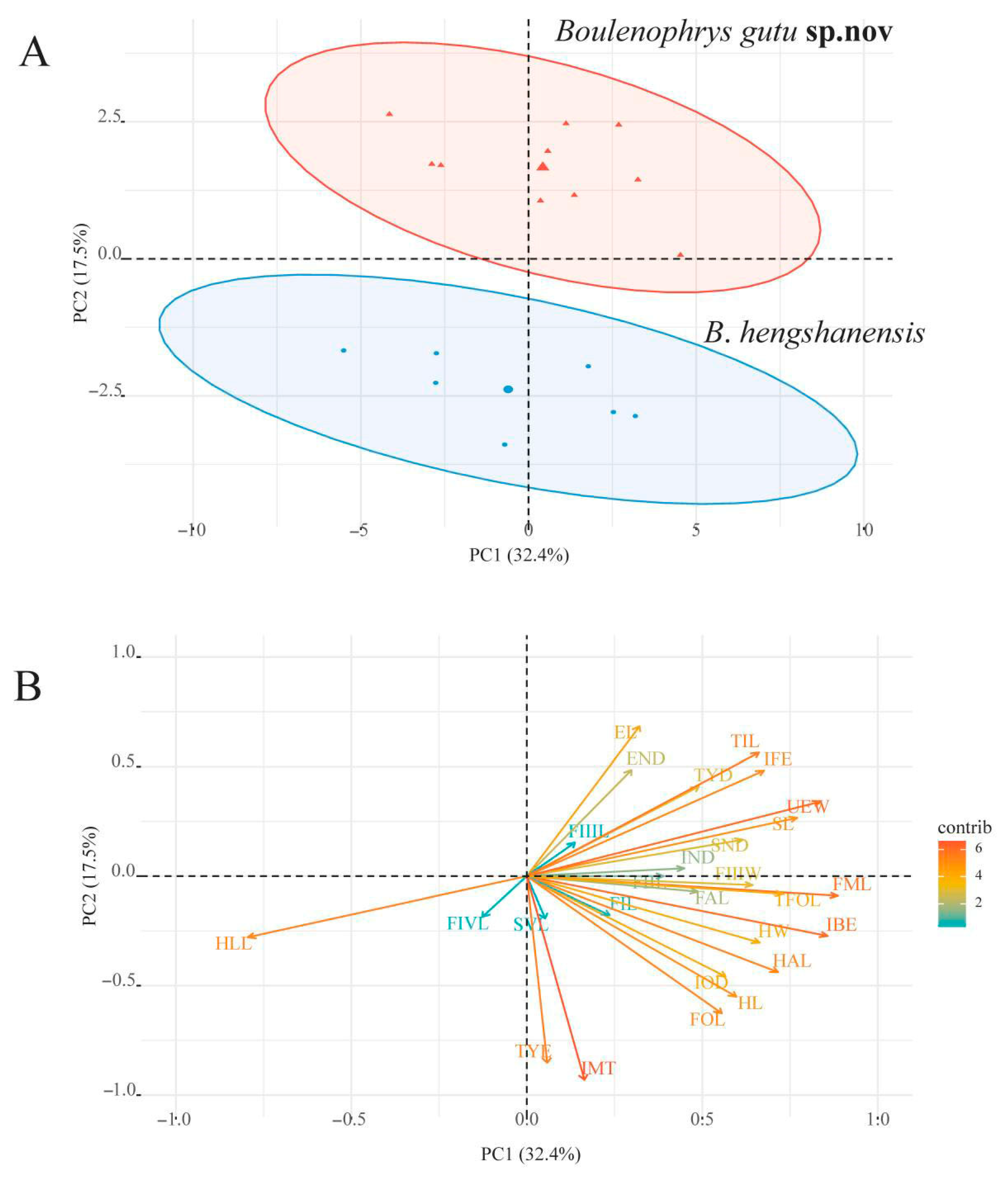
3.1. Phylogenetic Analyses
3.2. Morphological Analyses
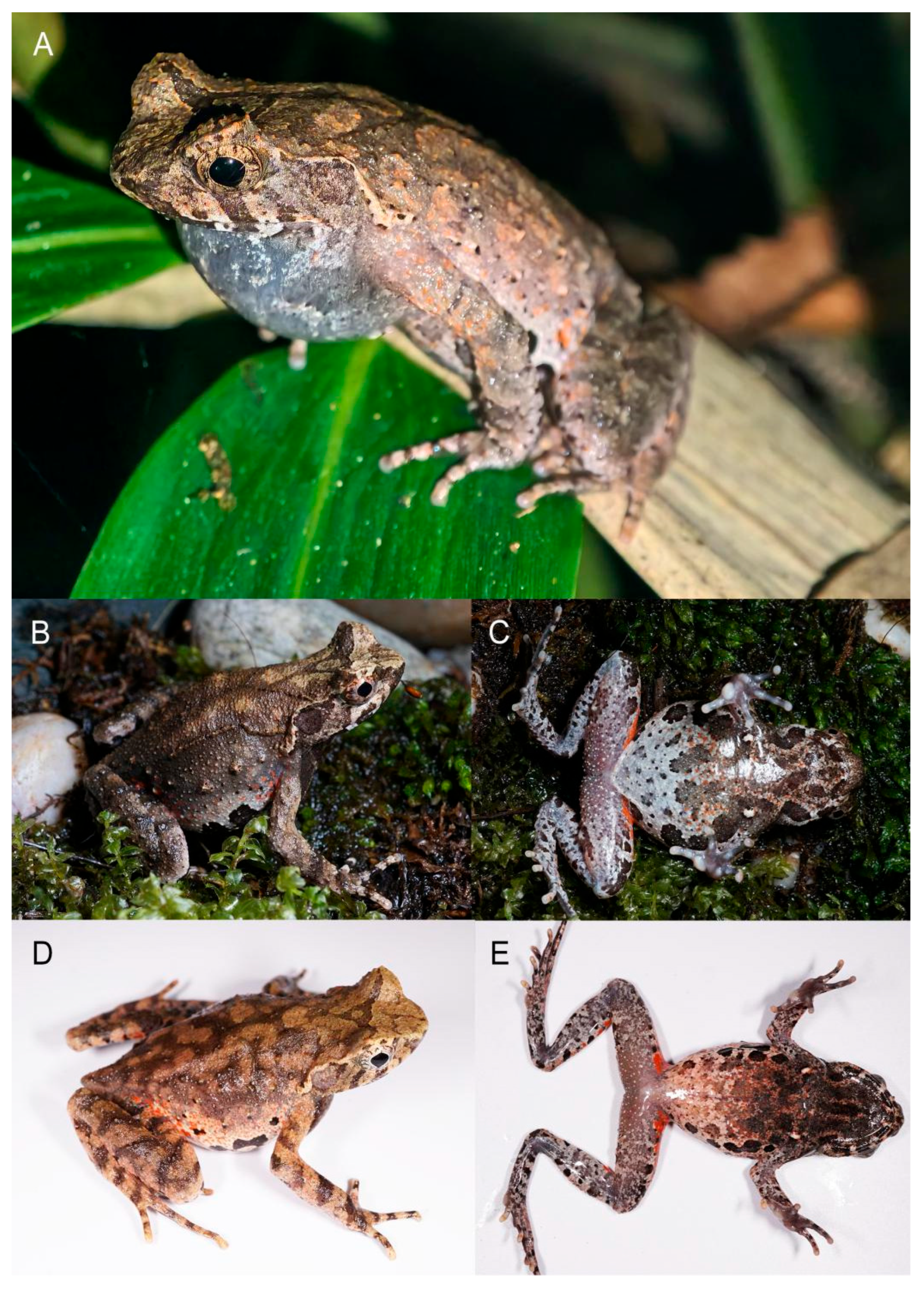
3.3. Taxonomic Account
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 27 July 2025).
- Lyu, Z.T.; Qi, S.; Wang, J.; Zhang, S.Y.; Zhao, J.; Zeng, Z.C.; Wan, H.; Yang, J.H.; Mo, Y.M.; Wang, Y.Y. Generic classification of Asian horned toads (Anura: Megophryidae: Megophryinae) and monograph of Chinese species. Zool Res. 2023, 44, 380–450. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, J.; Zhou, C.; Ma, L.; Wu, J.; Xie, C.; Zhu, Y.; Chen, S. A New Species of the Genus Boulenophrys from Fujian, China (Anura: Megophryidae). Chin. J. Wildl. 2025. Available online: https://link.cnki.net/urlid/23.1587.S.20250506.1103.002 (accessed on 9 July 2025). (In Chinese with English Abstract).
- Chen, J.M.; Zhou, W.W.; Poyarkov, N.A., Jr.; Stuart, B.L.; Brown, R.M.; Lathrop, A.; Wang, Y.Y.; Yuan, Z.Y.; Jiang, K.; Hou, M.; et al. A novel multilocus phylogenetic estimation reveals unrecognized diversity in Asian horned toads, genus Megophrys sensu lato (Anura: Megophryidae). Mol. Phylogenetics Evol. 2017, 106, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Chen, G.L.; Zhu, T.Q.; Zeng, Z.C.; Lyu, Z.T.; Wang, J.; Messenger, K.; Greenberg, A.J.; Guo, Z.; Yang, Z.H.; et al. Prevalence of cryptic species in morphologically uniform taxa—Fast speciation and evolutionary radiation in Asian frogs. Mol. Phylogenetics Evol. 2018, 127, 723–731. [Google Scholar] [CrossRef]
- Mahony, S.; Foley, N.M.; Biju, S.D.; Teeling, E.C. Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record. Mol. Biol. Evol. 2017, 34, 744–771. [Google Scholar] [CrossRef]
- Dubois, A.; Ohler, A.; Pyron, R.A. New concepts and methods for phylogenetic taxonomy and nomenclature in zoology, exemplified by a new ranked cladonomy of recent amphibians (Lissamphibia). Megataxa 2021, 5, 1–738. [Google Scholar] [CrossRef]
- Frost, D.R.; Grant, T.; Faivovich, J.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; De Sá, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 297, 1–291. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Cheng, Y.; Wei, G.; Wang, B.; Cheng, G. 2025. A new species of the genus Boulenophrys (Anura, Megophryidae) from Southwest China. Biodivers. Data J. 2025, 13, e153987. [Google Scholar] [CrossRef]
- Liu, J.; Feng, C.; Shen, T.; Li, S.Z.; Cheng, Y.; Wei, G.; Wang, B.; Su, H. A new species of the genus Boulenophrys (Anura, Megophryidae) from Guizhou, China. Herpetozoa 2025, 38, 117–136. [Google Scholar] [CrossRef]
- Xiao, B.; Xi, J.; Shi, S.; Li, H.; Liao, S.; Wang, B.; Mo, X.Y. A new species of the genus Boulenophrys (Anura, Megophryidae) from southern Hunan Province, central China. Animals 2025, 15, 440. [Google Scholar] [CrossRef]
- Li, S.; Shi, S.; Liu, J.; Zhao, J.; Gao, S.; Wang, B. A new species of the Boulenophrys (Anura, Megophryidae) from Hubei, China. Zoosyst. Evol. 2025, 101, 1213–1226. [Google Scholar]
- Qian, T.Y.; Hu, K.; Mo, X.Y.; Gao, Z.W.; Zhang, N.; Yang, D.D. A new species of Boulenophrys from central Hunan Province, China (Anura: Megophryidae). Vertebr. Zool. 2023, 73, 915–930. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Che, J.; Chen, H.M.; Yang, J.X.; Jin, J.Q.; Jiang, K.; Yuan, Z.Y.; Murphy, R.W.; Zhang, Y.P. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012, 12, 247–258. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Mahony, S.; Sengupta, S.; Kamei, R.G.; Biju, S.D. A new low altitude species of Megophrys Kuhl and van Hasselt (Amphibia: Megophryidae), from Assam, Northeast India. Zootaxa 2011, 3059, 36–46. [Google Scholar] [CrossRef]
- Fei, L.; Ye, C.Y. Amphibians of China; Chengdu Institute of Biology, Chinese Academy of Sciences, Science Press: Beijing, China, 2016; Volume 1. [Google Scholar]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef]
- Mo, J.W.; Yang, D.D.; Liu, S. Vertical distribution of amphibian resources of Nanyue Hengshan National Nature Reserve of Hunan Province. Sichuan J. Zool. 2009, 28, 145–146. [Google Scholar]
- Shen, Y.H.; Yang, D.D.; Mo, X.Y.; Li, H.H.; Chen, D. The Fauna of Hunan: Amphibia; Hunan Science and Technology Press: Changsha, China, 2014. [Google Scholar]
- Gao, Z.W.; Qian, T.Y.; Jiang, J.P.; Hou, D.J.; Deng, X.J.; Yang, D.D. Species diversity and distribution of amphibians and reptiles in Hunan Province, China. Biodivers. Sci. 2022, 30, 21290. [Google Scholar] [CrossRef]
- Li, Y.L.; Jin, M.J.; Zhao, J.; Liu, Z.Y.; Wang, Y.Y.; Pang, H. Description of two new species of the genus Megophrys (Amphibia: Anura: Megophryidae) from Heishiding Nature Reserve, Fengkai, Guangdong, China, based on molecular and morphological data. Zootaxa 2014, 3795, 449–471. [Google Scholar] [CrossRef]
- Wu, Y.H.; Suwannapoom, C.; Poyarkov, N.A., Jr.; Pawangkhanant, P.; Xu, K.; Jin, J.Q.; Murphy, R.W.; Che, J. A new species of the genus Xenophrys Anura Megophryidae from northern Thailand. Zool. Res. 2019, 40, 564–574. [Google Scholar] [CrossRef]
- Li, S.Z.; Lu, N.N.; Liu, J.; Wang, B. Description of a new Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from Guizhou Province, China. Zookeys 2020, 986, 101–126. [Google Scholar]
- Wu, Y.; Li, S.; Liu, W.; Wang, B.; Wu, J. Description of a new horned toad of Megophrys Kuhl & Van Hasselt, 1822 (Amphibia, Megophryidae) from Zhejiang Province, China. ZooKeys 2020, 1005, 73–102. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Pham, C.T.; Nguyen, T.T.; Luong, A.M.; Ziegler, T. A new species of Megophrys (Amphibia: Anura: Megophryidae) from Vietnam. Zootaxa 2020, 4722, 401–422. [Google Scholar] [CrossRef]
- Shen, Y.H. A new pelobatid toad of the genus Megophrys from China (Anura: Pelobatidae). In The 60th Anniversary of the Foundation of the Zoological Society of China, Nanking (China); Zoological Society of China, Ed.; China Science and Technology Publishing House: Beijing, China, 1994; pp. 603–606. [Google Scholar]
- Wang, Y.Y.; Zhao, J.; Yang, J.H.; Zhou, Z.; Chen, G.L.; Liu, Y. Morphology, molecular genetics, and bioacoustics support two new sympatric Xenophrys toads (Amphibia: Anura: Megophryidae) in southeast China. PLoS ONE 2014, 9, e93075. [Google Scholar] [CrossRef]
- Xu, N.; Li, S.Z.; Liu, J.; Wei, G.; Wang, B. A new species of the horned toad Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 943, 119–144. [Google Scholar]
- Luo, T.; Wang, Y.; Wang, S.; Lu, X.; Wang, W.; Deng, H.; Zhou, J. A species of the genus Panophrys (Anura, Megophryidae) from southeastern Guizhou Province, China. Zookeys 2021, 1047, 27–60. [Google Scholar] [CrossRef]
- Lyu, Z.T.; Zeng, Z.C.; Wang, J.; Liu, Z.Y.; Huang, Y.Q.; Li, W.Z.; Wang, Y.Y. Four new species of Panophrys (Anura, Megophryidae) from eastern China, with discussion on the recognition of Panophrys as a distinct genus. Zootaxa 2021, 4927, 009–040. [Google Scholar] [CrossRef]
- Rao, D.Q.; Yang, D.T. The karyotypes of Megophryinae (Pelobatidae) with a discussion on their classification and phylogenetic relationships. Asian Herpetol. Res. 1997, 7, 93–102. [Google Scholar]
- Zeng, Z.C.; Wang, J.; Chen, H.H.; Xiao, W.W.; Zhan, B.B.; Li, Y.H.; Lin, S.S. A New Species of the Genus Boulenophrys (Anura, Megophryidae) from Eastern Guangdong, China. Asian Herpetol. Res. 2024, 15, 12–21. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, L.; Ran, H.; Shen, Z.X. Megophrys binlingensis fanjingmontis: A New Subspecies of Megophryidae from Guizhou, China. Chin. J. Zool. 2012, 47, 135–138, (In Chinese with English abstract). [Google Scholar]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Luong, A.M.; Le, D.T.; Nguyen, T.T.; Nguyen, T.Q.; Portway, C.; et al. Two new and potentially highly threatened Megophrys Horned frogs (Amphibia: Megophryidae) from Indochina’s highest mountains. Zootaxa 2018, 4508, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, Z.C.; Lyu, Z.T.; Qi, S.; Liu, Z.Y.; Chen, H.H.; Lu, Y.H.; Xiao, H.W.; Lin, C.R.; Chen, K.; et al. Description of three new Boulenophrys species from eastern Guangdong, China, emphasizing the urgency of ecological conservation in this region (Anura, Megophryidae). Zootaxa 2022, 5099, 91–119. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.P.; Nguyen, L.T.; Portway, C.; Mahony, S.; Nguyen, C.T.; Harding, L.; Luong, H.V.; Rowley, J.J.L. A new potentially Endangered species of Megophrys (Amphibia: Megophryidae) from Mount Ky Quan San, north-west Vietnam. J. Nat. Hist. 2021, 54, 2543–2575. [Google Scholar] [CrossRef]
- Song, H.M.; Wang, H.T.; Qi, S.; Wang, N.; Wang, Y.Y. A new species of the genus Boulenophrys (Anura: Megophryidae: Megophryinae) from Zhuhai, Guangdong, China. Asian Herpetol. Res. 2024, 15, 251–264. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.Y.; Lyu, Z.T.; Zeng, Z.C.; Wang, Y.Y. A new species of the genus Xenophrys (Amphibia: Anura: Megophryidae) from an offshore island in Guangdong Province, southeastern China. Zootaxa 2017, 4324, 541–556. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.Z.; Wei, G.; Xu, N.; Cheng, Y.L.; Wang, B.; Wu, J. A New Species of the Asian Toad Genus Megophrys sensu lato (Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2020, 11, 1–18. [Google Scholar]
- Fei, L.; Ye, C.Y.; Huang, Y.Z. Two new subspecies of Megophrys omeimontis Liu from China (Amphibia, Pelobatidae). Acta Herpetol. Sin. 1983, 2, 49–52, (In Chinese with English Abstract). [Google Scholar]
- Li, S.Z.; Xu, N.; Liu, J.; Jiang, J.P.; Wei, G.; Wang, B. A New Species of the Asian Toad Genus Megophrys sensu lato (Amphibia: Anura: Megophryidae) from Guizhou Province, China. Asian Herpetol. Res. 2018, 9, 224–239. [Google Scholar]
- Zhang, Y.N.; Li, G.; Xiao, N.; Li, J.Q.; Pan, T.; Wang, H.; Zhang, B.W.; Zhou, J. A New Species of the Genus Xenophrys (Amphibia: Anura: Megophryidae) from Libo County, Guizhou, China. Asian Herpetol. Res. 2017, 8, 75–85. [Google Scholar]
- Lin, S.S.; Chen, H.H.; Li, Y.H.; Peng, Z.N.; Zeng, Z.C.; Wang, J. A new Boulenophrys species (Anura, Megophryidae) from the coastal hills of eastern Fujian Province, China. Zookeys 2024, 1216, 1–15. [Google Scholar] [CrossRef]
- Shi, S.C.; Li, D.H.; Zhu, W.B.; Wang, J. Description of a new toad of Megophrys Kuhl & Van Hasselt, 1822 (Amphibia: Anura: Megophryidae) from western Yunnan Province, China. Zootaxa 2021, 4942, 351–381. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, Z.T.; Liu, Z.Y.; Liao, C.K.; Zeng, Z.C.; Zhao, J.; Li, Y.L.; Wang, Y.Y. Description of six new species of the subgenus Panophrys within the genus Megophrys (Anura, Megophryidae) from southeastern China based on molecular and morphological data. Zookeys 2019, 851, 113–164. [Google Scholar] [CrossRef]
- Messenger, K.R.; Dahn, H.A.; Liang, Y.; Xie, P.; Wang, Y.; Lu, C. A new species of the genus Megophrys Gunther, 1864 (Amphibia: Anura: Megophryidae) from Mount Wuyi, China. Zootaxa 2019, 4554, 561–583. [Google Scholar] [CrossRef]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica. Amphibia (Vol. 2). Anura; Science Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Wang, J.; Lin, S.S.; Gan, J.S.; Chen, H.H.; Yu, L.M.; Pan, Z.; Xiao, J.J.; Zeng, Z.C. A new species of the genus Boulenophrys from South China (Anura, Megophryidae). Zootaxa 2024, 5514, 451–468. [Google Scholar] [CrossRef]
- Su, H.J.; Shi, S.C.; Wu, Y.Q.; Li, G.R.; Yao, X.G.; Wang, B.; Li, S.Z. Description of a new horned toad of Megophrys Kuhl & Van Hasselt, 1822 (Anura, Megophryidae) from southwest China. Zookeys 2020, 974, 131–159. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Mahony, S.; Nguyen, C.T.; Dau, V.Q.; Nguyen, T.T.; Luong, H.V.; Rowley, J.J.L. The Vietnamese population of Megophrys kuatunensis (Amphibia: Megophryidae) represents a new species of Asian horned frog from Vietnam and southern China. Zootaxa 2017, 4344, 465–492. [Google Scholar] [CrossRef]
- Tapley, B.; Cutajar, T.; Nguyen, L.T.; Nguyen, C.T.; Harding, L.; Portway, C.; Van Luong, H.; Rowley, J.J. A new locality and elevation extension for Megophrys rubrimera (Tapley et al.; 2017) in Bat Xat Nature Reserve, Lao Cai Province, northern Vietnam. Herpetol. Notes 2018, 11, 865–868. [Google Scholar]
- Tian, W.S.; Hu, Q.X. Taxonomic study on genus Megophrys, with descriptions of two genera. Acta Herpetol. Sin. 1983, 2, 41–48. [Google Scholar]
- Hu, S.C.; Djao, E.M.; Liu, C.C. A survey of amphibians and reptiles in Kweichow province, including a herpetofauna analysis. Acta Zool. Sin. 1973, 19, 149–171. [Google Scholar]
- Mo, X.Y.; Shen, Y.H.; Li, H.H.; Wu, X.S. A new species of Megophrys (Amphibia: Anura: Megophryidae) from the northwestern Hunan Province, China. Curr. Zool. 2010, 56, 432–436. [Google Scholar] [CrossRef]
- Lyu, Z.T.; Li, Y.Q.; Zeng, Z.C.; Zhao, J.; Liu, Z.Y.; Guo, G.X.; Wang, Y.Y. Four new species of Asian horned toads (Anura, Megophryidae, Megophrys) from southern China. ZooKeys 2020, 942, 105–140. [Google Scholar] [CrossRef]
- Qi, S.; Lyu, Z.T.; Wang, J.; Mo, Y.M.; Zeng, Z.C.; Zeng, Y.J.; Dai, K.Y.; Li, Y.Q.; Grismer, L.L.; Wang, Y.Y. Three new species of the genus Boulenophrys (Anura, Megophryidae) from southern China. Zootaxa 2021, 5072, 401–438. [Google Scholar] [CrossRef]




| Measurements | B. gutu sp. nov | B. hengshanensis | p-Values from ANOVA in Males * | |||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 7) | Females (n = 8) | Males (n = 10) | Females (n = 1) | |||||
| Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | |||
| SVL | 34.4–44.7 | 38.8 ± 3.5 | 36.2–52.8 | 45 ± 7.1 | 34.4–39.3 | 37.4 ± 1.2 | 48.4 | 0.24 |
| HL | 10.3–13.7 | 12.2 ± 1.1 | 12–15.5 | 13.7 ± 1.3 | 10.6–13.5 | 12.2 ± 0.8 | 12.6 | 0.13 |
| HW | 11.7–15.9 | 14.3 ± 1.3 | 13.4–17.9 | 15.5 ± 1.9 | 12.7–15.2 | 14 ± 0.7 | 16.5 | 0.44 |
| SL | 4.1–5.5 | 4.7 ± 0.5 | 4.4–5.9 | 5.2 ± 0.6 | 3.8–4.8 | 4.3 ± 0.3 | 5 | 0.14 |
| SND | 2.1–2.6 | 2.4 ± 0.2 | 2.2–3.1 | 2.7 ± 0.3 | 1.6–2.6 | 2.2 ± 0.3 | 2 | 0.19 |
| END | 2.1–3.0 | 2.4 ± 0.3 | 2.1–3.1 | 2.6 ± 0.4 | 1.7–2.4 | 2.1 ± 0.2 | 2.3 | 0.04 |
| IND | 3.4–4.6 | 4.2 ± 0.4 | 3.7–5.2 | 4.4 ± 0.6 | 3.4–4.9 | 4 ± 0.4 | 5.5 | 0.53 |
| IOD | 3.5–4.5 | 4.1 ± 0.3 | 3.5–5.4 | 4.3 ± 0.7 | 3.8–4.6 | 4.2 ± 0.3 | 4.8 | 0.10 |
| EL | 4.3–5.4 | 5.1 ± 0.4 | 4.4–6.2 | 5.2 ± 0.7 | 3.8–4.9 | 4.3 ± 0.3 | 6.2 | 0.00 |
| UEW | 2.9–4.2 | 3.6 ± 0.4 | 3.3–5.1 | 4.2 ± 0.7 | 2.6–3.7 | 3.1 ± 0.4 | 3.7 | 0.08 |
| IFE | 6–8.3 | 7.3 ± 0.8 | 4.3–9.2 | 7.7 ± 1.8 | 5.9–7.4 | 6.5 ± 0.4 | 8.1 | 0.02 |
| IBE | 9.1–12.1 | 11.1 ± 1 | 10–14 | 12.1 ± 1.7 | 9.5–12.1 | 10.9 ± 0.8 | 13.8 | 0.57 |
| TYD | 1.8–3 | 2.7 ± 0.4 | 2.1–4.2 | 2.9 ± 0.8 | 1.9–2.9 | 2.3 ± 0.3 | 3.3 | 0.05 |
| TYE | 1.4–2.8 | 1.9 ± 0.4 | 1.7–2.9 | 2.2 ± 0.5 | 2.2–3.2 | 2.7 ± 0.4 | 3.6 | 0.00 |
| FAL | 6.3–9.7 | 8.3 ± 1 | 7.7–9.8 | 8.8 ± 0.9 | 7.5–8.7 | 8.1 ± 0.4 | 10.1 | 0.66 |
| HAL | 8.3–10.4 | 9.5 ± 0.9 | 8.9–12.2 | 10.6 ± 1.3 | 8.5–10.3 | 9.4 ± 0.6 | 10 | 0.27 |
| FIL | 2–2.9 | 2.6 ± 0.3 | 2.5–3.6 | 3.1 ± 0.4 | 2–2.9 | 2.6 ± 0.3 | 2.3 | 0.82 |
| FIIL | 2.2–2.9 | 2.6 ± 0.3 | 2.6–3.5 | 3.1 ± 0.3 | 2.3–2.9 | 2.6 ± 0.2 | 2.4 | 0.96 |
| FIIIL | 4–5.4 | 4.7 ± 0.5 | 4.3–5.7 | 5.1 ± 0.5 | 3.8–4.9 | 4.4 ± 0.4 | 5 | 0.52 |
| FIVL | 2.7–3.5 | 3.1 ± 0.3 | 2.9–4.2 | 3.7 ± 0.4 | 2.7–3.9 | 3.1 ± 0.3 | 3.4 | 0.55 |
| FIIIW | 0.6–1 | 0.9 ± 0.1 | 0.6–1 | 0.8 ± 0.1 | 0.6–1.1 | 0.8 ± 0.2 | 1 | 0.62 |
| HLL | 47.4–61.5 | 54.8 ± 4.8 | 51.7–68.6 | 60.5 ± 6.4 | 46–55.5 | 51 ± 3.4 | 54.6 | 0.13 |
| FML | 14.8–18.8 | 17.3 ± 1.5 | 16–21.7 | 19.2 ± 2.4 | 14.2–18.1 | 16.5 ± 1.3 | 19.7 | 0.65 |
| TIL | 15.3–19.4 | 17.1 ± 1.4 | 16.3–20.7 | 18.6 ± 1.8 | 14.5–16.6 | 15.6 ± 0.7 | 18.1 | 0.00 |
| TFOL | 19.8–27 | 23.7 ± 2.7 | 22.2–30.4 | 26.2 ± 3 | 20.6–24.2 | 22.6 ± 1.2 | 26.2 | 0.63 |
| FOL | 12.6–16.6 | 14.8 ± 1.6 | 14.2–19 | 16.9 ± 1.7 | 13.4–16.5 | 15 ± 1.1 | 18.7 | 0.06 |
| IMT | 1.5–2.2 | 1.8 ± 0.2 | 1.7–2.6 | 2.1 ± 0.3 | 2–3.1 | 2.5 ± 0.4 | 3.6 | 0.00 |
| TYD/EL | 0.42–0.58 | 0.53 ± 0.05 | 0.47–0.79 | 0.56 ± 0.11 | 0.42–0.61 | 0.54 ± 0.05 | 0.53 | \ |
| TIL/SVL | 0.43–0.46 | 0.44 ± 0.01 | 0.37–0.45 | 0.42 ± 0.03 | 0.40–0.44 | 0.42 ± 0.01 | 0.37 | \ |
| FOL/SVL | 0.36–0.40 | 0.38 ± 0.02 | 0.35–0.43 | 0.38 ± 0.03 | 0.37–0.43 | 0.40 ± 0.02 | 0.39 | \ |
| TFOL/SVL | 0.57–0.67 | 0.61 ± 0.03 | 0.54–0.66 | 0.59 ± 0.04 | 0.57–0.64 | 0.60 ± 0.02 | 0.54 | \ |
| IMT/SVL | 0.04–0.05 | 0.05 ± 0.005 | 0.04–0.06 | 0.05 ± 0.006 | 0.06–0.08 | 0.07 ± 0.008 | 0.07 | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, D.-Y.; Wei, Y.-F.; Luo, Y.-S.; Pei, K.-W.; Cao, Y.-Y.; Zhang, M.-F.; Huang, T.-F.; Pu, L.; Shi, S.-C. A New Species of Boulenophrys (Megophridae) from Mt. Hengshan, Hunan Province, China, with Re-Description on B. hengshanensis. Animals 2025, 15, 2745. https://doi.org/10.3390/ani15182745
Kuang D-Y, Wei Y-F, Luo Y-S, Pei K-W, Cao Y-Y, Zhang M-F, Huang T-F, Pu L, Shi S-C. A New Species of Boulenophrys (Megophridae) from Mt. Hengshan, Hunan Province, China, with Re-Description on B. hengshanensis. Animals. 2025; 15(18):2745. https://doi.org/10.3390/ani15182745
Chicago/Turabian StyleKuang, Dai-Yong, Yi-Fu Wei, Yi-Sha Luo, Kang-Wen Pei, Ying-Yue Cao, Meng-Fei Zhang, Tai-Fu Huang, Ling Pu, and Sheng-Chao Shi. 2025. "A New Species of Boulenophrys (Megophridae) from Mt. Hengshan, Hunan Province, China, with Re-Description on B. hengshanensis" Animals 15, no. 18: 2745. https://doi.org/10.3390/ani15182745
APA StyleKuang, D.-Y., Wei, Y.-F., Luo, Y.-S., Pei, K.-W., Cao, Y.-Y., Zhang, M.-F., Huang, T.-F., Pu, L., & Shi, S.-C. (2025). A New Species of Boulenophrys (Megophridae) from Mt. Hengshan, Hunan Province, China, with Re-Description on B. hengshanensis. Animals, 15(18), 2745. https://doi.org/10.3390/ani15182745





