Importance and Characterisation of Concurrent Pathogens in Diarrhoeic Calves from North-Western Spain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Detection of Pathogens
2.3. Statistical Analyses
3. Results
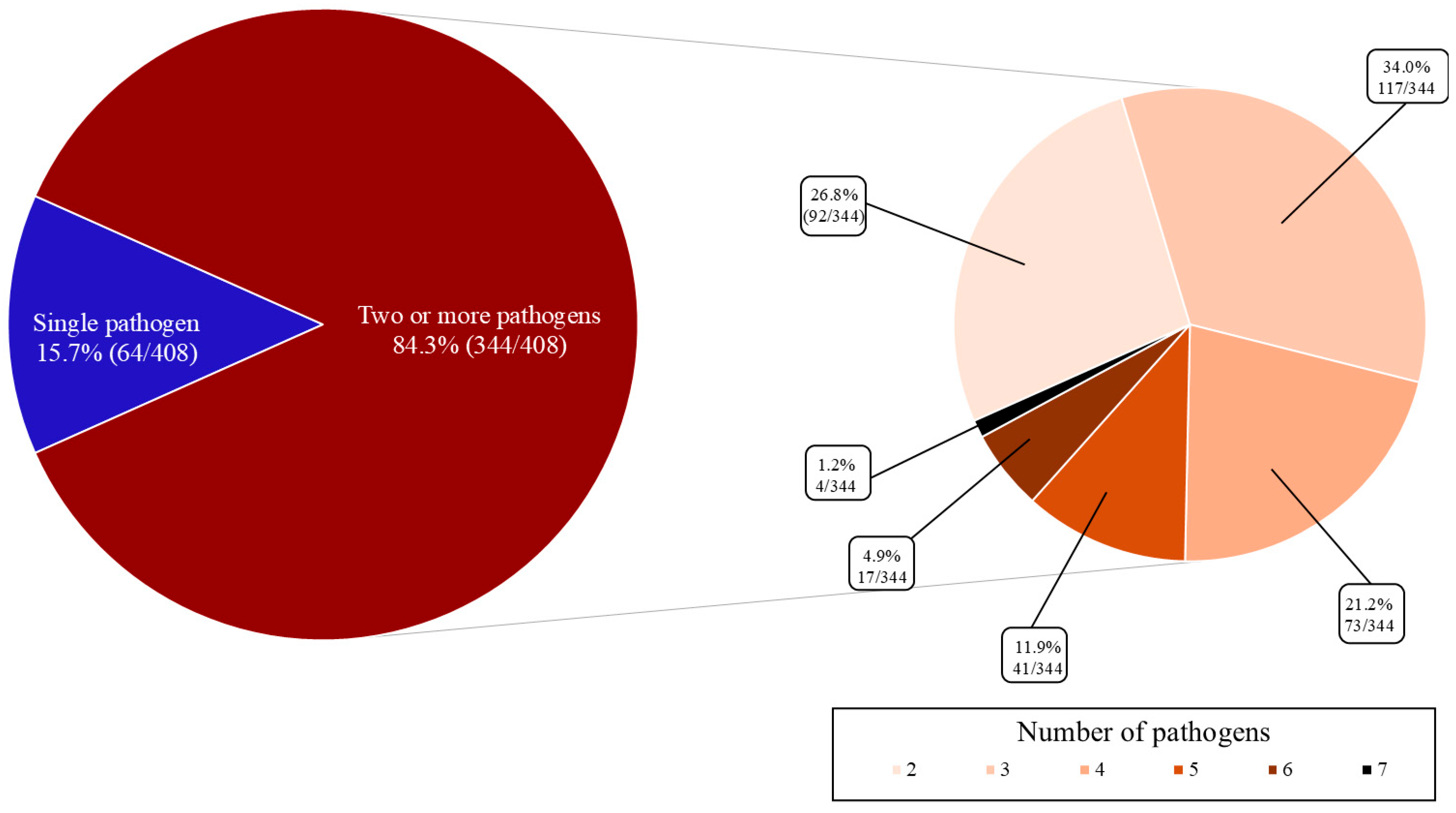
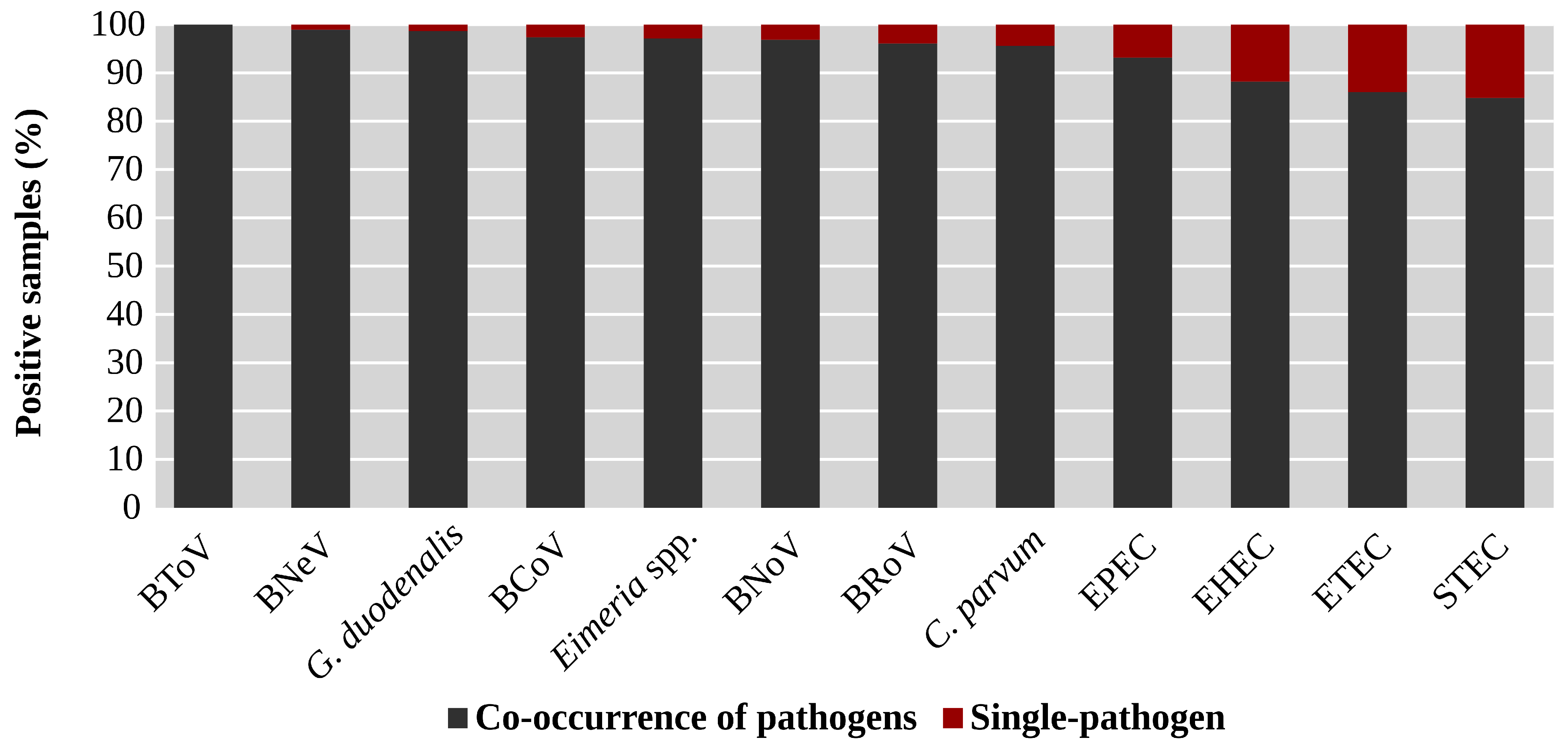
3.1. Prevalence of the Target Enteropathogens
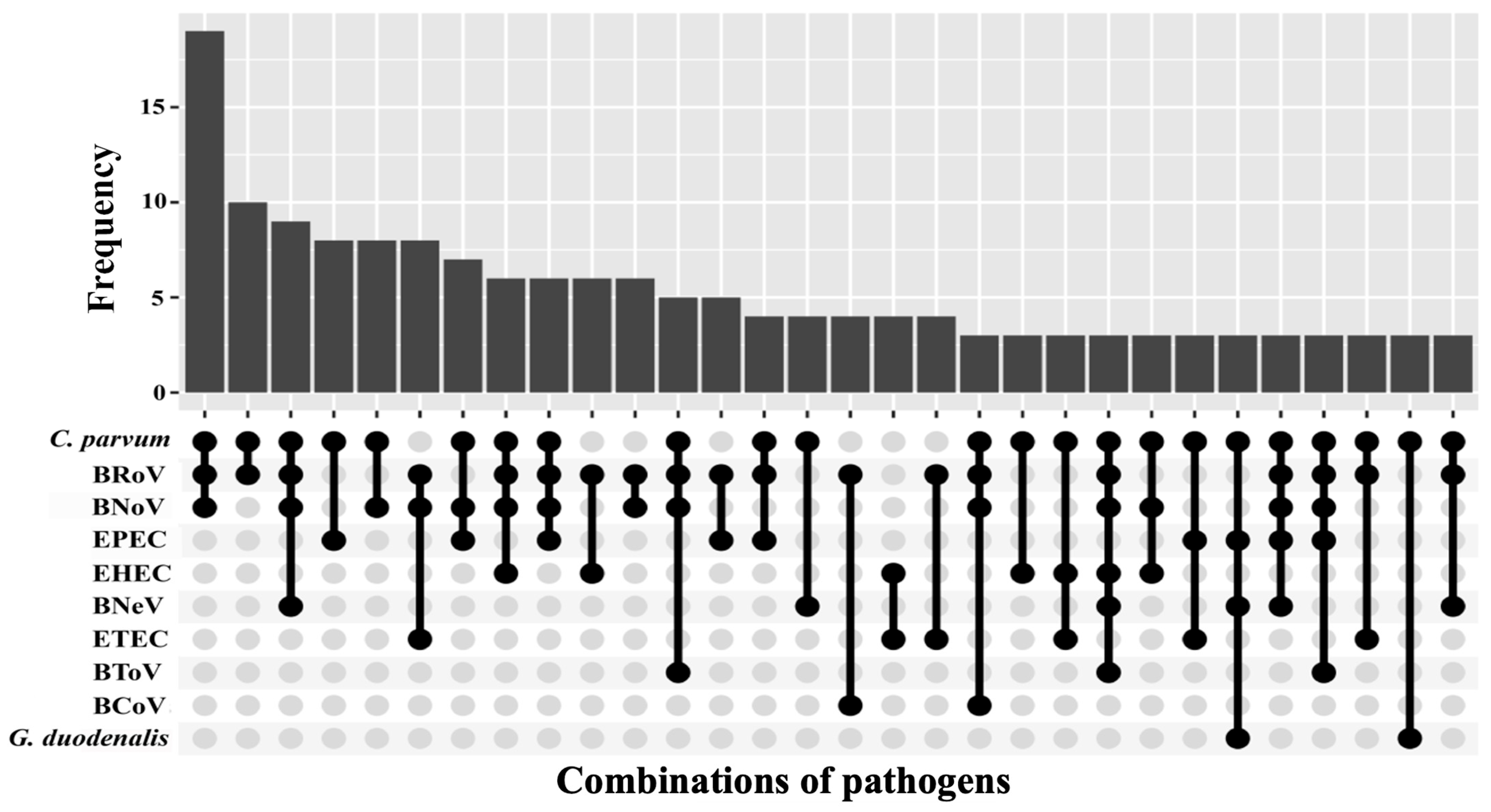
3.2. Analysis of Co-Occurrence Between Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez, D.E.; Weese, J.S. Viral Enteritis in Calves. Can. Vet. J. 2017, 58, 1267–1274. [Google Scholar]
- Cho, Y.-I.; Yoon, K.-J. An Overview of Calf Diarrhea—Infectious Etiology, Diagnosis, and Intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef]
- McGuirk, S.M. Disease Management of Dairy Calves and Heifers. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef]
- Bendali, F.; Bichet, H.; Schelcher, F.; Sanaa, M. Pattern of Diarrhoea in Newborn Beef Calves in South-West France. Vet. Res. 1999, 30, 61–74. [Google Scholar]
- de Graaf, D.C.; Vanopdenbosch, E.; Ortega-Mora, L.M.; Abbassi, H.; Peeters, J.E. A Review of the Importance of Cryptosporidiosis in Farm Animals. Int. J. Parasitol. 1999, 29, 1269–1287. [Google Scholar] [CrossRef] [PubMed]
- Gillhuber, J.; Rügamer, D.; Pfister, K.; Scheuerle, M.C. Giardiosis and Other Enteropathogenic Infections: A Study on Diarrhoeic Calves in Southern Germany. BMC Res. Notes 2014, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Mohler, V.L.; Izzo, M.M.; House, J.K. Salmonella in Calves. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C.L.; Fairbrother, J.M. Escherichia coli . In Pathogenesis of Bacterial Infections in Animals; Gyles, C.L., Prescott, J.F., Songer, G., Thoen, C.O., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 267–308. [Google Scholar]
- Taghipour, A.; Sharbatkhori, M.; Tohidi, F.; Ghanbari, M.R.; Karanis, P.; Olfatifar, M.; Majidiani, H.; Khazaei, S.; Bahadory, S.; Javanmard, E. Global Prevalence of Giardia duodenalis in Cattle: A Systematic Review and Meta-Analysis. Prev. Vet. Med. 2022, 203, 105632. [Google Scholar] [CrossRef]
- Castells, M.; Colina, R. Viral Enteritis in Cattle: To Well Known Viruses and Beyond. Microbiol. Res. 2021, 12, 663–682. [Google Scholar] [CrossRef]
- Cho, Y.-I.; Han, J.I.; Wang, C.; Cooper, V.; Schwartz, K.; Engelken, T.; Yoon, K.J. Case-Control Study of Microbiological Etiology Associated with Calf Diarrhea. Vet. Microbiol. 2013, 166, 375–385. [Google Scholar] [CrossRef]
- Guo, Z.; He, Q.; Zhang, B.; Yue, H.; Tang, C. Detection and Molecular Characteristics of Neboviruses in Dairy Cows in China. J. Gen. Virol. 2019, 100, 35–45. [Google Scholar] [CrossRef]
- Abuelo, A.; Havrlant, P.; Wood, N.; Hernandez-Jover, M. An Investigation of Dairy Calf Management Practices, Colostrum Quality, Failure of Transfer of Passive Immunity, and Occurrence of Enteropathogens among Australian Dairy Farms. J. Dairy Sci. 2019, 102, 8352–8366. [Google Scholar] [CrossRef]
- Lanz Uhde, F.; Kaufmann, T.; Sager, H.; Albini, S.; Zanoni, R.; Schelung, E.; Meylan, M. Prevalence of Four Enteropathogens in the Faeces of Young Diarrhoeic Dairy Calves in Switzerland. Vet. Rec. 2008, 163, 362–366. [Google Scholar] [CrossRef]
- Izzo, M.M.; Kirkland, P.D.; Mohler, V.L.; Perkins, N.R.; Gunn, A.A.; House, J.K. Prevalence of Major Enteric Pathogens in Australian Dairy Calves with Diarrhoea. Aust. Vet. J. 2011, 89, 167–173. [Google Scholar] [CrossRef]
- Caffarena, R.D.; Casaux, M.L.; Schild, C.O.; Fraga, M.; Castells, M.; Colina, R.; Maya, L.; Corbellini, L.G.; Riet-correa, F.; Giannitti, F. Causes of Neonatal Calf Diarrhea and Mortality in Pasture-Based Dairy Herds in Uruguay: A Farm-Matched Case-Control Study. Braz. J. Microbiol. 2021, 52, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y.; Choi, E.W.; Kim, D. Causative Agents and Epidemiology of Diarrhea in Korean Native Calves. J. Vet. Sci. 2019, 20, e64. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.A.; Monteagudo, L.V.; Arnal, J.L.; Baselga, C.; Quílez, J. Occurrence and Genetic Diversity of Rotavirus A in Faeces of Diarrheic Calves Submitted to a Veterinary Laboratory in Spain. Prev. Vet. Med. 2020, 185, 105196. [Google Scholar] [CrossRef]
- Brar, A.P.S.; Sood, N.K.; Kaur, P.; Singla, L.D.; Sandhu, B.S.; Gupta, K.; Narang, D.; Singh, C.K.; Chandra, M. Periurban Outbreaks of Bovine Calf Scours in Northern India Caused by Cryptosporidium in Association with Other Enteropathogens. Epidemiol. Infect. 2017, 145, 2717–2726. [Google Scholar] [CrossRef]
- De La Fuente, R.; García, A.; Ruiz-Santa-Quiteria, J.A.; Luzón, M.; Cid, D.; García, S.; Orden, J.A.; Gómez-Bautista, M. Proportional Morbidity Rates of Enteropathogens among Diarrheic Dairy Calves in Central Spain. Prev. Vet. Med. 1998, 36, 145–152. [Google Scholar] [CrossRef]
- García-Meniño, I.; Díaz, P.; Gómez, V.; Prieto, A.; Fernández, G.; Díez-Baños, P.; Morrondo, P.; Mora, A. Estudio de Prevalencia de Enteropatógenos Implicados En La Diarrea Del Ternero En Galicia. Boletín ANEMBE 2015, 108, 33–37. [Google Scholar]
- Reynolds, D.J.; Morgan, J.H.; Chanter, N.; Jones, P.W.; Bridger, J.C.; Debney, T.G.; Bunch, K.J. Microbiology of Calf Diarrhoea in Southern Britain. Vet. Rec. 1986, 119, 34–39. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Ruiz-Santa-Quiteria, J.A.; Orden, J.A.; Cid, D.; Sanz, R.; Gómez-Bautista, M.; De La Fuente, R. Rotavirus and Concurrent Infections with Other Enteropathogens in Neonatal Diarrheic Dairy Calves in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Gonzalez, E.A.; Garcia, S.; Blanco, M.; Regueiro, B.; Bernardez, I. Production of Toxins by Escherichia coli Strains Isolated from Calves with Diarrhoea in Galicia (North-Western Spain). Vet. Microbiol. 1988, 18, 297–311. [Google Scholar] [CrossRef]
- Blanco, M.; Blanco, J.; Blanco, J.E.; Ramos, J. Enterotoxigenic, Verotoxigenic, and Necrotoxigenic Escherichia Coli Isolated from Cattle in Spain. Am. J. Vet. Res. 1993, 54, 1446–1451. [Google Scholar] [CrossRef]
- Consellería do Medio Rural Anuario de Estatística Agraria 2019. 2021. Available online: https://agacal.xunta.gal/sites/default/files/estatisticas/Sector_Gandeiro_BOVINO_2019.xlsx (accessed on 3 September 2025).
- Larson, L.L.; Owen, F.G.; Albright, J.L.; Appleman, R.D.; Lamb, R.C.; Muller, L.D. Guidelines Toward More Uniformity in Measuring and Reporting Calf Experimental Data I. J. Dairy Sci. 1977, 60, 989–991. [Google Scholar] [CrossRef]
- López-Novo, C.; Couso-Pérez, S.; Prieto, A.; Díaz-Cao, J.M.; García-Dios, D.; López-Lorenzo, G.; Remesar, S.; Ares-Mazás, E.; López, C.; Morrondo, P.; et al. Prevalence of Cryptosporidium parvum, Giardia duodenalis and Eimeria spp. in Diarrhoeic Suckling Calves from North-Western Spain and Analysis of Their Interactions. Int. J. Vet. Sci. Med. 2025, 13, 1–14. [Google Scholar] [CrossRef]
- Yamagishi, J.; Sato, Y.; Shinozaki, N.; Ye, B.; Tsuboi, A.; Nagasaki, M.; Yamashita, R. Comparison of Boiling and Robotics Automation Method in DNA Extraction for Metagenomic Sequencing of Human Oral Microbes. PLoS ONE 2016, 11, e0154389. [Google Scholar] [CrossRef]
- Naylor, S.W.; Gally, D.L.; Low, C.J. Enterohaemorrhagic E. Coli in Veterinary Medicine. Int. J. Med. Microbiol. 2005, 295, 419–441. [Google Scholar] [CrossRef]
- Díaz, P.; Varcasia, A.; Pipia, A.P.; Tamponi, C.; Sanna, G.; Prieto, A.; Ruiu, A.; Spissu, P.; Díez-Baños, P.; Morrondo, P.; et al. Molecular Characterisation and Risk Factor Analysis of Cryptosporidium spp. in Calves from Italy. Parasitol. Res. 2018, 117, 3081–3090. [Google Scholar] [CrossRef]
- Ministry of Agriculture Fisheries and Food. Manual of Veterinary Parasitological Laboratory Techniques; HSMO: London, UK, 1986. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Barkley, J.A.; Pempek, J.A.; Bowman, A.S.; Nolting, J.M.; Lee, J.; Lee, S.; Habing, G.G. Longitudinal Health Outcomes for Enteric Pathogens in Preweaned Calves on Ohio Dairy Farms. Prev. Vet. Med. 2021, 190, 105323. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Zhai, J.; Chen, X.; Qi, Y. Prevalence of Bovine Rotavirus among Cattle in Mainland China: A Meta-Analysis. Microb. Pathog. 2022, 170, 105727. [Google Scholar] [CrossRef]
- Brunauer, M.; Roch, F.F.; Conrady, B. Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis. Animals 2021, 11, 1014. [Google Scholar] [CrossRef]
- Jessop, E.; Li, L.; Renaud, D.L.; Verbrugghe, A.; Macnicol, J.; Gamsjäger, L.; Gomez, D.E. Neonatal Calf Diarrhea and Gastrointestinal Microbiota: Etiologic Agents and Microbiota Manipulation for Treatment and Prevention of Diarrhea. Vet. Sci. 2024, 11, 108. [Google Scholar] [CrossRef]
- Kennedy, A. Bovine Neonatal Enteritis. In All-Island Animal Disease Surveillance Report; McCarthy, M.C., Sánchez-Miguel, C., Eds.; Department of Agriculture, Food and the Marine of Ireland, Agri-Food & Biosciences Institute of Northern Ireland and Animal Health Ireland: Dublin, UK, 2024. [Google Scholar]
- Benito, A.A.; Arnal, J.L.; García, B.; Serrano, J.D.; Barrios, J.; Ondarra, M.; Chacón, G. Identificación y Genotipado de Rotavirus A Circulante En Terneros Con Problemas Digestivos En España. In Proceedings of the XXIV Congreso Internacional ANEMBE Medicina Bovina, Sevilla, Spain, 22–24 May 2019; p. 360. [Google Scholar]
- Quílez, J.; Sánchez-Acedo, C.; Del Cacho, E.; Clavel, A.; Causapé, A.C. Prevalence of Cryptosporidium and Giardia Infections in Cattle in Aragón (Northeastern Spain). Vet. Parasitol. 1996, 66, 139–146. [Google Scholar] [CrossRef]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Robinson, G.; Del Cacho, E.; Sanchez-Acedo, C. Cryptosporidium Species and Subtype Analysis from Dairy Calves in Spain. Parasitology 2008, 135, 1613–1620. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; González-Losada, Y.A.; Ares-Mazás, E. Prevalence of and Risk Factors Involved in the Spread of Neonatal Bovine Cryptosporidiosis in Galicia (NW Spain). Vet. Parasitol. 2002, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Quílez, J.; Chalmers, R.M.; Panadero, R.; López, C.; Sánchez-Acedo, C.; Morrondo, P.; Díez-Baños, P. Genotype and Subtype Analysis of Cryptosporidium Isolates from Calves and Lambs in Galicia (NW Spain). Parasitology 2010, 137, 1187–1193. [Google Scholar] [CrossRef]
- Díaz, P.; Pedreira García, J.; Morrondo, P.; Díez-Baños, P.; López, C.; Panadero, R.; Fernández, G.; Prieto, A.; Remesar, S.; Díaz-Cao, J.M.; et al. Servet Update: Diarrea Neonatal en Terneros; Servet: Zaragoza, Spain, 2019. [Google Scholar]
- Brainard, J.; Hammer, C.C.; Hunter, P.R.; Katzer, F.; Hurle, G.; Tyler, K. Efficacy of Halofuginone Products to Prevent or Treat Cryptosporidiosis in Bovine Calves: A Systematic Review and Meta-Analyses. Parasitology 2021, 148, 408–409. [Google Scholar] [CrossRef]
- Parreño, V.; Béjar, C.; Vagnozzi, A.; Barrandeguy, M.; Costantini, V.; Craig, M.I.; Yuan, L.; Hodgins, D.; Saif, L.; Fernández, F. Modulation by Colostrum-Acquired Maternal Antibodies of Systemic and Mucosal Antibody Responses to Rotavirus in Calves Experimentally Challenged with Bovine Rotavirus. Vet. Immunol. Immunopathol. 2004, 100, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, J.; Fremy, C.; Bernard, S.; Rehby, L.; Aho, S.; Pothier, P.; Ambert-Balay, K. Impact of Rotavirus Vaccine on Rotavirus Genotypes and Caliciviruses Circulating in French Cattle. Vaccine 2013, 31, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, D.R.; Browning, G. Enteric vaccines for farm animals and horses. In Vaccines for Veterinary Applications; Peters, A.R., Ed.; Butterworth-Heinemann: Oxford, UK, 1993; pp. 59–81. [Google Scholar]
- Maier, G.U.; Breitenbuecher, J.; Pablo Gomez, J.; Samah, F.; Fausak, E.; Van Noord, M. Vaccination for the Prevention of Neonatal Calf Diarrhea in Cow-Calf Operations: A Scoping Review. Vet. Anim. Sci. 2022, 15, 100238. [Google Scholar] [CrossRef]
- da Silva Medeiros, T.N.; Lorenzetti, E.; Alfieri, A.F.; Alfieri, A.A. Phylogenetic Analysis of a G6P[5] Bovine Rotavirus Strain Isolated in a Neonatal Diarrhea Outbreak in a Beef Cattle Herd Vaccinated with G6P[1] and G10P[11] Genotypes. Arch. Virol. 2015, 160, 447–451. [Google Scholar] [CrossRef]
- Gomes Rocha, T.; Dornelas, F.; Silva, F.; Gregori, F.; Alfieri, A.A.; Buzinaro, M.d.G.; Fagliari, J.J. Longitudinal Study of Bovine Rotavirus Group A in Newborn Calves from Vaccinated and Unvaccinated Dairy Herds. Trop. Anim. Health Prod. 2017, 49, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Karayel, I.; Fehér, E.; Marton, S.; Coskun, N.; Bányai, K.; Alkan, F. Putative Vaccine Breakthrough Event Associated with Heterotypic Rotavirus Infection in Newborn Calves, Turkey, 2015. Vet. Microbiol. 2017, 201, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, J.T.T.; Oliveira, M.V.; Lorenzetti, E.; Miyabe, F.M.; Viziack, M.P.; Rodrigues, C.A.; Ayres, H.; Alfieri, A.F.; Alfieri, A.A. Longitudinal Surveillance of Rotavirus A Genotypes Circulating in a High Milk Yield Dairy Cattle Herd after the Introduction of a Rotavirus Vaccine. Vet. Microbiol. 2019, 230, 260–264. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Blanchard, P.C. Diagnostics of Dairy and Beef Cattle Diarrhea. Vet. Clin. N. Am.—Food Anim. Pract. 2012, 28, 443–464. [Google Scholar] [CrossRef]
- Viidu, D.A.; Mõtus, K. Implementation of a Pre-Calving Vaccination Programme against Rotavirus, Coronavirus and Enterotoxigenic Escherichia coli (F5) and Association with Dairy Calf Survival. BMC Vet. Res. 2022, 18, 59. [Google Scholar] [CrossRef]
- Deng, Y.; Batten, C.A.; Liu, B.L.; Lambden, P.R.; Elschner, M.; Günther, H.; Otto, H.P.; Schnürch, P.; Eichhorn, W.; Herbst, W.; et al. Studies of Epidemiology and Seroprevalence of Bovine Noroviruses in Germany. J. Clin. Microbiol. 2003, 41, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Milnes, A.S.; Binns, S.H.; Oliver, S.L.; Bridger, J.C. Retrospective Study of Noroviruses in Samples of Diarrhoea from Cattle, Using the Veterinary Laboratories Agency’s Farmfile Database. Vet. Rec. 2007, 160, 326–330. [Google Scholar] [CrossRef]
- Di Bartolo, I.; Ponterio, E.; Monini, M.; Ruggeri, F.M. A Pilot Survey of Bovine Norovirus in Northern Italy. Vet. Rec. 2011, 169, 73. [Google Scholar] [CrossRef]
- Kaplon, J.; Guenau, E.; Asdrubal, P.; Pothier, P.; Ambert-Balay, K. Possible Novel Nebovirus Genotype in Cattle, France. Emerg. Infect. Dis. 2011, 17, 1120–1123. [Google Scholar] [CrossRef]
- Tråvén, M.; Axén, C.; Svensson, A.; Björkman, C.; Emanuelson, U. Prevalence of Bovine Norovirus and Nebovirus and Risk Factors of Infection in Swedish Dairy Herds. Dairy 2022, 3, 137–147. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Martella, V.; Ceci, C.; Marsilio, F. Evidence for Recombination in Neboviruses. Vet. Microbiol. 2011, 153, 367–372. [Google Scholar] [CrossRef]
- Liebler, E.M.; Klüver, S.; Pohlenz, J.; Koopmans, M. Importance of Breda Torovirus as a Cause of Diarrhoea in Calves in Lower Saxony, Germany. Dtsch. Tierärztl. Wochenschr. 1992, 99, 195–200. [Google Scholar] [PubMed]
- Matiz, K.; Kecskeméti, S.; Kiss, I.; Ádám, Z.; Tanyi, J.; Nagy, B. Torovirus Detection in Faecal Specimens of Calves and Pigs in Hungary: Short Communication. Acta Vet. Hung. 2002, 50, 293–296. [Google Scholar] [CrossRef]
- Haschek, B.; Klein, D.; Benetka, V.; Herrera, C.; Sommerfeld-Stur, I.; Vilcek, Š.; Moestl, K.; Baumgartner, W. Detection of Bovine Torovirus in Neonatal Calf Diarrhoea in Lower Austria and Styria (Austria). J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Orden, J.A.; Ruiz-Santa-Quiteria, J.A.; Cid, D.; García, S.; Sanz, R.; de la Fuente, R. Verotoxin-Producing Escherichia Coli (VTEC) and Eae-Positive Non-VTEC in 1–30-Days-Old Diarrhoeic Dairy Calves. Vet. Microbiol. 1998, 63, 239–248. [Google Scholar] [CrossRef]
- Lichtmannsperger, K.; Hinney, B.; Joachim, A.; Wittek, T. Molecular Characterization of Giardia intestinalis and Cryptosporidium parvum from Calves with Diarrhoea in Austria and Evaluation of Point-of-Care Tests. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101333. [Google Scholar] [CrossRef] [PubMed]
- Jor, E.; Myrmel, M.; Jonassen, C.M. SYBR Green Based Real-Time RT-PCR Assay for Detection and Genotype Prediction of Bovine Noroviruses and Assessment of Clinical Significance in Norway. J. Virol. Methods 2010, 169, 1–7. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Shin, S.-U.; Choi, K.-S. Molecular Surveillance of Viral Pathogens Associated with Diarrhea in Pre-Weaned Korean Native Calves. Trop. Anim. Health Prod. 2020, 52, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Mafura, M.; Carter, B.; Lynch, K.; Anjum, M.F.; Woodward, M.J.; Pritchard, G.C. Genes Associated with Escherichia coli Isolates from Calves with Diarrhoea and/or Septicaemia. Vet. Rec. 2010, 166, 691–692. [Google Scholar] [CrossRef]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A Systematic Review and Meta-Analysis of the Epidemiology of Pathogenic Escherichia coli of Calves and the Role of Calves as Reservoirs for Human Pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Ngeleka, M.; Godson, D.; Vanier, G.; Desmarais, G.; Wojnarowicz, C.; Sayi, S.; Yanyun, H.; Movasseghi, R.; Fairbrother, J.M. Frequency of Escherichia coli Virotypes in Calf Diarrhea and Intestinal Morphologic Changes Associated with These Virotypes or Other Diarrheagenic Pathogens. J. Vet. Diagn. Investig. 2019, 31, 611–615. [Google Scholar] [CrossRef]
- Quílez, J.; Sánchez-Acedo, C.; Clavel, A.; Del Cacho, E.; López-Bernad, F. Comparison of an Acid-Fast Stain and a Monoclonal Antibody-Based Immunofluorescence Reagent for the Detection of Cryptosporidium Oocysts in Faecal Specimens from Cattle and Pigs. Vet. Parasitol. 1996, 67, 75–81. [Google Scholar] [CrossRef]
- Huetink, R.E.C.; Van der Giessen, J.W.B.; Noordhuizen, J.P.T.M.; Ploeger, H.W. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a Dairy Farm. Vet. Parasitol. 2001, 102, 53–67. [Google Scholar] [CrossRef]
- Björkman, C.; Svensson, C.; Christensson, B.; De Verdier, K. Cryptosporidium parvum and Giardia intestinalis in Calf Diarrhoea in Sweden. Acta Vet. Scand. 2003, 44, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.P.; Dooley, J.S.G.; Kenny, J.; McCoy, M.; Lowery, C.J.; Moore, J.E.; Xiao, L. Genotypes and Subtypes of Cryptosporidium spp. in Neonatal Calves in Northern Ireland. Parasitol. Res. 2007, 100, 619–624. [Google Scholar] [CrossRef]
- ARSIA Organization. ARSIA Rapport Annuel 2023; Association for Asian Studies: Ann Arbor, MI, USA, 2024. [Google Scholar]
- Delling, C.; Daugschies, A. Literature Review: Coinfection in Young Ruminant Livestock- Cryptosporidium spp. and Its Companions. Pathogens 2022, 11, 103. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella-Part 1: Detection of Salmonella spp. 1st ed. International Organization for Standardization: Geneva, Switzerland, 2017.
- Bartels, C.J.M.; Holzhauer, M.; Jorritsma, R.; Swart, W.A.J.M.; Lam, T.J.G.M. Prevalence, Prediction and Risk Factors of Enteropathogens in Normal and Non-Normal Faeces of Young Dutch Dairy Calves. Prev. Vet. Med. 2010, 93, 162–169. [Google Scholar] [CrossRef]
- Busato, A.; Lentze, T.; Hofer, D.; Burnens, A.; Hentrich, B.; Gaillard, C. A Case Control Study of Potential Enteric Pathogens for Calves Raised in Cow-Calf Herds. J. Vet. Med. B Infect. Dis. Vet. Public Health 1998, 45, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hur, J.; Stein, B.D. Occurrence and Characteristics of Enterohemorrhagic Escherichia coli O26 and O111 in Calves Associated with Diarrhea. Vet. J. 2008, 176, 205–209. [Google Scholar] [CrossRef]
- Younis, E.E.; Ahmed, A.M.; El-Khodery, S.A.; Osman, S.A.; El-Naker, Y.F.I. Molecular Screening and Risk Factors of Enterotoxigenic Escherichia coli and Salmonella spp. in Diarrheic Neonatal Calves in Egypt. Res. Vet. Sci. 2009, 87, 373–379. [Google Scholar] [CrossRef]
- Shams, Z.; Tahamtan, Y.; Pourbakhsh, A. Detection of Enterotoxigenic K99 (F5) and F41 from Fecal Sample of Calves by Molecular and Serological Methods. Comp. Clin. Pathol. 2012, 21, 475–478. [Google Scholar] [CrossRef]
- Shahrani, M.; Dehkordi, F.S.; Momtaz, H. Characterization of Escherichia coli Virulence Genes, Pathotypes and Antibiotic Resistance Properties in Diarrheic Calves in Iran. Biol. Res. 2014, 47, 28. [Google Scholar] [CrossRef] [PubMed]
- Engelen, F.; Thiry, D.; Devleesschauwer, B.; Heyndrickx, M.; Mainil, J.; De Zutter, L.; Cox, E. Pathogenic Potential of Escherichia coli O157 and O26 Isolated from Young Belgian Dairy Calves by Recto-Anal Mucosal Swab Culturing. J. Appl. Microbiol. 2021, 131, 964–972. [Google Scholar] [CrossRef]
- O’Handley, R.M.; Olson, M.E. Giardiasis and Cryptosporidiosis in Ruminants. Vet. Clin. N. Am. Food. Anim. Pract. 2006, 22, 623–643. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Novo, C.; Díaz, P.; Díaz-Cao, J.M.; Couso-Pérez, S.; García-Dios, D.; López-Lorenzo, G.; Remesar, S.; Ares-Mazás, E.; Morrondo, P.; Gómez-Couso, H.; et al. Importance and Characterisation of Concurrent Pathogens in Diarrhoeic Calves from North-Western Spain. Animals 2025, 15, 2735. https://doi.org/10.3390/ani15182735
López-Novo C, Díaz P, Díaz-Cao JM, Couso-Pérez S, García-Dios D, López-Lorenzo G, Remesar S, Ares-Mazás E, Morrondo P, Gómez-Couso H, et al. Importance and Characterisation of Concurrent Pathogens in Diarrhoeic Calves from North-Western Spain. Animals. 2025; 15(18):2735. https://doi.org/10.3390/ani15182735
Chicago/Turabian StyleLópez-Novo, Cynthia, Pablo Díaz, José Manuel Díaz-Cao, Seila Couso-Pérez, David García-Dios, Gonzalo López-Lorenzo, Susana Remesar, Elvira Ares-Mazás, Patrocinio Morrondo, Hipólito Gómez-Couso, and et al. 2025. "Importance and Characterisation of Concurrent Pathogens in Diarrhoeic Calves from North-Western Spain" Animals 15, no. 18: 2735. https://doi.org/10.3390/ani15182735
APA StyleLópez-Novo, C., Díaz, P., Díaz-Cao, J. M., Couso-Pérez, S., García-Dios, D., López-Lorenzo, G., Remesar, S., Ares-Mazás, E., Morrondo, P., Gómez-Couso, H., & Prieto, A. (2025). Importance and Characterisation of Concurrent Pathogens in Diarrhoeic Calves from North-Western Spain. Animals, 15(18), 2735. https://doi.org/10.3390/ani15182735




