Comparative Analyses of Gene and Protein Expressions and the Lipid Contents in Intramuscular and Subcutaneous Fat Tissues in Fattening Steers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics and Management
2.2. Animals, Management, and Sample Collection
2.3. RNA Sequencing Analysis
2.4. Tandem Mass Tag (TMT) Quantitative Protein Analysis
2.5. Lipid Extraction and Lipidomic Analysis
2.6. Bioinformatics Analysis
3. Results
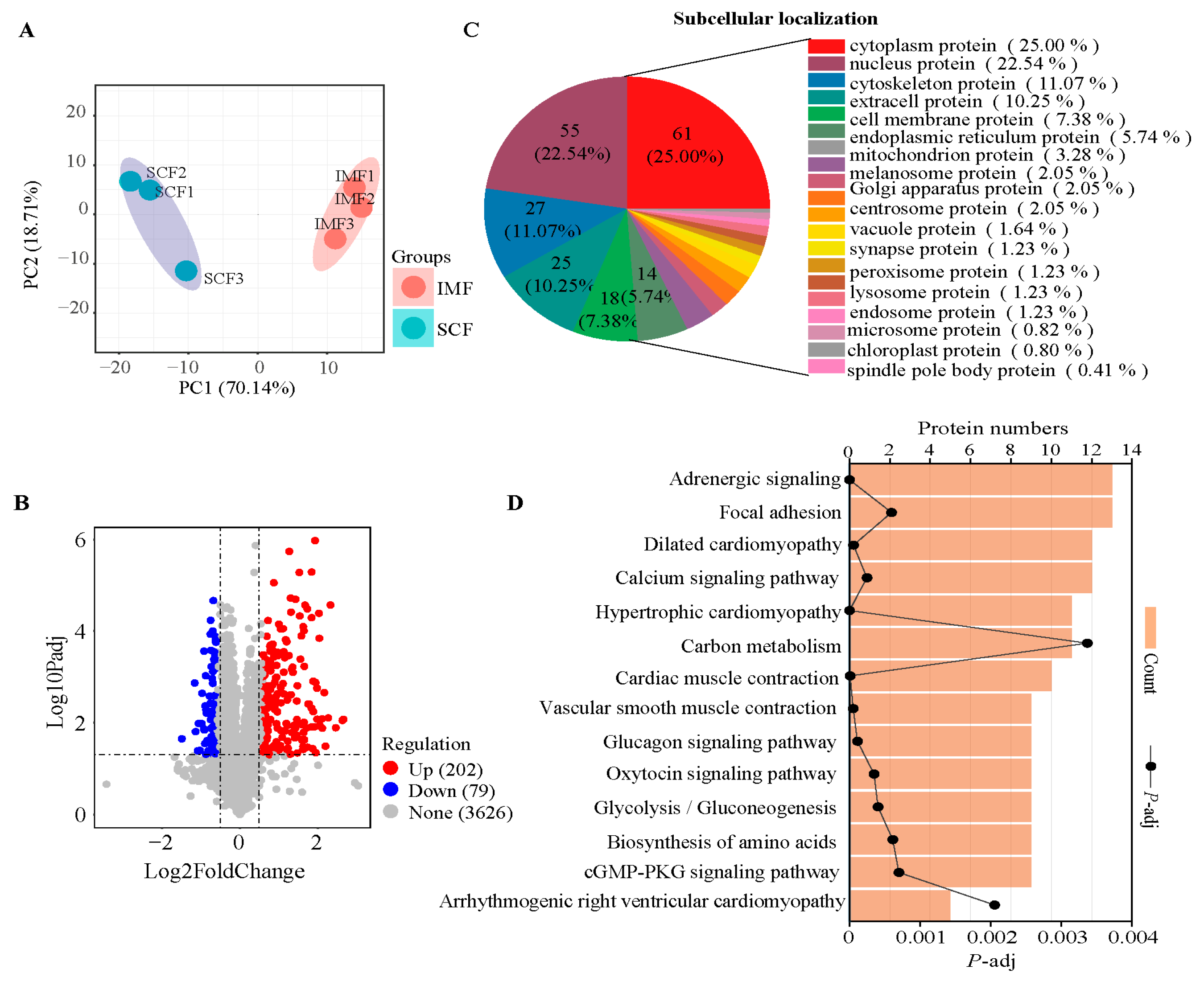
3.1. Transcriptomic Profiles and Differentially Expressed Genes (DEGs)
3.2. Proteomic Analysis and Differentially Expressed Proteins (DEPs)
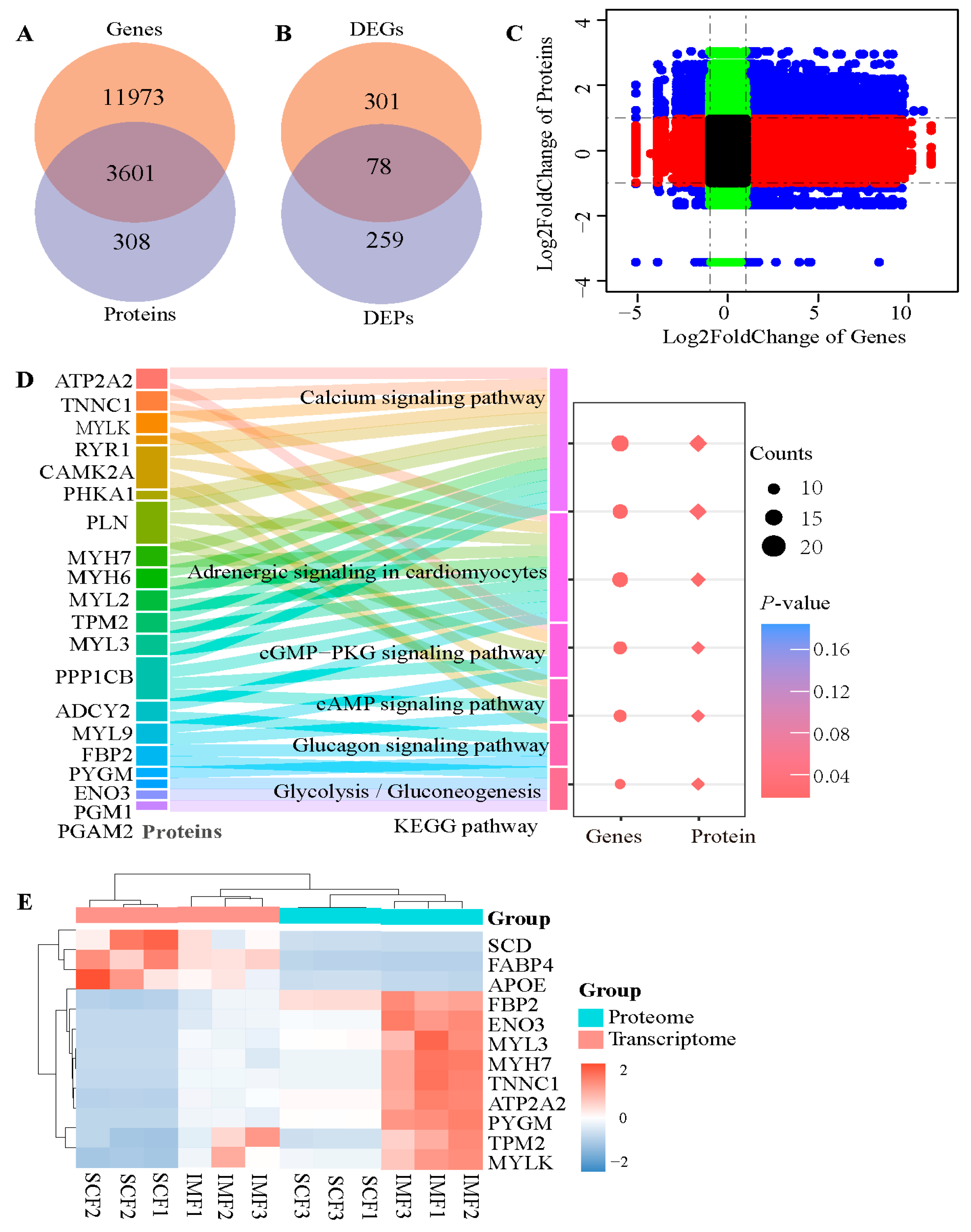
3.3. Integrating Analysis of Transcriptome and Proteome
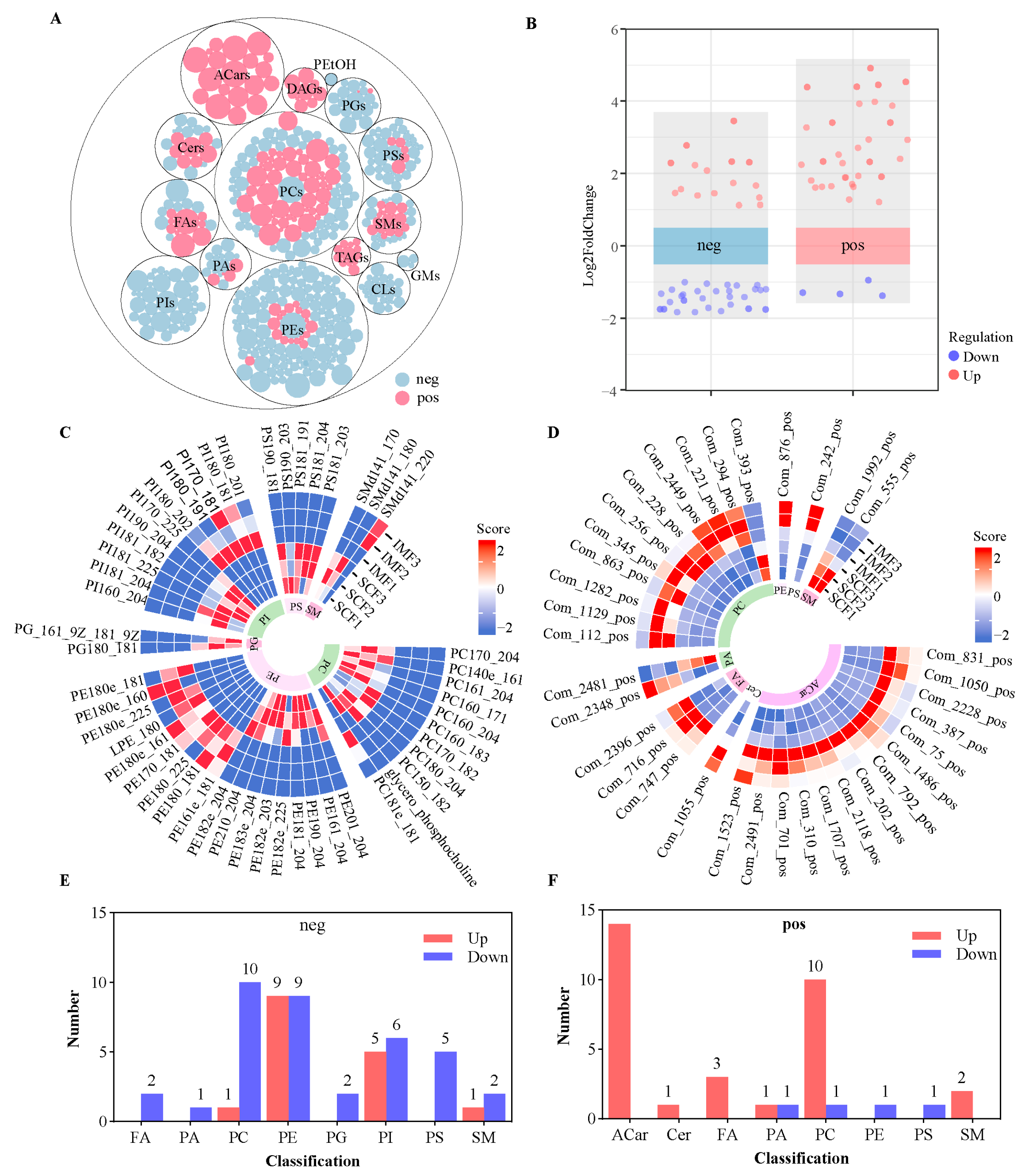
3.4. Untargeted Lipidomics and Differential Abundance Lipids (DALs)
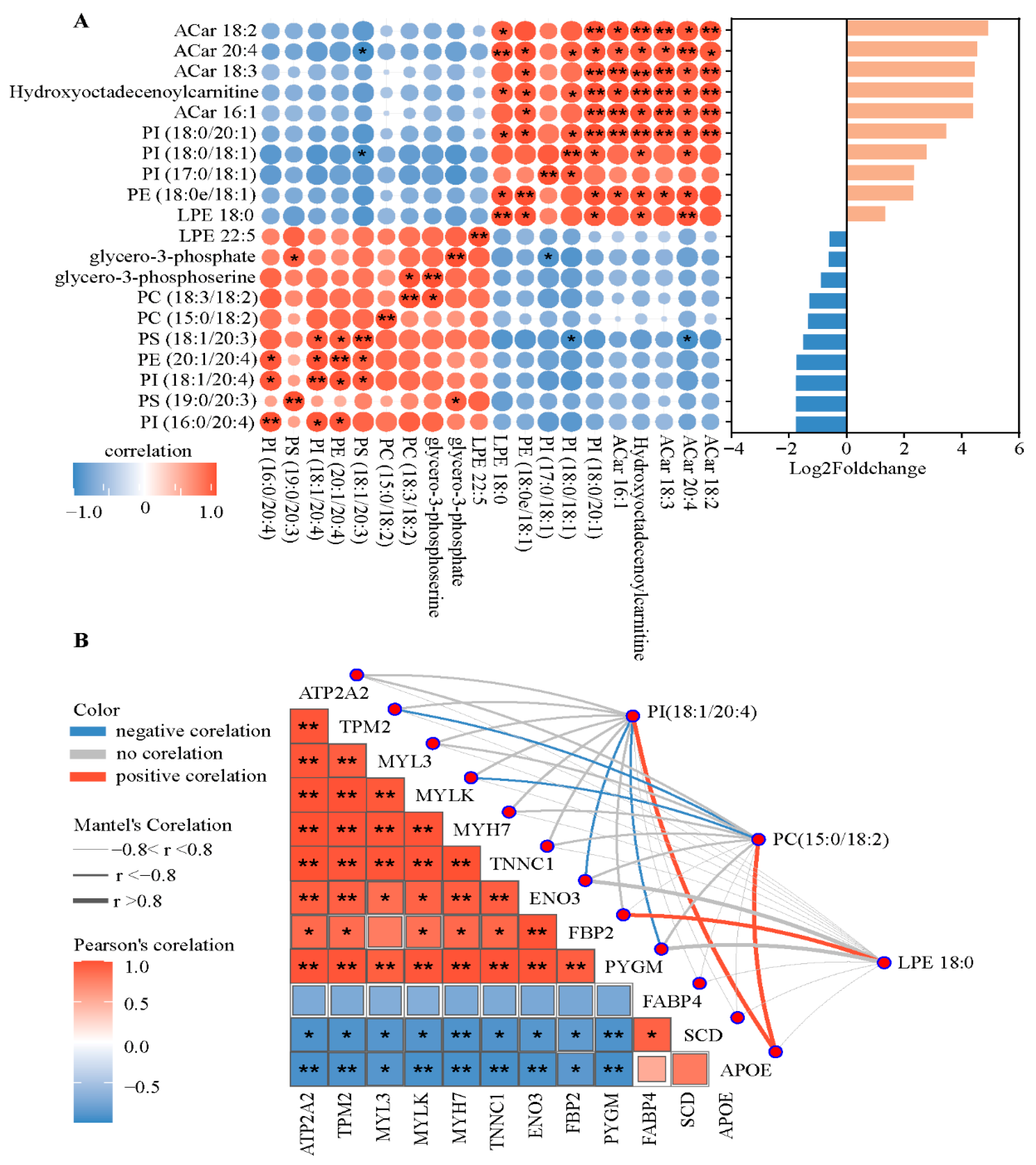
3.5. Pearson Correlation Analysis
4. Discussion
4.1. The Tissue-Specific Factors Enriched in the Calcium Pathway Between IMF and SCF in Steers
4.2. The Tissue-Specific Factors Related to Glycolysis and Lipid Metabolism in IMF and SCF of Steers
4.3. The Tissue-Specific Lipids in IMF and SCF of Steers
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iida, F.; Saitou, K.; Kawamura, T.; Yamaguchi, S.; Nishimura, T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim. Sci. J. 2015, 86, 707–715. [Google Scholar] [CrossRef]
- Hirai, S.; Kawai, A.; Mizuno, Y.; Sasaki, S.; Iida, F. Effect of intramuscular fat content on the sensory characteristics and dynamic flavor attributes of Japanese black cattle beef. Anim. Sci. J. 2023, 94, e13841. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Anim. Int. J. Anim. Biosci. 2020, 14, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Wu, Z.; Xiong, X.; Yang, B. Subcutaneous and intramuscular fat transcriptomes show large differences in network organization and associations with adipose traits in pigs. Sci. China. Life Sci. 2021, 64, 1732–1746. [Google Scholar] [CrossRef]
- Smith, S.B.; Crouse, J.D. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 1984, 114, 792–800. [Google Scholar] [CrossRef]
- Yamada, T.; Kamiya, M.; Higuchi, M. Fat depot-specific effects of body fat distribution and adipocyte size on intramuscular fat accumulation in Wagyu cattle. Anim. Sci. J. Nihon Chikusan Gakkaiho 2020, 91, e13449. [Google Scholar] [CrossRef]
- Miller, M.F.; Cross, H.R.; Lunt, D.K.; Smith, S.B. Lipogenesis in acute and 48-hour cultures of bovine intramuscular and subcutaneous adipose tissue explants. J. Anim. Sci. 1991, 69, 162–170. [Google Scholar] [CrossRef]
- Gondret, F.; Guitton, N.; Guillerm-Regost, C.; Louveau, I. Regional differences in porcine adipocytes isolated from skeletal muscle and adipose tissues as identified by a proteomic approach. J. Anim. Sci. 2008, 86, 2115–2125. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Huang, Q.; Meng, F.; Hong, C.; Li, B.; Yang, Y.; Qu, Z.; Wu, J.; Li, F.; Xin, H.; et al. Analysis of transcriptome differences between subcutaneous and intramuscular adipose tissue of tibetan pigs. Genes 2025, 16, 246. [Google Scholar] [CrossRef]
- Arrighi, N.; Moratal, C.; Clément, N.; Giorgetti-Peraldi, S.; Peraldi, P.; Loubat, A.; Kurzenne, J.Y.; Dani, C.; Chopard, A.; Dechesne, C.A. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015, 6, e1733. [Google Scholar] [CrossRef] [PubMed]
- Siebert, B.D.; Deland, M.P.; Pitchford, W.S. Breed differences in the fatty acid composition of subcutaneous and intramuscular lipid of early and late maturing, grain-finished cattle. Aust. J. Agric. Res. 1996, 47, 202–213. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, S.; Wang, Z.; Zhu, X.; Shu, G.; Liao, W.; Yu, K.; Gao, P.; Xi, Q.; Wang, X.; et al. Global comparison of gene expression profiles between intramuscular and subcutaneous adipocytes of neonatal landrace pig using microarray. Meat Sci. 2010, 86, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Bonneau, M. Compared development of intermuscular and subcutaneous fat in carcass and primal cuts of growing pigs from 30 to 140kg body weight. Meat Sci. 2009, 81, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gardan, D.; Gondret, F.; Louveau, I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E372–E380. [Google Scholar] [CrossRef]
- Ortiz-Colón, G.; Grant, A.C.; Doumit, M.E.; Buskirk, D.D. Bovine intramuscular, subcutaneous, and perirenal stromal-vascular cells express similar glucocorticoid receptor isoforms, but exhibit different adipogenic capacity. J. Anim. Sci. 2009, 87, 1913–1920. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, G.; Shu, G.; Wang, L.; Zhu, X.; Gao, P.; Xi, Q.; Zhang, Y.; Yuan, L.; Jiang, Q. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cell Physiol. Biochem. 2013, 31, 981–996. [Google Scholar] [CrossRef]
- Wu, W.; Ji, M.; Xu, K.; Zhang, D.; Yin, Y.; Huang, X.; Peng, Y.; Zhang, J. Knockdown of CTRP6 reduces the deposition of intramuscular and subcutaneous fat in pigs via different signaling pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158729. [Google Scholar] [CrossRef]
- Yi, X.; Feng, M.; Zhu, J.; Yu, H.; He, Z.; Zhang, Z.; Zhao, T.; Zhang, Q.; Pang, W. Adipocyte progenitor pools composition and cellular niches affect adipogenesis divergence in porcine subcutaneous and intramuscular fat. J. Agric. Food Chem. 2024, 72, 14044–14056. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Liu, L.; Cui, H.; Dong, N.; Zhu, X.; Li, S.; Ma, X.; Niu, D. Effects of phosphatidylethanolamine on intramuscular fat deposition and key gene identification by transcriptome sequencing in broiler chickens. Poult. Sci. 2025, 104, 104914. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Yang, M.; Niu, N.; Zong, W.; Yang, L.; Li, H.; Hou, R.; Wang, X.; Wang, L.; et al. Characteristics of transcriptome and metabolome concerning intramuscular fat content in beijing black pigs. J. Agric. Food Chem. 2023, 71, 15874–15883. [Google Scholar] [CrossRef]
- Meng, W.; Yan, J.; Zhao, Y.; Ye, Z.; Ma, X.; Wei, W.; Chen, J.; Zhang, L. Identifying the specific lipid biomarkers and LYPLA1 as a novel candidate in intramuscular fat deposition of Erhualian pigs. BMC Genom. 2025, 26, 407. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Wu, J.; Qiao, M.; Xu, Z.; Peng, X.; Mei, S. Proteomic and lipidomic analyses reveal saturated fatty acids, phosphatidylinositol, phosphatidylserine, and associated proteins contributing to intramuscular fat deposition. J. Proteom. 2021, 241, 104235. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Chai, W.; Wang, Y.; Fan, D.; Lv, M.; Jiang, X.; Liu, Y.; Wei, Q.; Wang, C. Determination of lipid profiles of Dezhou donkey meat using an LC-MS-based lipidomics method. J. Food Sci. 2021, 86, 4511–4521. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124 Pt 21, 3654–3664. [Google Scholar] [CrossRef]
- Braun, T.; Arnold, H.H. Myf-5 and myoD genes are activated in distinct mesenchymal stem cells and determine different skeletal muscle cell lineages. Embo J. 1996, 15, 310–318. [Google Scholar]
- Li, W.; Qiu, L.; Guan, J.; Sun, Y.; Zhao, J.; Du, M. Comparative transcriptome analysis of longissimus dorsi tissues with different intramuscular fat contents from Guangling donkeys. BMC Genom. 2022, 23, 644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Q.; Wu, Y.; Zhang, Y.; Zhang, B.; Zhang, H. Identification of candidate genes that specifically regulate subcutaneous and intramuscular fat deposition using transcriptomic and proteomic profiles in Dingyuan pigs. Sci. Rep. 2022, 12, 2844. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Wei, H.; Song, T.; Yang, Y.; Jian, P.; Siwen, J.; Qiong, W. Transcriptome comparison between porcine subcutaneous andintramuscular stromal vascular cells during adipogenic differentiation. PLoS ONE 2013, 8, e77094. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeong, J.Y.; Lee, R.H.; Park, M.N.; Kim, S.H.; Park, S.M.; Shin, J.C.; Jeon, Y.J.; Shim, J.H.; Choi, N.J.; et al. Regional differences of proteins expressing in adipose depots isolated from cows, steers and bulls as identified by a proteomic approach. Asian-Australas. J. Anim. Sci. 2016, 29, 1197–1206. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, H.G.; Han, J.A.; Kim, E.B.; Kang, S.K.; Yin, J.; Baik, M.; Shen, Y.; Kim, S.H.; Seo, K.S.; et al. Differentially expressed proteins during fat accumulation in bovine skeletal muscle. Meat Sci. 2010, 86, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Qasawa, A.H.; Ferrara, P.J.; Malik, A.N.; Funai, K.; McDonagh, B.; Mendias, C.L. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J. 2019, 33, 7863–7881. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Cheng, F.; Chen, K.; Lei, G.; Deng, Z.; Wu, X.; Liu, C.; Si, J.; Liang, J. Screening for genes related to meat production traits in duroc × bama xiang crossbred pigs by whole transcriptome sequencing. Animals 2024, 14, 2347. [Google Scholar] [CrossRef] [PubMed]
- Pierzchala, M.; Hoekman, A.J.; Urbanski, P.; Kruijt, L.; Kristensen, L.; Young, J.F.; Oksbjerg, N.; Goluch, D.; Te Pas, M.F. Validation of biomarkers for loin meat quality (M. longissimus) of pigs. J. Anim. Breed. Genet. 2014, 131, 258–270. [Google Scholar] [CrossRef]
- Feng, X.; Pan, C.; Liu, S.; Hu, H.; Ma, Y. Identification of core genes affecting IMF deposition in bovine. Anim. Biotechnol. 2023, 34, 2887–2899. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, B.; Shang, P.; Fu, Y.; Nie, R.; Chamba, Y.; Zhang, H. Comparative transcriptomic profiles of differentiated adipocytes provide insights into adipogenesis mechanisms of subcutaneous and intramusculcar fat tissues in pigs. Cells 2022, 11, 499. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Wang, X. Transcriptomic analysis of different intramuscular fat contents on the flavor of the longissimus dorsi tissues from Guangling donkey. Genomics 2024, 116, 110905. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al-Jammas, M.; De Koning, L.; Valais, A.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Muscle type effect. PeerJ 2018, 6, e4891. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, L.; Yao, K.; Wang, Y.; Shao, W.; Yang, M.; Zhang, X.; Wei, Y.; Ren, W. Exploration of genes related to intramuscular fat deposition in xinjiang brown cattle. Genes 2024, 15, 1121. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, L.; Wang, J.; Zhang, L.; Hou, X.; Yan, H.; Wang, L. Integrative analysis of nanopore and illumina sequencing reveals alternative splicing complexity in pig longissimus dorsi muscle. Front. Genet. 2022, 13, 877646. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Robotti, E.; Manfredi, M.; Barberis, E.; Marengo, E.; Novelli, E.; Cecconi, D. Kohonen artificial neural network and multivariate analysis in the identification of proteome changes during early and long aging of bovine longissimus dorsi muscle using swath mass spectrometry. J. Agric. Food Chem. 2021, 69, 11512–11522. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Y.; Li, X.; Wang, J.; Wang, B.; Lin, Y.; Xiong, Y. Cloning of goat PGAM2 gene and its overexpression promotes the differentiation of intramuscular preadipocytes. Anim. Biotechnol. 2023, 34, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.; Wang, M.; Han, H.; Liu, Y.; Sun, X.; Tian, T.; Pang, W.; Cai, R. Profiling of m(6)A methylation in porcine intramuscular adipocytes and unravelling PHKG1 represses porcine intramuscular lipid deposition in an m(6)A-dependent manner. Int. J. Biol. Macromol. 2024, 272 Pt 1, 132728. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Cheng, G.; Cheng, Z.X.; Bao, C.; Yamada, T.; Cao, G.F.; Bao, S.Q.; Schreurs, N.M.; Zan, L.S.; Tong, B. Association of variants in FABP4, FASN, SCD, SREBP1 and TCAP genes with intramuscular fat, carcass traits and body size in Chinese Qinchuan cattle. Meat Sci. 2022, 192, 108882. [Google Scholar] [CrossRef]
- Ros-Freixedes, R.; Gol, S.; Pena, R.N.; Tor, M.; Ibáñez-Escriche, N.; Dekkers, J.C.; Estany, J. Genome-wide association study singles out SCD and LEPR as the two main loci influencing intramuscular fat content and fatty acid composition in duroc pigs. PLoS ONE 2016, 11, e0152496. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Yang, D.; Jiang, C.; Raza, S.H.A.; Pant, S.D.; Ma, Y.; Zan, L.; Wei, D. APOE mediates the coupling of myogenesis and lipid metabolism in skeletal muscle: Decoding intercellular crosstalk via a cell co-culture model. Int. J. Biol. Macromol. 2025, 315 Pt 1, 144549. [Google Scholar] [CrossRef]
- Bosch, L.; Tor, M.; Reixach, J.; Estany, J. Age-related changes in intramuscular and subcutaneous fat content and fatty acid composition in growing pigs using longitudinal data. Meat Sci. 2012, 91, 358–363. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Li, A.; Xie, L.; Miao, X. Genome-wide analysis of mRNAs and lncRNAs of intramuscular fat related to lipid metabolism in two pig breeds. Cell Physiol. Biochem. 2018, 50, 2406–2422. [Google Scholar] [CrossRef]
- Chen, D.; Su, M.; Zhu, H.; Zhong, G.; Wang, X.; Ma, W.; Wanapat, M.; Tan, Z. Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum. Animals 2022, 12, 2243. [Google Scholar] [CrossRef]
- Tian, J.; Wu, Y.; Zhao, W.; Zhang, G.; Zhang, H.; Xue, L.; Yang, L.; Zhang, T.; Gu, Y.; Zhang, J. Transcriptomic and metabolomic-based revelation of the effect of fresh corn extract on meat quality of Jingyuan chicken. Poult. Sci. 2025, 104, 104814. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Yoon, Y.C.; Im, H.; Son, Y.; Kim, M.; Saha, A.; Choi, C.; Lee, J.; Lee, S.; Kim, J.H.; et al. Adipocyte lysoplasmalogenase TMEM86A regulates plasmalogen homeostasis and protein kinase A-dependent energy metabolism. Nat. Commun. 2022, 13, 4084. [Google Scholar] [CrossRef] [PubMed]

| Groups | Samples | Raw Bases | Clean Bases | Clean Reads | Mapping Reads to Genome | Mapping Ratio to Genome | Unique Reads | Unique Mapping Ratio | Error Rate | Q30 |
|---|---|---|---|---|---|---|---|---|---|---|
| SCF | SCF1 | 7.24 G | 7.05 G | 47,018,464 | 41,812,006 | 88.93% | 41,058,526 | 87.32% | 0.03 | 91.29% |
| SCF2 | 6.92 G | 6.69 G | 44,590,924 | 35,766,667 | 80.21% | 35,101,512 | 78.72% | 0.03 | 91.48% | |
| SCF3 | 7.03 G | 6.72 G | 44,787,368 | 39,627,734 | 88.48% | 38,924,720 | 86.91% | 0.03 | 91.70% | |
| IMF | IMF1 | 6.89 G | 6.75 G | 44,984,872 | 39,267,491 | 87.29% | 38,519,307 | 85.63% | 0.03 | 91.37% |
| IMF2 | 6.84 G | 6.65 G | 44,301,772 | 37,477,298 | 84.60% | 36,686,678 | 82.81% | 0.03 | 91.96% | |
| IMF3 | 6.78 G | 6.66 G | 44,424,366 | 39,131,318 | 88.09% | 38,414,966 | 86.47% | 0.03 | 91.74% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, K.; Yang, M.; Tan, Z.; Zhao, H.; Zhang, X. Comparative Analyses of Gene and Protein Expressions and the Lipid Contents in Intramuscular and Subcutaneous Fat Tissues in Fattening Steers. Animals 2025, 15, 2733. https://doi.org/10.3390/ani15182733
Ji K, Yang M, Tan Z, Zhao H, Zhang X. Comparative Analyses of Gene and Protein Expressions and the Lipid Contents in Intramuscular and Subcutaneous Fat Tissues in Fattening Steers. Animals. 2025; 15(18):2733. https://doi.org/10.3390/ani15182733
Chicago/Turabian StyleJi, Kaixi, Ming Yang, Ziying Tan, Hongbo Zhao, and Xianglun Zhang. 2025. "Comparative Analyses of Gene and Protein Expressions and the Lipid Contents in Intramuscular and Subcutaneous Fat Tissues in Fattening Steers" Animals 15, no. 18: 2733. https://doi.org/10.3390/ani15182733
APA StyleJi, K., Yang, M., Tan, Z., Zhao, H., & Zhang, X. (2025). Comparative Analyses of Gene and Protein Expressions and the Lipid Contents in Intramuscular and Subcutaneous Fat Tissues in Fattening Steers. Animals, 15(18), 2733. https://doi.org/10.3390/ani15182733










