Nanozinc Ecotoxicity in the Freshwater Invasive Bivalve Limnoperna fortunei Under a Climate Change Scenario
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanozinc Characterization
2.2. Bivalves and Exposure Conditions
2.3. Experimental Designs
2.4. Tissue Damage and Oxidative Stress-Related Biomarkers
2.5. Filtration Rate
2.6. Statistical Analysis
3. Results
3.1. Nanozinc Characterization
3.2. Tissue Damage and Oxidative Stress-Related Biomarkers
3.3. Filtration Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ale, A.; Andrade, V.S.; Gutierrez, M.F.; Ayech, A.; Monserrat, J.M.; Desimone, M.F.; Cazenave, J. Metal-based nanomaterials in aquatic environments: What do we know so far about their ecotoxicity? Aquat. Toxicol. 2024, 275, 107069. [Google Scholar] [CrossRef]
- Wu, F.; Deng, Y.; Sokolov, E.P.; Falfushynska, H.; Glänzer, A.; Xie, L.; Sokolova, I.M. Nanopollutants (nZnO) amplify hypoxia-induced cellular stress in a keystone marine bivalve, Mytilus edulis. Environ. Res. 2025, 274, 121346. [Google Scholar] [CrossRef]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.N.; Mouneyrac, C.; Leung, K.M.Y. Ecotoxicity of Zinc Oxide Nanoparticles in the Marine Environment. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hong, H.; Adam, V.; Nowack, B. Form-Specific and Probabilistic Environmental Risk Assessment of 3 Engineered Nanomaterials (Nano-Ag, Nano-TiO2, and Nano-ZnO) in European Freshwaters. Environ. Toxicol. Chem. 2021, 40, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.W.S.; Zhou, G.J.; Yung, M.M.N.; Djurišić, A.B.; Leung, K.M.Y. Interactive effects of temperature and salinity on toxicity of zinc oxide nanoparticles towards the marine mussel Xenostrobus securis. Sci. Total Environ. 2023, 889, 164254. [Google Scholar] [CrossRef]
- Wu, F.; Sokolova, I.M. Immune responses to ZnO nanoparticles are modulated by season and environmental temperature in the blue mussels Mytilus edulis. Sci. Total Environ. 2021, 801, 149786. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Ale, A.; Andrade, V.; Bacchetta, C.; Rossi, A.; Cazenave, J. Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: An update and basis for the use of new test species. Water Environ. Res. 2021, 93, 2505–2526. [Google Scholar] [CrossRef]
- Skawina, A.; Agnieszka, D.; Bonk, A.; Paterczyk, B.; Nowakowska, J. Tracking the micro- and nanoplastics in the terrestrial-freshwater food webs. Bivalves as sentinel species. Sci. Total Environ. 2024, 917, 170468. [Google Scholar] [CrossRef]
- Cazenave, J.; Ale, A.; Bacchetta, C.; Rossi, A.S. Nanoparticles Toxicity in Fish Models. Curr. Pharm. Des. 2019, 25, 3927–3942. [Google Scholar] [CrossRef]
- Auclair, J.; Turcotte, P.; Gagnon, C.; Gagné, F. The influence of surface waters on the bioavailability and toxicity of copper oxide nanoparticles to freshwater mussels. Invertebr. Surviv. J. 2021, 18, 33–45. [Google Scholar] [CrossRef]
- Girardello, F.; Leite, C.C.; Touguinha, L.B.; Roesch-Ely, M.; da Silva, C.K.H.; de Oliveira, R.M.; Borges, D.L.G.; Villela, I.V.; Fernandes, A.N.; Salvador, M.; et al. ZnO nanoparticles alter redox metabolism of Limnoperna fortunei. Environ. Sci. Pollut. Res. 2021, 28, 69416–69425. [Google Scholar] [CrossRef]
- Qadermarzi, A.; Pouladi, M.; Rezamand, A.; Hoseinifar, S.H.; Hedayati, A.A. Investigation of sub-acute levels of zinc oxide nanoparticles on the filtration rate of Mytilaster lineatus and Dressina polymorpha in the short term. Nusant. Biosci. 2018, 10, 53–57. [Google Scholar] [CrossRef]
- Johnson, M.F.; Albertson, L.K.; Algar, A.C.; Dugdale, S.J.; Edwards, P.; England, J.; Gibbins, C.; Kazama, S.; Komori, D.; MacColl, A.D.C.; et al. Rising water temperature in rivers: Ecological impacts and future resilience. Wiley Interdiscip. Rev. Water 2024, 11, e1724. [Google Scholar] [CrossRef]
- Van Vliet, M.T.; Thorslund, J.; Strokal, M.; Hofstra, N.; Flörke, M.; Ehalt Macedo, H.; Nkwasa, A.; Tang, T.; Kaushal, S.S.; Kumar, R.; et al. Global river water quality under climate change and hydroclimatic extremes. Nat. Rev. Earth Environ. 2023, 4, 687–702. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Eyasu, G.; Meresa, E.; Abrehe, S.; Darcha, G.; Manaye, A.; Tesfamariam, B. Modeling the ecological niche of rhamnus prinoides L. under climate change scenarios: A MaxEnt modeling approach. Geol. Ecol. Landsc. 2024, 1–17. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymarkers: Synthesis Andreport. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Andrade, V.S.; Ale, A.; Monserrat, J.M.; Roa-Fuentes, C.A.; Herrera-Martínez, Y.; Bacchetta, C.; Cazenave, J.; Rossi, A.S.; Nandini, S.; et al. Responses of freshwater organisms to multiple stressors in a climate change scenario: A review on small-scale experiments. Environ. Sci. Pollut. Res. 2025, 32, 4431–4444. [Google Scholar] [CrossRef]
- Barbosa, F.G. The scientific literature on Limnoperna fortune (Dunker 1857) from 1982 to 2012. An. Acad. Bras. Cienc. 2014, 86, 1373–1383. [Google Scholar] [CrossRef]
- Castro, M.; Garreta, C.; Arocena, R. Urban effluents affect the invasive bivalve Limnoperna fortunei (Dunker 1857) fitness in a large Pampasic river (Río Negro, Uruguay). Environ. Monit. Assess. 2024, 196, 48. [Google Scholar] [CrossRef]
- Cazenave, J.; Rossi, A.S.; Ale, A.; Montalto, L.; Gutierrez, M.F.; Rojas Molina, F. Does temperature influence on biomarker responses to copper exposure? The invasive bivalve Limnoperna fortunei (Dunker 1857) as a model. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2025, 287, 110059. [Google Scholar] [CrossRef] [PubMed]
- Girardello, F.; Leite, C.C.; Branco, C.S.; Roesch-Ely, M.; Fernandes, A.N.; Salvador, M.; Henriques, J.A.P. Antioxidant defences and haemocyte internalization in Limnoperna fortunei exposed to TiO2 nanoparticles. Aquat. Toxicol. 2016, 176, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Perepelizin, P.V.; Boltovskoy, D. Thermal tolerance of Limnoperna fortunei to gradual temperature increase and its applications for biofouling control in industrial and power plants. Biofouling 2011, 27, 667–674. [Google Scholar] [CrossRef]
- Iriondo, M.H.; Paira, A.R. Physical Geography of the Basin. In The Middle Paraná River; Iriondo, M.H., Paggi, J.C., Parma, M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Boltovskoy, D.; Paolucci, E.; MacIsaac, H.J.; Zhan, A.; Xia, Z.; Correa, N. What We Know and Don’t Know About the Invasive Golden Mussel Limnoperna Fortunei; Springer International Publishing: Cham, Switzerland, 2025; Volume 852, ISBN 0123456789. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Gagnon, C.; Turcotte, P.; Trépanier, S.; Gagné, F.; Cejka, P.J. Impacts of municipal wastewater oxidative treatments: Changes in metal physical speciation and bioavailability. Chemosphere 2014, 97, 86–91. [Google Scholar] [CrossRef]
- Clara, M.; Windhofer, G.; Weilgony, P.; Gans, O.; Denner, M.; Chovanec, A.; Zessner, M. Identification of relevant micropollutants in Austrian municipal wastewater and their behaviour during wastewater treatment. Chemosphere 2012, 87, 1265–1272. [Google Scholar] [CrossRef]
- Andrade, V.S.; Ale, A.; Antezana, P.E.; Desimone, M.F.; Cazenave, J.; Gutierrez, M.F. Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ. Sci. Pollut. Res. 2023, 30, 27137–27149. [Google Scholar] [CrossRef]
- Bacchetta, C.; Cazenave, J.; Parma, M.J.; Biancucci, G.F. Biochemical stress responses in tissues of the cichlid fish Cichlasoma dimerus exposed to a commercial formulation of endosulfan. Arch. Environ. Contam. Toxicol. 2011, 61, 453–460. [Google Scholar] [CrossRef]
- Reglero, M.M.; Taggart, M.A.; Monsalve-González, L.; Mateo, R. Heavy metal exposure in large game from a lead mining area: Effects on oxidative stress and fatty acid composition in liver. Environ. Pollut. 2009, 157, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Poletta, G.L.; Simoniello, M.F.; Mudry, M.D. Biomarkers of oxidative damage and antioxidant defense capacity in Caiman latirostris blood. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2016, 179, 29–36. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 212–216. [Google Scholar] [CrossRef]
- Barker Jørgensen, C. Bivalve filter feeding revisited. Mar. Ecol. Prog. Ser. 1996, 142, 287–302. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Khani, O.; Mohammadi, M.; Khaz’ali, A.R.; Aghdam, M.A. Effect of pH value and zeta potential on the stability of CO2 foam stabilized by SDS surfactant and SiO2, ZnO and Fe2O3 nanoparticles. Sci. Rep. 2025, 15, 10302. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Zam, S.Z.; Park, D.R.; Yeom, I.T. Assessment of key environmental factors influencing the sedimentation and aggregation behavior of zinc oxide nanoparticles in aquatic environment. Water 2018, 10, 660. [Google Scholar] [CrossRef]
- Wu, F.; Sokolov, E.P.; Timm, S.; Sokolova, I.M. Synergistic impacts of nanopollutants (nZnO) and hypoxia on bioenergetics and metabolic homeostasis in a marine bivalve Mytilus edulis. Environ. Sci. Nano 2024, 12, 576–596. [Google Scholar] [CrossRef]
- Andrade, V.S.; Ale, A.; Antezana, P.E.; Desimone, M.F.; Cazenave, J.; Gutierrez, M.F. Environmental factors modify silver nanoparticles ecotoxicity in Chydorus eurynotus (Cladocera). Ecotoxicology 2024, 33, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Laban, G.; Nies, L.F.; Turco, R.F.; Bickham, J.W.; Sepúlveda, M.S. The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos. Ecotoxicology 2010, 19, 185–195. [Google Scholar] [CrossRef]
- Lee, W.M.; Ha, S.W.; Yang, C.Y.; Lee, J.K.; An, Y.J. Effect of fluorescent silica nanoparticles in embryo and larva of Oryzias latipes: Sonic effect in nanoparticle dispersion. Chemosphere 2011, 82, 451–459. [Google Scholar] [CrossRef]
- Kokhan, A.S.; Soldatov, A.A.; Golovina, I.V.; Bogdanovich, V.Y.; Shalagina, N.E.; Rychkova, V.N. Parameters of Energy Metabolism and Adenylate System in Mytilus galloprovincialis Tissues under Moderate Hypoxia. J. Evol. Biochem. Phys. 2023, 59, 1986–1994. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Z.; Liu, H.; Shen, H.; Yang, J. Biochemical response of the mussel Mytilus coruscus (Mytiloida: Mytilidae) exposed to in vivo sub-lethal copper concentrations. Chin. J. Oceanol. Limnol. 2012, 30, 738–745. [Google Scholar] [CrossRef]
- Blasco, J.; Puppo, J. Effect of heavy metals (Cu, Cd and Pb) on aspartate and alanine aminotransferase in Ruditapes philippinarum (Mollusca: Bivalvia). Comp. Biochem. Physiol.-C Pharmacol. Toxicol. Endocrinol. 1999, 122, 253–263. [Google Scholar] [CrossRef]
- Narvia, M.; Rantamäki, P. Aminotransferases in the bivalve mollusc Mytilus edulis L. and short term effects of crude oil in brackish water. Biomarkers 1997, 2, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; Abdel-Ghaffar, F.; Bakry, F.A.; Sayed, D.A. Ecotoxicological effect of sublethal exposure to zinc oxide nanoparticles on freshwater snail Biomphalaria alexandrina. Arch. Environ. Contam. Toxicol. 2014, 67, 192–202. [Google Scholar] [CrossRef]
- Rasheed, A.; Javed Iqbal, K.; Safdar, A.; Nasir, A.; Jabeen, R.; sami, A.; Tara, N.; Ali, S.; Zeeshan, M.; Abbas, S.; et al. Toxicological effects of zinc oxide nanoparticles on hemato-biochemical profile of common carp (Cyprinus carpio). J. King Saud Univ.-Sci. 2023, 35, 102835. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, X.; Zhu, R.; Luo, Z.; Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016, 180, 56–70. [Google Scholar] [CrossRef]
- Falfushynska, H.; Gnatyshyna, L.; Yurchak, I.; Sokolova, I.; Stoliar, O. The effects of zinc nanooxide on cellular stress responses of the freshwater mussels Unio tumidus are modulated by elevated temperature and organic pollutants. Aquat. Toxicol. 2015, 162, 82–93. [Google Scholar] [CrossRef]
- Marisa, I.; Matozzo, V.; Munari, M.; Binelli, A.; Parolini, M.; Martucci, A.; Franceschinis, E.; Brianese, N.; Marin, M.G. In vivo exposure of the marine clam Ruditapes philippinarum to zinc oxide nanoparticles: Responses in gills, digestive gland and haemolymph. Environ. Sci. Pollut. Res. 2016, 23, 15275–15293. [Google Scholar] [CrossRef]
- Morosetti, B.; Freitas, R.; Pereira, E.; Hamza, H.; Andrade, M.; Coppola, F.; Maggioni, D.; Della Torre, C. Will temperature rise change the biochemical alterations induced in Mytilus galloprovincialis by cerium oxide nanoparticles and mercury? Environ. Res. 2020, 188, 109778. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Hummel, H.; Amiard-Triquet, C.; Larroux, C.; Sukhotin, A. Trace metals and variations of antioxidant enzymes in arctic bivalve populations. Arch. Environ. Contam. Toxicol. 1998, 35, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, A.M.; Saadeldin, I.M.; Swelum, A.A.A.; Afifi, M.M.; Alkaladi, A. Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxid. Med. Cell. Longev. 2018, 2018, 6926712. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Wang, J.; Wu, J.; Wan, J.; He, Y.; Liu, J. Zinc oxide nanoparticles cause hepatotoxicity in rare minnow (Gobiocypris rarus) via ROS-mediated oxidative stress and apoptosis activation and inhibition of lipid peroxidation. Aquac. Rep. 2024, 38, 102317. [Google Scholar] [CrossRef]
- Borase, H.P.; Patil, S.V.; Singhal, R.S. Moina macrocopa as a non-target aquatic organism for assessment of ecotoxicity of silver nanoparticles: Effect of size. Chemosphere 2019, 219, 713–723. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Yang, Y. Assessing the effects of environmental factors on filtration rate of golden mussel (Limnoperna fortunei). Ecol. Indic. 2024, 158, 111544. [Google Scholar] [CrossRef]
- Casas, S.M.; Filgueira, R.; Lavaud, R.; Comeau, L.A.; La Peyre, M.K.; La Peyre, J.F. Combined effects of temperature and salinity on the physiology of two geographically-distant eastern oyster populations. J. Exp. Mar. Bio. Ecol. 2018, 506, 82–90. [Google Scholar] [CrossRef]
- Quevedo, L.; Ibáñez, C.; Caiola, N.; Mateu, D. Effects of thermal pollution on benthic macroinvertebrate communities of a large Mediterranean River. J. Entomol. Zool. Stud. 2018, 6, 500–507. [Google Scholar]
- Basti, L.; Nagai, S.; Watanabe, S.; Oda, T.; Tanaka, Y. Neuroenzymatic activity and physiological energetics in Manila clam, Ruditapes philippinarum, during short-term sublethal exposure to harmful alga, Heterocapsa circularisquama. Aquat. Toxicol. 2016, 176, 76–87. [Google Scholar] [CrossRef]
- Rojas Molina, F.; Paggi, J.C.; Devercelli, M. Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei. Biol. Invasions 2010, 12, 1647–1659. [Google Scholar] [CrossRef]
- Frau, D.; Molina, F.R.; Mayora, G. Feeding selectivity of the invasive mussel Limnoperna fortunei (Dunker, 1857) on a natural phytoplankton assemblage: What really matters? Limnology 2016, 17, 47–57. [Google Scholar] [CrossRef]
- Bourgeault, A.; Legros, V.; Gonnet, F.; Daniel, R.; Paquirissamy, A.; Bénatar, C.; Spalla, O.; Chanéac, C.; Renault, J.P.; Pin, S. Interaction of TiO2 nanoparticles with proteins from aquatic organisms: The case of gill mucus from blue mussel. Environ. Sci. Pollut. Res. 2017, 24, 13474–13483. [Google Scholar] [CrossRef] [PubMed]
- Manske Nunes, S.; Josende, M.E.; González-Durruthy, M.; Pires Ruas, C.; Gelesky, M.A.; Romano, L.A.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Different crystalline forms of titanium dioxide nanomaterial (rutile and anatase) can influence the toxicity of copper in golden mussel Limnoperna fortunei? Aquat. Toxicol. 2018, 205, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Saidani, W.; Sellami, B.; Khazri, A.; Mezni, A.; Dellali, M.; Joubert, O.; Sheehan, D.; Beyrem, H. Metal accumulation, biochemical and behavioral responses on the Mediterranean clams Ruditapes decussatus exposed to two photocatalyst nanocomposites (TiO2 NPs and AuTiO2NPs). Aquat. Toxicol. 2019, 208, 71–79. [Google Scholar] [CrossRef]

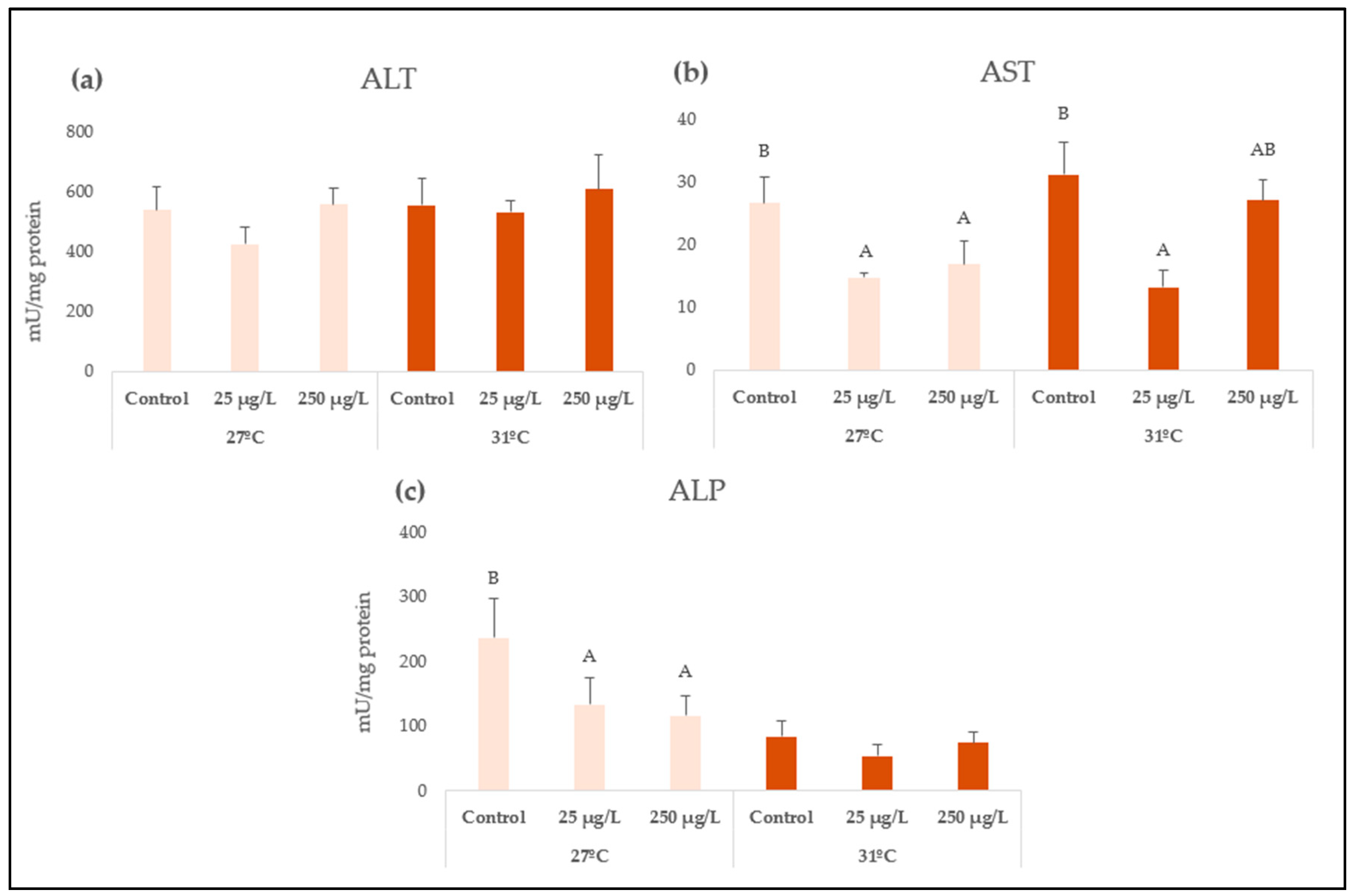
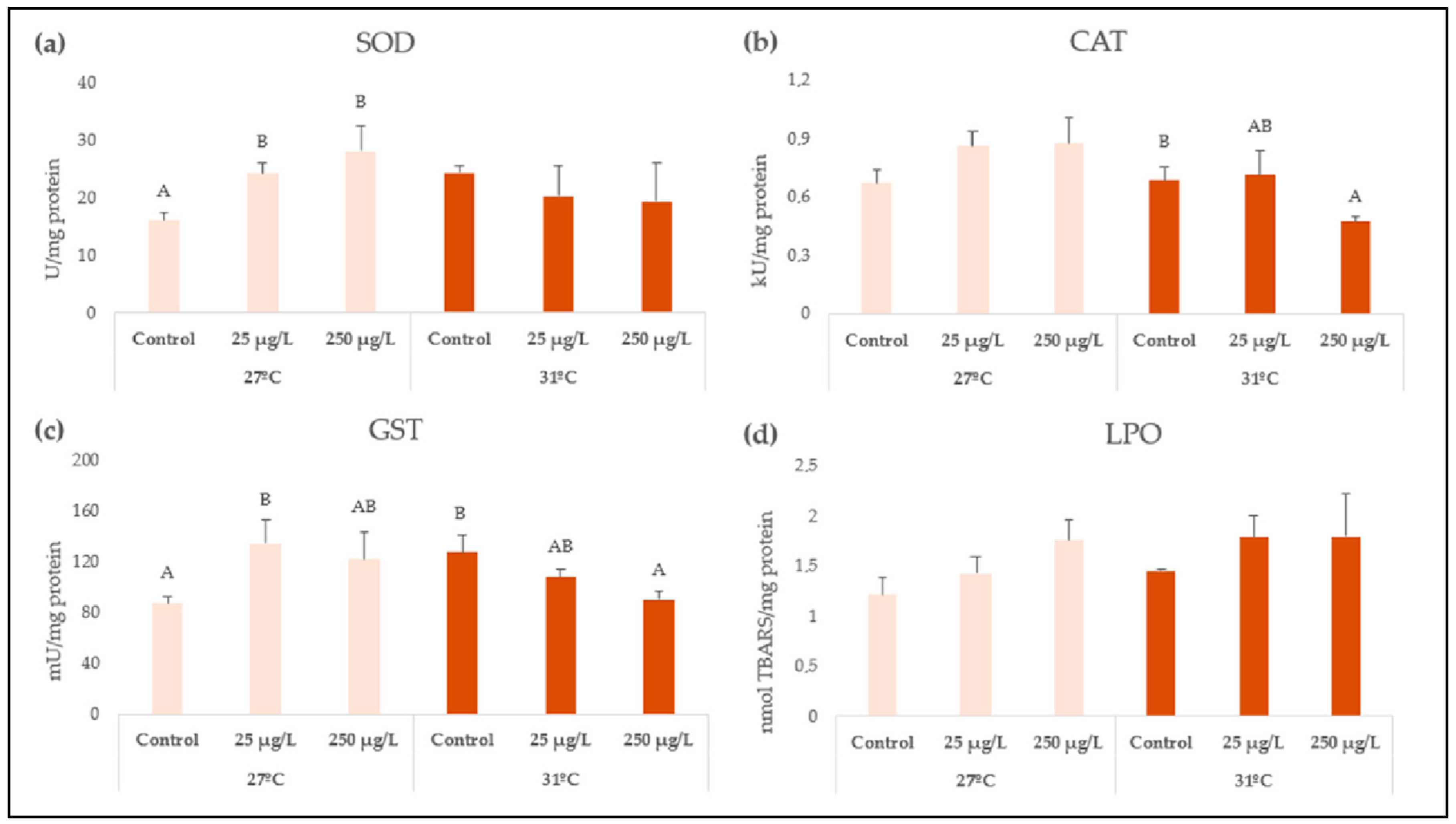

| Biomarker | Factor | df | F Value | p Value |
|---|---|---|---|---|
| ALT | Concentration | 2 | 0.89 | 0.4246 |
| Temperature | 1 | 0.81 | 0.3786 | |
| Conc × Temp | 2 | 0.16 | 0.8555 | |
| AST | Concentration | 2 | 8.5 | 0.0018 |
| Temperature | 1 | 2.27 | 0.1462 | |
| Conc × Temp | 2 | 1.41 | 0.2655 | |
| ALP | Concentration | 2 | 2.28 | 0.1242 |
| Temperature | 1 | 9.95 | 0.0043 | |
| Conc × Temp | 2 | 1.27 | 0.3001 | |
| SOD | Concentration | 2 | 1.41 | 0.2663 |
| Temperature | 1 | 0.64 | 0.4325 | |
| Conc × Temp | 2 | 8.11 | 0.0026 | |
| CAT | Concentration | 2 | 1.69 | 0.2091 |
| Temperature | 1 | 12.41 | 0.002 | |
| Conc × Temp | 2 | 3.86 | 0.0373 | |
| GST | Concentration | 2 | 1.79 | 0.1917 |
| Temperature | 1 | 2.21 | 0.152 | |
| Conc × Temp | 2 | 8.96 | 0.0015 | |
| LPO | Concentration | 2 | 1.9 | 0.1713 |
| Temperature | 1 | 1.26 | 0.2727 | |
| Conc × Temp | 2 | 0.26 | 0.7705 |
| Contrast | Ratio | SE | t Ratio | p Value | ||
|---|---|---|---|---|---|---|
| 3 h | ||||||
| Control | 27 °C/0.025 µg/L | 27 °C | 1.177 | 0.445 | 0.431 | 0.9979 |
| Control | 27 °C/0.250 µg/L | 27 °C | 1.511 | 0.665 | 0.938 | 0.9337 |
| Control | 27 °C/control | 31 °C | 0.766 | 0.242 | −0.843 | 0.9570 |
| Control | 27 °C/0.025 µg/L | 31 °C | 1.074 | 0.388 | 0.197 | 1.0000 |
| Control | 27 °C/0.250 µg/L | 31 °C | 0.906 | 0.304 | −0.295 | 0.9997 |
| 0.025 µg/L | 27 °C/0.250 µg/L | 27 °C | 1.284 | 0.595 | 0.539 | 0.9941 |
| 0.025 µg/L | 31 °C/control | 31 °C | 0.650 | 0.226 | −1.236 | 0.8160 |
| 0.025 µg/L | 31 °C/0.025 µg/L | 31 °C | 0.912 | 0.355 | −0.236 | 0.9999 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.769 | 0.281 | −0.717 | 0.9785 |
| 0.250 µg/L | 31 °C/control | 31 °C | 0.507 | 0.211 | −1.631 | 0.5845 |
| 0.250 µg/L | 31 °C/0.025 µg/L | 31 °C | 0.711 | 0.321 | −0.756 | 0.9729 |
| 0.250 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.599 | 0.258 | −1.188 | 0.8395 |
| Control | 31 °C/0.025 µg/L | 31 °C | 1.402 | 0.459 | 1.033 | 0.9035 |
| Control | 31 °C/0.250 µg/L | 31 °C | 1.183 | 0.353 | 0.562 | 0.0028 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.844 | 0.292 | −0.491 | 0.9962 |
| 6 h | ||||||
| Control | 27 °C/0.025 µg/L | 27 °C | 1.170 | 0.563 | 0.327 | 0.9995 |
| Control | 27 °C/0.250 µg/L | 27 °C | 1.123 | 0.532 | 0.245 | 0.9999 |
| Control | 27 °C/control | 31 °C | 0.317 | 0.107 | −3.404 | 0.0196 |
| Control | 27 °C/0.025 µg/L | 31 °C | 1.279 | 0.646 | 0.488 | 0.9963 |
| Control | 27 °C/0.250 µg/L | 31 °C | 1.177 | 0.567 | 0.339 | 0.9994 |
| 0.025 µg/L | 27 °C/0.250 µg/L | 27 °C | 0.959 | 0.488 | −0.081 | 1.0000 |
| 0.025 µg/L | 31 °C/control | 31 °C | 0.271 | 0.1044 | −3.392 | 0.0202 |
| 0.025 µg/L | 31 °C/0.025 µg/L | 31 °C | 1.093 | 0.588 | 0.165 | 1.0000 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 1.006 | 0.519 | 0.011 | 1.0000 |
| 0.250 µg/L | 31 °C/control | 31 °C | 0.282 | 0.107 | −3.352 | 0.0223 |
| 0.250 µg/L | 31 °C/0.025 µg/L | 31 °C | 1.139 | 0.606 | 0.245 | 0.9999 |
| 0.250 µg/L | 31 °C/0.250 µg/L | 31 °C | 1.048 | 0.535 | 0.093 | 1.0000 |
| Control | 31 °C/0.025 µg/L | 31 °C | 4.038 | 1.670 | 3.366 | 0.0215 |
| Control | 31 °C/0.250 µg/L | 31 °C | 3.712 | 1.430 | 3.400 | 0.0198 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.920 | 0.496 | −0.154 | 1.0000 |
| 12 h | ||||||
| Control | 27 °C/0.025 µg/L | 27 °C | 0.998 | 0.239 | −0.007 | 1.0000 |
| Control | 27 °C/0.250 µg/L | 27 °C | 2.042 | 0.736 | 1.981 | 0.3736 |
| Control | 27 °C/control | 31 °C | 1.035 | 0.251 | 0.143 | 1.0000 |
| Control | 27 °C/0.025 µg/L | 31 °C | 1.407 | 0.397 | 1.211 | 0.8284 |
| Control | 27 °C/0.250 µg/L | 31 °C | 1.353 | 0.373 | 1.095 | 0.8798 |
| 0.025 µg/L | 27 °C/0.250 µg/L | 27 °C | 2.045 | 0.736 | 1.987 | 0.3704 |
| 0.025 µg/L | 31 °C/control | 31 °C | 1.037 | 0.251 | 1.150 | 1.0000 |
| 0.025 µg/L | 31 °C/0.025 µg/L | 31 °C | 1.409 | 0.397 | 1.218 | 0.8252 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 1.355 | 0.374 | 1.102 | 0.8771 |
| 0.250 µg/L | 31 °C/control | 31 °C | 0.507 | 0.184 | −1.867 | 0.4386 |
| 0.250 µg/L | 31 °C/0.025 µg/L | 31 °C | 0.689 | 0.268 | −0.959 | 0.9277 |
| 0.250 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.663 | 0.255 | −1.071 | 0.8893 |
| Control | 31 °C/0.025 µg/L | 31 °C | 1.359 | 0.388 | 1.075 | 0.8879 |
| Control | 31 °C/0.250 µg/L | 31 °C | 1.307 | 0.365 | 0.958 | 0.9280 |
| 0.025 µg/L | 31 °C/0.250 µg/L | 31 °C | 0.962 | 0.301 | −0.125 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ale, A.; Andrade, V.S.; Rojas Molina, F.M.; Montalto, L.; Odetti, L.M.; Antezana, P.E.; Desimone, M.F.; Simoniello, M.F. Nanozinc Ecotoxicity in the Freshwater Invasive Bivalve Limnoperna fortunei Under a Climate Change Scenario. Animals 2025, 15, 2734. https://doi.org/10.3390/ani15182734
Ale A, Andrade VS, Rojas Molina FM, Montalto L, Odetti LM, Antezana PE, Desimone MF, Simoniello MF. Nanozinc Ecotoxicity in the Freshwater Invasive Bivalve Limnoperna fortunei Under a Climate Change Scenario. Animals. 2025; 15(18):2734. https://doi.org/10.3390/ani15182734
Chicago/Turabian StyleAle, Analía, Victoria S. Andrade, Florencia M. Rojas Molina, Luciana Montalto, Lucía M. Odetti, Pablo E. Antezana, Martín F. Desimone, and María Fernanda Simoniello. 2025. "Nanozinc Ecotoxicity in the Freshwater Invasive Bivalve Limnoperna fortunei Under a Climate Change Scenario" Animals 15, no. 18: 2734. https://doi.org/10.3390/ani15182734
APA StyleAle, A., Andrade, V. S., Rojas Molina, F. M., Montalto, L., Odetti, L. M., Antezana, P. E., Desimone, M. F., & Simoniello, M. F. (2025). Nanozinc Ecotoxicity in the Freshwater Invasive Bivalve Limnoperna fortunei Under a Climate Change Scenario. Animals, 15(18), 2734. https://doi.org/10.3390/ani15182734







