Do Peri-Urban Areas Act as Refuges for the European Rabbit (Oryctolagus cuniculus L.)?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
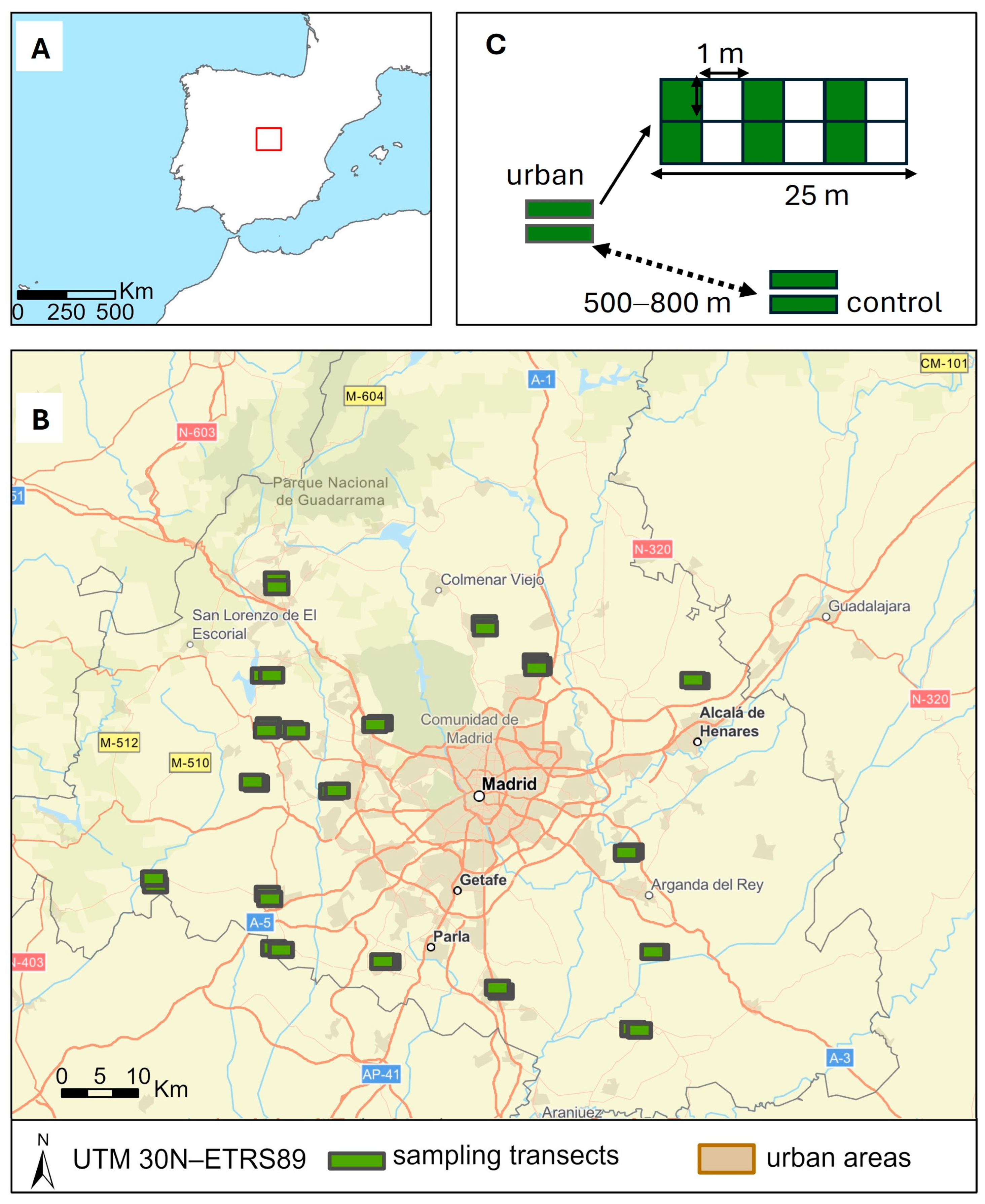
2.1. Study Area and Site Selection
2.2. Rabbit Sampling and Explanatory Variables
2.3. Data Analysis
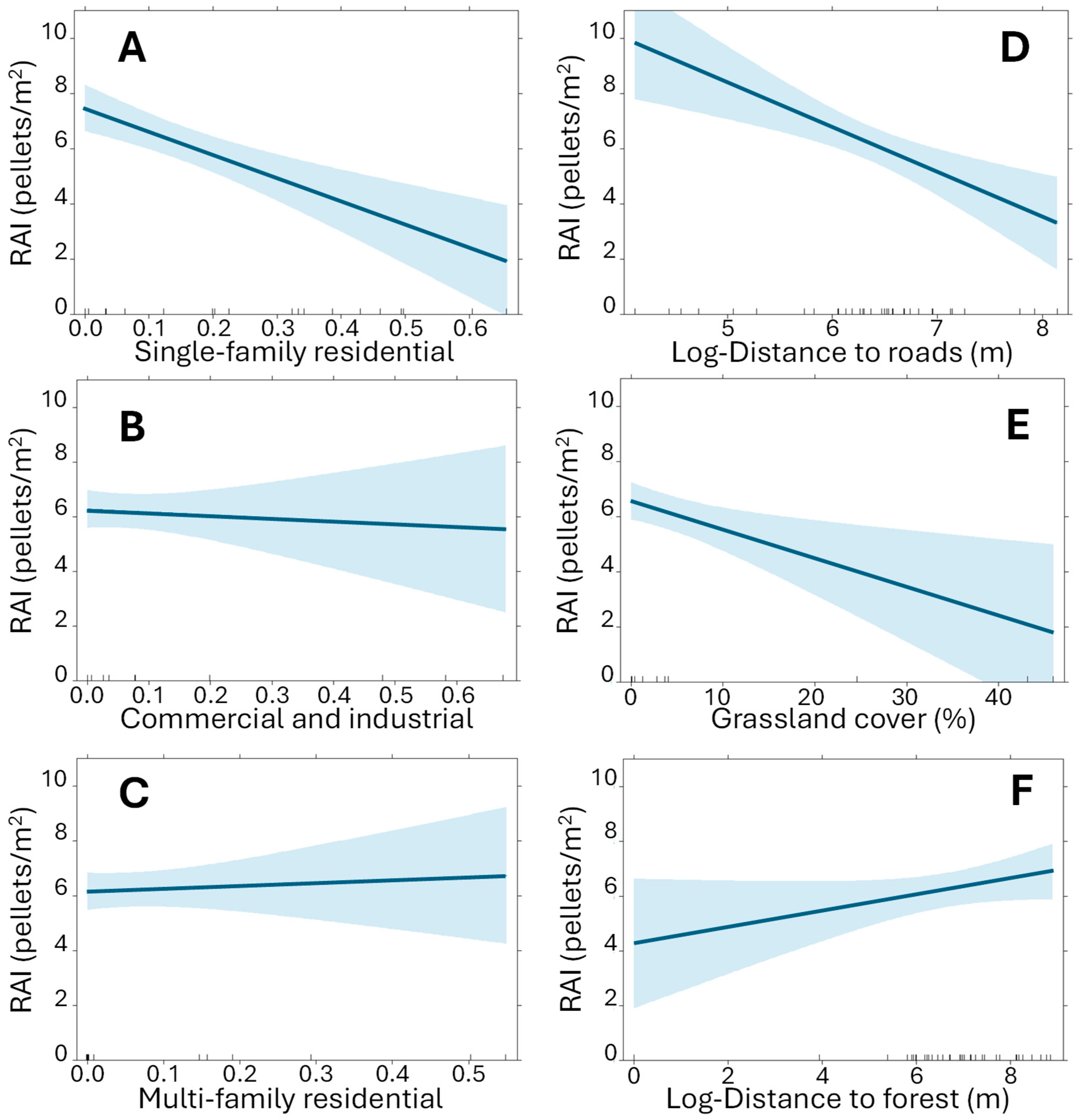
3. Results
4. Discussion
4.1. Human Determinants of Rabbit Abundance
4.2. Landscape Determinants of Rabbit Abundance
4.3. Final Remarks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RAI | Rabbit Abundance Index |
| AIC | Akaike’s Information Criterium |
References
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 125985. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Gupta, J.; Qin, D.; Lade, S.J.; Abrams, J.F.; Andersen, L.S.; McKay, D.I.A.; Bai, X.; Bala, G.; Bunn, S.E.; et al. Safe and just Earth system boundaries. Nature 2023, 619, 102–111. [Google Scholar] [CrossRef]
- Sage, R.F. Global change biology: A primer. Glob. Change Biol. 2020, 26, 3–30. [Google Scholar] [CrossRef]
- Thomas, C.D. The development of Anthropocene biotas. Phil. Trans. R. Soc. B 2020, 375, 20190113. [Google Scholar] [CrossRef]
- O’Brien, L.E.; Urbanek, R.E.; Gregory, J.G. Ecological functions and human benefits of urban forests. Urban For. Urban Green. 2022, 75, 127707. [Google Scholar] [CrossRef]
- Roth, A.T.; Kleemann, J.; Spyra, M. Policy-making for peri-urban landscapes as arenas of human-wildlife interactions. Urban Ecosys. 2024, 27, 1707–1721. [Google Scholar] [CrossRef]
- Ravetz, J.; Sahana, M. Where is the peri-urban? Mapping the areas ‘around, beyond and between’. Front. Sustain. Cities 2025, 7, 1436287. [Google Scholar] [CrossRef]
- Wiig, A.; Silver, J. Turbulent presents, precarious futures: Urbanization and the deployment of global infrastructure. Reg. Stud. 2019, 53, 912–923. [Google Scholar] [CrossRef]
- Maes, J.; Barbosa, A.; Baranzelli, C.; Zulian, G.; Batista e Silva, F.; Vandecasteele, I.; Lavalle, C. More green infrastructure is required to maintain ecosystem services under current trends in land-use change in Europe. Landsc. Ecol. 2015, 30, 517–534. [Google Scholar] [CrossRef]
- Hulme-Beaman, A.; Dobney, K.; Cucchi, T.; Searle, J.B. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol. Evol. 2016, 31, 633–645. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, J.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Luniak, M. Synurbization—Adaptation of animal wildlife to urban development. In Proceedings of the 4th International Symposium Urban Wildlife Conservation, Tucson, AZ, USA, 1–5 May 1999; Shaw, W.W., Harris, L.K., VanDruff, A.L., Eds.; University of Arizona: Tucson, AZ, USA, 2004; pp. 50–55. [Google Scholar]
- Soulsbury, C.D.; White, P.C.L. Human–wildlife interactions in urban areas: A review of conflicts, benefits and opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Hansen, C.P.; Parsons, A.W.; Kays, R.; Millspaugh, J.J. Does Use of Backyard Resources Explain the Abundance of Urban Wildlife? Front. Ecol. Evol. 2020, 8, 570771. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. What makes a species synurbic? Appl. Geogr. 2011, 32, 514–521. [Google Scholar] [CrossRef]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef]
- Wilson, M.W.; Ridlon, A.D.; Gaynor, K.M.; Gaines, S.D.; Stier, A.C.; Halpern, B.S. Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 2020, 23, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.D.; Cleeton, S.H.; Lyons, T.P.; Miller, J.R. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. BioScience 2012, 62, 809–818. [Google Scholar] [CrossRef]
- Muhly, T.B.; Semeniuk, C.; Massolo, A.; Hickman, L.; Musiani, M. Human activity helps prey win the predator-prey space race. PLoS ONE 2011, 6, e17050. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Gálvez-Bravo, L. El papel del conejo como especie clave multifuncional en el ecosistema mediterráneo de la Península Ibérica. Ecosistemas 2009, 18, 14–25. [Google Scholar]
- Lees, A.C.; Bell, B.J. A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Rev. 2008, 38, 304–320. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Ferreras, P.; Villafuerte, R. European rabbit population trends and associated factors: A review of the situation in the Iberian Peninsula. Mammal Rev. 2009, 39, 124–140. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Farfán, M.A.; Olivero, J.; Vargas, J.M. Land-use changes as a critical factor for long-term wild rabbit conservation in the Iberian Peninsula. Environ. Conserv. 2010, 37, 169–176. [Google Scholar] [CrossRef]
- Villafuerte, R.; Delibes-Mateos, M. Oryctolagus cuniculus (errata version published in 2020). IUCN Red List. Threat. Species 2019, e.T41291A170619657. [Google Scholar] [CrossRef]
- Lombardi, L.; Fernández, N.; Moreno, S. Habitat use and spatial behaviour in the European rabbit in three Mediterranean environments. Basic Appl. Ecol. 2007, 8, 453–463. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Carpio, A.J.; Ruiz-Aizpurúa, L.; Tortosa, F.S. Restocking a keystone species in a biodiversity hotspot: Recovering the European rabbit on a landscape scale. J. Nat. Conserv. 2013, 21, 444–448. [Google Scholar] [CrossRef]
- Virgós, E.; Cabezas-Díaz, S.; Malo, A.; Lozano, J.; López-Huertas, D. Factors shaping European rabbit abundance. Acta Theriol. 2003, 48, 113–122. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L. What are the keys to the adaptive success of European wild rabbit (Oryctolagus cuniculus) in the Iberian Peninsula? Animals 2021, 11, 2453. [Google Scholar] [CrossRef]
- Planillo, A.; Malo, J.E. Infrastructure features outperform environmental variables explaining rabbit abundance around motorways. Ecol. Evol. 2018, 8, 942–952. [Google Scholar] [CrossRef]
- Fernández-López, F.; López-Galán, N.; Acevedo, P.; Blanco-Aguiar, J.A.; Vicente, J.; Santamaría, A.E.; Truchado-Quintana, G.; Pinedo, S.O.; Gabaldón, L.; Pérez de Ayala, R. Rabbits on the road: Disentangling the factors driving the warren’s abundance on motorways. Glob. Ecol. Conserv. 2025, 60, e03598. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Farfán, M.A.; Rouco, C.; Olivero, J.; Márquez, A.L.; Fa, J.E.; Villafuerte, R. A large-scale assessment of European rabbit damage to agriculture in Spain. Pest Manag. Sci. 2018, 74, 111–119. [Google Scholar] [CrossRef]
- Vía Libre. Adif instalará 450 kilómetros de vallado de protección contra la entrada de conejos en las vías. Vía Libre 2018, 632, e25100. Available online: https://vialibre-ffe.com/noticias.asp?not=25100 (accessed on 23 July 2025).
- Ziege, M.; Babitsch, D.; Brix, M.; Kristen, S.; Straskraba, S.; Wenninger, S.; Wronsk, T.; Plath, M. Extended diurnal activity patterns of European rabbits along a rural-to-urban gradient. Mamm. Biol. 2016, 81, 534–541. [Google Scholar] [CrossRef]
- Suárez-Tangil, B.D.; Rodríguez, A. Environmental filtering drives the assembly of mammal communities in a heterogeneous Mediterranean region. Ecol. Appl. 2023, 33, e2801. [Google Scholar] [CrossRef] [PubMed]
- Dunagan, S.P.; Karels, T.J.; Moriarty, J.G.; Brown, J.L.; Riley, S.P.D. Bobcat and rabbit habitat use in an urban landscape. J. Mammal. 2019, 100, 401–409. [Google Scholar] [CrossRef]
- Sogliani, D.; Cerri, J.; Turetta, R.; Crema, M.; Corsini, M.; Mori, E. Feral rabbit populations in a peri-urban area: Insights about invasion dynamics and potential management strategies. Eur. J. Wildl. Res. 2021, 67, 60. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Fantle-Lepczyk, J.E.; Dunham, K.D.; Bonnaud, E.; Lindner, J.; Doherty, T.S.; Woinarski, J.C.Z. A global synthesis and assessment of free-ranging domestic cat diet. Nat. Commun. 2023, 14, 7809. [Google Scholar] [CrossRef]
- Thomas, R.L.; Papworth, S.K.; Fellowes, M.D.E. Unleashed: Walking dogs off the lead greatly increases habitat disturbance in UK lowland heathlands. Urban Ecosyst. 2024, 27, 2037–2046. [Google Scholar] [CrossRef]
- Kent, E.; Schwartz, A.L.W.; Perkins, S.E. Life in the fast lane: Roadkill risk along an urban–rural gradient. J. Urban Ecol. 2021, 7, juaa039. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Magura, T.; Lövei, G.L. A meta-analysis indicates reduced predation pressure with increasing urbanization. Landsc. Urban Plan. 2018, 180, 54–59. [Google Scholar] [CrossRef]
- Zlender, V.; Ward-Thompson, C.D. Accessibility and use of peri-urban green space for inner-city dwellers: A comparative study. Landsc. Urban Plan. 2017, 165, 193–205. [Google Scholar] [CrossRef]
- Bischof, R.; Hansen, N.R.; Nyheim, Ø.S.; Kisen, A.; Prestmoen, L.; Haugaasen, T. Mapping the “catscape” formed by a population of pet cats with outdoor access. Sci. Rep. 2022, 12, 5964. [Google Scholar] [CrossRef]
- Gálvez-Bravo, L. Conejo—Oryctolagus cuniculus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: http://www.vertebradosibericos.org/ (accessed on 3 May 2025).
- Iberpix. Visualizador de Mapas e Imágenes (IBERPIX). Centro Nacional de Información Geográfica, Ministerio de Transportes y Movilidad Sostenible, Madrid, Spain. 2023. Available online: https://www.ign.es/iberpix/ (accessed on 3 August 2025).
- IDEM. Infraestructura de Datos Espaciales de la Comunidad de Madrid (IDEM). Comunidad de Madrid, Madrid, Spain. 2023. Available online: https://idem.comunidad.madrid/visor/ (accessed on 3 August 2025).
- Iborra, O.; Lumaret, J.P. Validity limits of the pellet group counts in wild rabbit (Oryctolagus cuniculus). Mammalia 1997, 61, 205–218. [Google Scholar] [CrossRef]
- Fernandez-de-Simón, J.; Díaz-Ruiz, F.; Cirilli, F.; Tortosa, F.S.; Villafuerte, R.; Delibes-Mateos, M.; Ferreras, P. Towards a standardized index of European rabbit abundance in Iberian Mediterranean habitats. Eur. J. Wildl. Res. 2011, 57, 1091–1100. [Google Scholar] [CrossRef]
- Fa, J.E.; Sharples, C.M.; Bell, D.J. Habitat correlates of European rabbit (Oryctolagus cuniculus) distribution after the spread of RVHD in Cadiz Province, Spain. J. Zool. 1999, 249, 83–96. [Google Scholar] [CrossRef]
- SIGPAC. Sistema de Información Geográfica de Parcelas Agrícolas (SIGPAC). Ministerio de Agricultura, Pesca y Alimentación, Madrid, Spain. 2023. Available online: https://www.mapa.gob.es/es/agricultura/temas/ (accessed on 5 June 2025).
- Alberti, M. The effects of urban patterns on ecosystem function. Int. Reg. Sci. Rev. 2005, 28, 168–192. [Google Scholar] [CrossRef]
- Narce, M.; Meloni, R.; Beroud, T.; Pléney, A.; Ricci, J.C. Landscape ecology and wild rabbit (Oryctolagus cuniculus) habitat modeling in the Mediterranean region. Anim. Biodiv. Conserv. 2012, 35, 277–283. [Google Scholar] [CrossRef]
- Virgós, E. Relative value of riparian woodlands in landscapes with different forest cover for medium-sized Iberian carnivores. Biodivers. Conserv. 2001, 10, 1039–1049. [Google Scholar] [CrossRef]
- Mangas, J.G.; Lozano, J.; Cabezas-Díaz, S.; Virgós, E. The priority value of scrubland habitats for carnivore conservation in Mediterranean ecosystems. Biodivers. Conserv. 2008, 17, 43–51. [Google Scholar] [CrossRef]
- QGIS. QGIS Geographic Information System. QGIS Association. 2022. Available online: http://www.qgis.org (accessed on 3 August 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 3 August 2025).
- Aziz, A.; Anwar, M.M.; Majeed, M.; Fatima, S.; Mehdi, S.S.; Mangrio, W.M.; Elbouzidi, A.; Abdullah, M.; Shaukat, S.; Zahid, N.; et al. Quantifying Landscape and Social Amenities as Ecosystem Services in Rapidly Changing Peri-Urban Landscape. Land 2023, 12, 477. [Google Scholar] [CrossRef]
- Weston, M.A.; Stankowich, T. Dogs as agents of disturbance. In Free-Ranging Dogs and Wildlife Conservation; Gompper, M.E., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; pp. 94–116. ISBN 978-0-19-966321-7. [Google Scholar]
- Lewis, J.S.; Spaulding, S.; Swanson, H.; Keeley, W.; Gramza, A.R.; VandeWoude, S.; Crooks, K.R. Human activity influences wildlife populations and activity patterns: Implications for spatial and temporal refuges. Ecosphere 2021, 12, e03487. [Google Scholar] [CrossRef]
- Lenth, B.E.; Knight, R.L.; Brennan, M.E. The effects of dogs on wildlife communities. Nat. Areas J. 2008, 28, 218–227. [Google Scholar] [CrossRef]
- Mahar, N.; Habib, B.; Hussain, S.A. Do we need to unfriend a few friends? Free-ranging dogs affect wildlife and pastoralists in the Indian Trans-Himalaya. Anim. Conserv. 2024, 27, 53–64. [Google Scholar] [CrossRef]
- Pirie, T.J.; Thomas, R.L.; Fellowes, M.D.E. Pet cats (Felis catus) from urban boundaries use different habitats, have larger home ranges and kill more prey than cats from the suburbs. Landsc. Urban Plan. 2022, 220, 104338. [Google Scholar] [CrossRef]
- Marchesi, L.; Sergio, F.; Pedrini, P. Costs and benefits of breeding in human-altered landscapes for the Eagle Owl Bubo bubo. Ibis 2002, 144, E164–E177. [Google Scholar] [CrossRef]
- Palomino, D.; Carrascal, L.M. Habitat associations of a raptor community in a mosaic landscape of Central Spain under urban development. Landsc. Urban Plan. 2007, 83, 268–274. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The landscape of fear: Ecological implications of being afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Rodríguez, D.A.; Evenson, K.R.; Diez Roux, A.V.; Brines, S.J. Land use, residential density, and walking. The multi-ethnic study of atherosclerosis. Am. J. Prev. Med. 2009, 37, 397–404. [Google Scholar] [CrossRef]
- Tapia, L.; Domínguez, J.; Regos, A.; Vidal, M. Using remote sensing data to model European wild rabbit (Oryctolagus cuniculus) occurrence in a highly fragmented landscape in northwestern Spain. Acta Theriol. 2014, 59, 289–298. [Google Scholar] [CrossRef]
- Ruiz-Capillas, P.; Mata, C.; Malo, J.E. Community response of mammalian predators and their prey to motorways: Implications for predator-prey dynamics. Ecosystems 2013, 16, 617–626. [Google Scholar] [CrossRef]
- Ziege, M.; Brix, M.; Schulze, M.; Seidemann, A.; Straskraba, S.; Wenninger, S.; Streit, B.; Wronsk, T.; Plath, M. From multifamily residences to studio apartments: Shifts in burrow structures of European rabbits along a rural-to-urban gradient. J. Zool. 2015, 295, 286–293. [Google Scholar] [CrossRef]
- Bakker, E.S.; Reiffers, R.C.; Olff, H.; Gleichman, J.M. Experimental manipulation of predation risk and food quality: Effect on grazing behaviour in a central-place foraging herbivore. Oecologia 2005, 146, 157–167. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.M.; Muhly, T.B.; Simi, S.; Musiani, M.; Pitt, J.A.; Boyce, M.S. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef]
- Barros, A.L.; Raposo, D.; Almeida, J.D.; Jesus, H.; Oliveira, M.A.; Fernandes, C.R.; MacKenzie, D.L.; Santos-Reis, M. An integrated assessment of niche partitioning reveals mechanisms of coexistence between mesocarnivores. Glob. Ecol. Conserv. 2024, 54, e03116. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Ferrer, X.; Jiménez, J.; Fernández–Calvo, I.C. The selection of anthropogenic habitat by wildlife as an ecological consequence of rural exodus: Empirical examples from Spain. Anim. Biodiver. Conserv. 2021, 44, 195–203. [Google Scholar] [CrossRef]
- Pongrácz, P.; Altbäcker, V.; Fenes, D. Human handling might interfere with conspecific recognition in the European rabbit (Oryctolagus cuniculus). Dev. Psychobiol. J. Int. Soc. Develop. Psychobiol. 2001, 39, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Samia, D.S.M.; Nakagawa, S.; Nomura, F.; Rangel, T.R.; Blumstein, D.T. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 2015, 6, 8877. [Google Scholar] [CrossRef]
- Griffin, S.C.; Valois, T.; Taper, M.L.; Mills, S. Effects of tourists on behavior and demography of Olympic marmots. Conserv. Biol. 2007, 21, 1070–1081. [Google Scholar] [CrossRef]
- Dickman, A.J.; Hazzah, L.; Carbone, C.; Durant, S.M. Carnivores, culture and ‘contagious conflict’: Multiple factors influence perceived problems with carnivores in Tanzania’s Ruaha landscape. Biol. Conserv. 2014, 178, 19–27. [Google Scholar] [CrossRef]
- Iranzo, E.; Ohrens, O.; Mata, C.; Traba, J.; Acebes, P.; González, B.; Tortato, F.; Hoogesteijn, R.; Goic, D.; Elbroch, L.M.; et al. More pumas (Puma concolor) does not change perceptions: The mismatched response of ranchers to the presence of a top carnivore. People Nat. 2025, in press. [Google Scholar] [CrossRef]
| Class | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Pellet range | 0 | 1–2 | 3–15 | 16–50 | 51–150 | If >150, estimated hundred range (e.g., 151–250, 251–350, 351–450…) |
| Ci | 0 | 1.5 | 9 | 33 | 100 | The median value (200, 300, 400…) |
| Variable Name | Description | Source |
|---|---|---|
| Urbanized area | ||
| Single-family residential | Residential areas composed of single-family houses with gardens | Visual categorization on 2021 aerial images and area measurements performed in GIS |
| Commercial or industrial | Industrial and commercial areas | |
| Multi-family residential | Residential areas of multi-dwelling blocks | |
| Vegetation of buffer surfaces not urbanized | ||
| Forest | Natural forest and tree plantations | Categories reclassified from [50] and checked on aerial images taken in 2021. Surfaces measured with GIS |
| Shrubland | Shrublands with grass cover underneath | |
| Grassland | Open grassland without shrub cover | |
| Woody crops | Vineyards, olive, almond, or other tree crops | |
| Herbaceous crops | Mainly cereal and fodder crops, including fallow land | |
| Barren | Areas without plant cover | |
| Variable | Estimate ± SE | F-Value | p |
|---|---|---|---|
| Intercept | 16.203 ± 3.241 | 24.98 | <0.0001 *** |
| Distance to transport infrastructures | −1.624 ± 0.425 | 14.61 | <0.0001 *** |
| Urbanized: single-family residential | −8.388 ± 1.840 | 20.77 | <0.0001 *** |
| Urbanized: commercial or industrial | −1.072 ± 2.354 | 0.21 | 0.652 |
| Urbanized: multi-family residential | 1.046 ± 2.362 | 0.20 | 0.661 |
| Grassland cover (%) | −0.103 ± 0.036 | 8.39 | 0.0071 ** |
| Distance to forest patches ≥ 5 ha | 0.295 ± 0.169 | 3.05 | 0.0915 · |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, A.; Mata, C.; Malo, J.E. Do Peri-Urban Areas Act as Refuges for the European Rabbit (Oryctolagus cuniculus L.)? Animals 2025, 15, 2719. https://doi.org/10.3390/ani15182719
Cabrera A, Mata C, Malo JE. Do Peri-Urban Areas Act as Refuges for the European Rabbit (Oryctolagus cuniculus L.)? Animals. 2025; 15(18):2719. https://doi.org/10.3390/ani15182719
Chicago/Turabian StyleCabrera, Ana, Cristina Mata, and Juan E. Malo. 2025. "Do Peri-Urban Areas Act as Refuges for the European Rabbit (Oryctolagus cuniculus L.)?" Animals 15, no. 18: 2719. https://doi.org/10.3390/ani15182719
APA StyleCabrera, A., Mata, C., & Malo, J. E. (2025). Do Peri-Urban Areas Act as Refuges for the European Rabbit (Oryctolagus cuniculus L.)? Animals, 15(18), 2719. https://doi.org/10.3390/ani15182719






