Antimicrobial Resistance and Genetic Characterization of Streptococcus equi subsp. zooepidemicus in Equines from Central Italy: Insights from a One Health Perspective
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Antimicrobial Susceptibility Test
2.3. Whole-Genome Sequence Analysis, MLST, Virulence Factor, and Antimicrobial Resistance Genetic Profile
3. Results
3.1. Samples
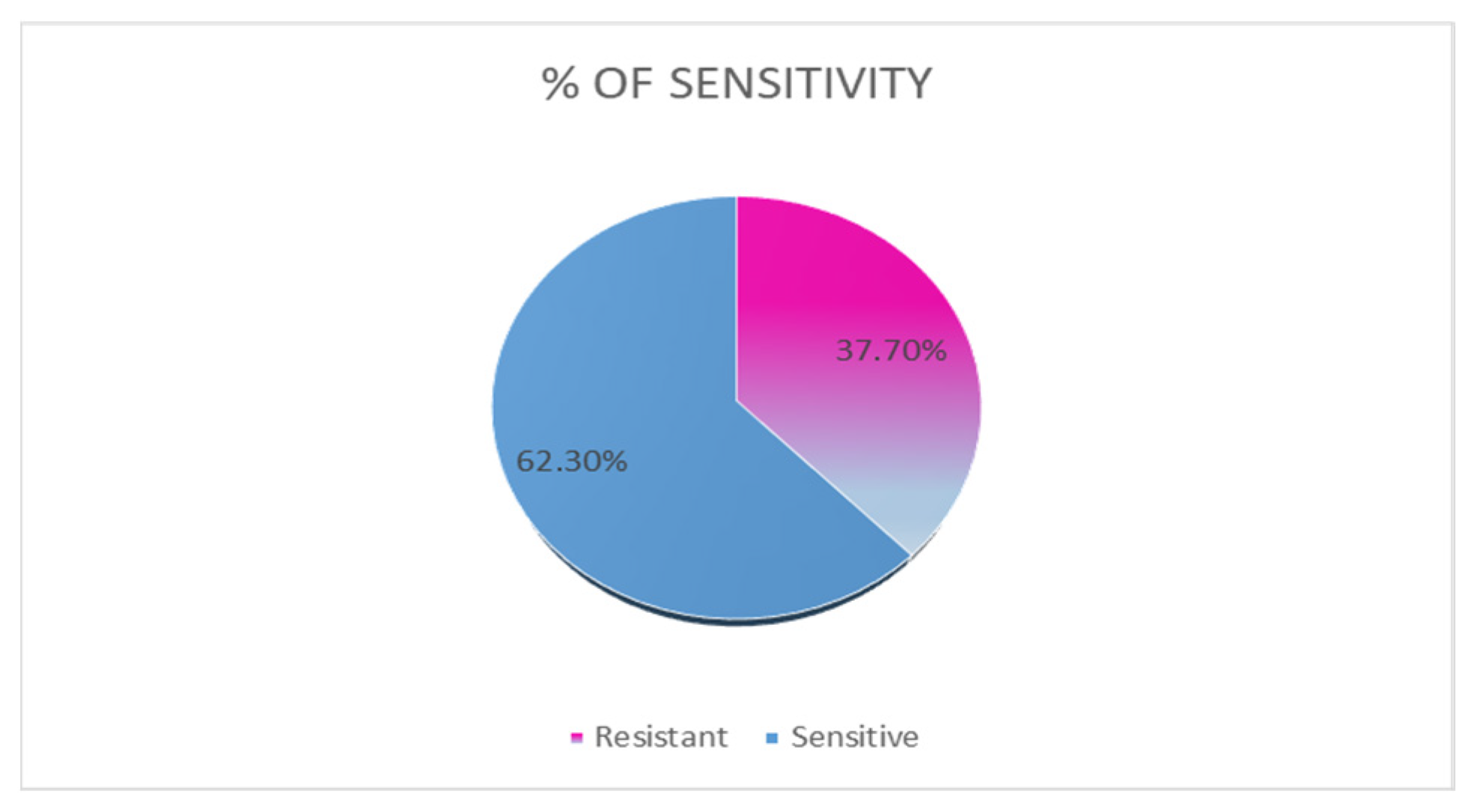
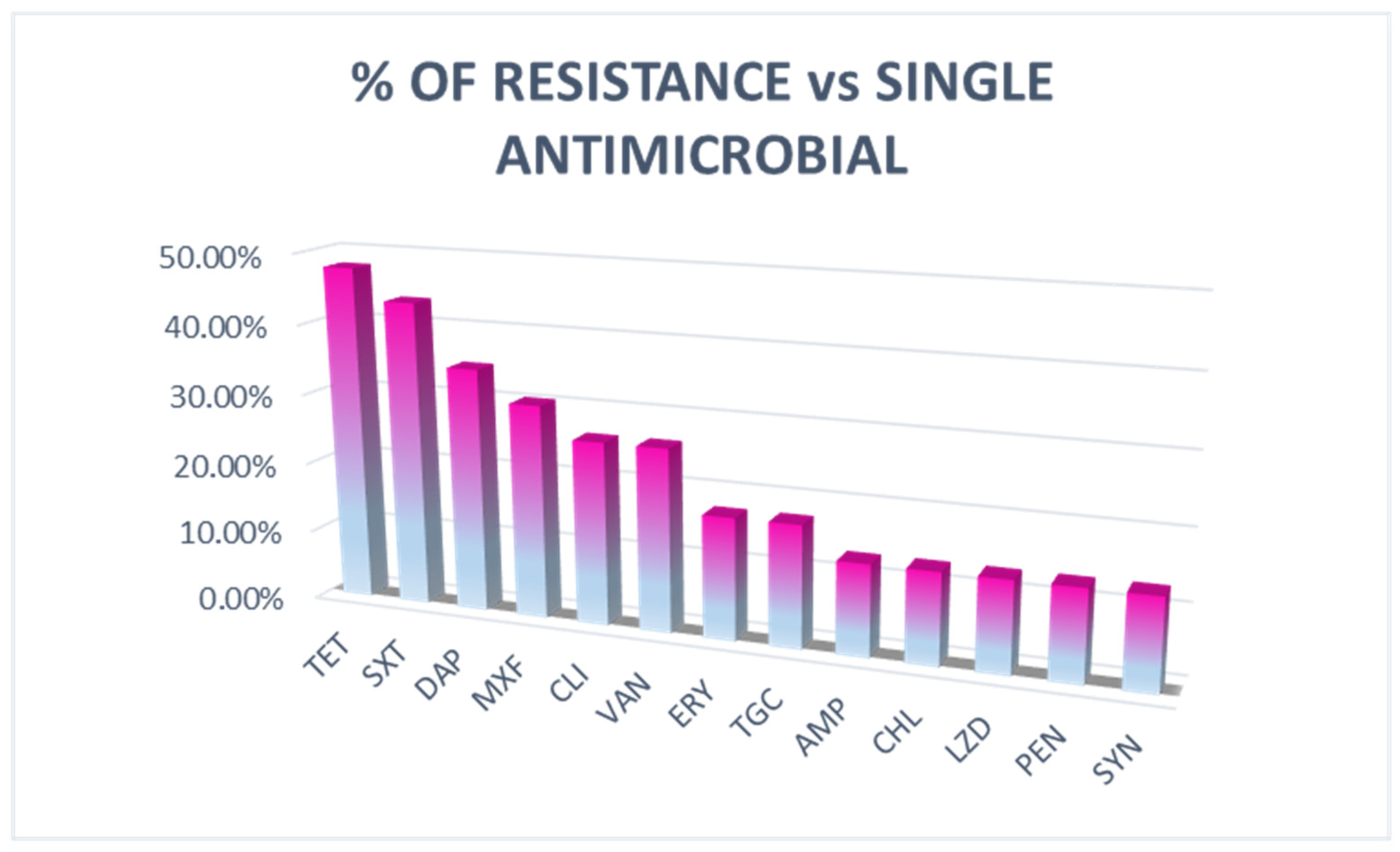
3.2. Antimicrobial Susceptibility Test
3.3. Whole-Genome Sequence Analysis, MLST, Virulence Factor, and Antimicrobial Resistance Genetic Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Pusterla, N.; Sandler-Burtness, E.; Barnum, S.; Hill, L.A.; Mendonsa, E.; Khan, R.; Portener, D.; Ridland, H.; Schumacher, S. Frequency of Detection of Respiratory Pathogens in Nasal Secretions from Healthy Sport Horses Attending a Spring Show in California. J. Equine Vet. Sci. 2022, 117, 104089. [Google Scholar] [CrossRef]
- Cantelmi, M.C.; Merola, C.; Averaimo, D.; Chiaverini, A.; Cito, F.; Cocco, A.; Di Teodoro, G.; De Angelis, M.E.; Di Bernardo, D.; Auzino, D.; et al. Identification of the Novel Streptococcus equi subsp. zooepidemicus Sequence Type 525 in Donkeys of Abruzzo Region, Italy. Pathogens 2023, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, M.F.; Burger, N.; Mazza, M.; Rodríguez, G.; Camou, T.; García Gabarrot, G. Characterization of Streptococcus equi subsp. zooepidemicus isolates containing lnuB gene responsible for the L phenotype. PLoS ONE 2023, 18, e0284869. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Capozzi, L.; Simone, D.; Pizzano, F.; Iovane, V.; Bianco, A.; Parisi, A.; De Martino, L. Multi-locus sequence typing and in vitro antimicrobial resistance of equine Streptococcus equi subspecies zooepidemicus strains. Vet. Res. Commun. Online 2023, 48, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Lamichhane, B.; Habib, T.; Adams, A.; El-Sheikh Ali, H.; Slovis, N.M.; Troedsson, M.H.T.; Helmy, Y.A. Antimicrobial Resistance in Equines: A Growing Threat to Horse Health and Beyond—A Comprehensive Review. Antibiotics 2024, 13, 713. [Google Scholar] [CrossRef]
- Glajzner, P.; Szewczyk, E.M.; Szemraj, M. Pathogenicity and drug resistance of animal streptococci responsible for human infections. J. Med. Microbiol. 2021, 70, 001339. [Google Scholar] [CrossRef]
- Mangano, E.R.; Jones, G.M.C.; Suarez-Bonnet, A.; Waller, A.S.; Priestnall, S.L. Streptococcus zooepidemicus in dogs: Exploring a canine pathogen through multilocus sequence typing. Vet. Microbiol. 2024, 292, 110059. [Google Scholar] [CrossRef]
- Watson Joshua, R.; Amy, L.; Sridhar, V.; Timoney John, F.; Ardura Monica, I. Recurrent Streptococcus equi subsp. zooepidemicus Bacteremia in an Infant. J. Clin. Microbiol. 2015, 53, 3096–3099. [Google Scholar] [CrossRef]
- Björnsdóttir, S.; Harris, S.R.; Svansson, V.; Gunnarsson, E.; Sigurðardóttir, Ó.G.; Gammeljord, K.; Steward, K.F.; Newton, J.R.; Robinson, C.; Charbonneau, A.R.L.; et al. Genomic Dissection of an Icelandic Epidemic of Respiratory Disease in Horses and Associated Zoonotic Cases. mBio 2017, 8, e00826-17. [Google Scholar] [CrossRef]
- Bosica, S.; Chiaverini, A.; De Angelis, M.E.; Petrini, A.; Averaimo, D.; Martino, M.; Rulli, M.; Saletti, M.A.; Cantelmi, M.C.; Ruggeri, F.; et al. Severe Streptococcus equi Subspecies zooepidemicus Outbreak from Unpasteurized Dairy Product Consumption, Italy. Emerg. Infect. Dis. 2023, 29, 1020–1024. [Google Scholar] [CrossRef]
- Cito, F.; Di Francesco, C.E.; Averaimo, D.; Chiaverini, A.; Alessiani, A.; Di Domenico, M.; Cresci, M.; Rulli, M.; Cantelmi, M.C.; Di Bernardo, M.D.; et al. Streptococcus equi subsp. zooepidemicus: Epidemiological and Genomic Findings of an Emerging Pathogen in Central Italy. Animals 2025, 15, 1351. [Google Scholar] [CrossRef]
- Isgren, C.M.; Williams, N.J.; Fletcher, O.D.; Timofte, D.; Newton, R.J.; Maddox, T.W.; Clegg, P.D.; Pinchbeck, G.L. Antimicrobial resistance in clinical bacterial isolates from horses in the UK. Equine Vet. J. 2022, 54, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Léon, A.; Castagnet, S.; Maillard, K.; Paillot, R.; Giard, J. Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019. Animals 2020, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S.; Meucci, V.; Sgorbini, M.; De Marchi, L.; Pirone, A.; Pretti, C.; Tognetti, R.; Intorre, L. Pattern of prescriptions and prudent use of antimicrobial in horse practice at a Veterinary Teaching Hospital. Res. Vet. Sci. 2024, 168, 105140. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Correcher, E.; Sitters, J.; Wassen, M.; Brion, N.; Olde Venterink, H. Herbivore dung quality affects plant community diversity. Sci. Rep. 2019, 9, 5675. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2024; Available online: https://clsi.org/shop/standards/m100/ (accessed on 12 December 2024).
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI supplement VET01S; CLSI: Wayne, PA, USA, 2024; Available online: https://clsi.org/shop/standards/vet01s/ (accessed on 12 December 2024).
- EUCAST Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 12 December 2024).
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Portmann, A.; Fournier, C.; Gimonet, J.; Ngom-Bru, C.; Barretto, C.; Baert, L. A Validation Approach of an End-to-End Whole Genome Sequencing Workflow for Source Tracking of Listeria monocytogenes and Salmonella enterica. Front. Microbiol. 2018, 9, 446. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Lukjancenko, O.; Saputra, D.; Rasmussen, S.; Hasman, H.; Sicheritz-Pontén, T.; Aarestrup, F.M.; Ussery, D.W.; Lund, O. Benchmarking of methods for genomic taxonomy. J. Clin. Microbiol. 2014, 52, 1529–1539. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Turner, C.E.; Bubba, L.; Efstratiou, A. Pathogenicity Factors in Group C and G Streptococci. Microbiol. Spectr. 2019, 7, 2018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paillot, R.; Darby, A.C.; Robinson, C.; Wright, N.L.; Steward, K.F.; Anderson, E.; Webb, K.; Holden, M.T.G.; Efstratiou, A.; Broughton, K.; et al. Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP. Infect. Immun. 2010, 78, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huang, Y.; Zhu, J.; Qiao, Y.; Xiao, N.; Jin, M.; Gao, H.; Huang, Y.; Hu, X.; Li, O. Advances in hyaluronic acid production: Biosynthesis and genetic engineering strategies based on Streptococcus—A review. Int. J. Biol. Macromol. 2024, 270, 132334. [Google Scholar] [CrossRef] [PubMed]
- Toyosaki, T.; Yoshioka, T.; Tsuruta, Y.; Yutsudo, T.; Iwasaki, M.; Suzuki, R. Definition of the mitogenic factor (MF) as a novel streptococcal superantigen that is different from streptococcal pyrogenic exotoxins A, B, and C. Eur. J. Immunol. 1996, 26, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Tsou, C.; Kuo, H.; Wang, J.; Wu, J.; Liao, P. Differential Secretomics of Streptococcus pyogenes Reveals a Novel Peroxide Regulator (PerR)-regulated Extracellular Virulence Factor Mitogen Factor3 (MF3)*. Mol. Cell. Proteom. 2011, 10, M110.007013. [Google Scholar] [CrossRef]
- Shaw, W.V. Chloramphenicol acetyltransferase: Enzymology and molecular biology. CRC Crit. Rev. Biochem. 1983, 14, 1–46. [Google Scholar] [CrossRef]
- Roberts, M.C. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 2005, 245, 195–203. [Google Scholar] [CrossRef]
- Pelkonen, S.; Lindahl, S.B.; Suomala, P.; Karhukorpi, J.; Vuorinen, S.; Koivula, I.; Väisänen, T.; Pentikäinen, J.; Autio, T.; Tuuminen, T. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg. Infect. Dis. 2013, 19, 1041–1048. [Google Scholar] [CrossRef]
- Nocera, F.P.; D’Eletto, E.; Ambrosio, M.; Fiorito, F.; Pagnini, U.; De Martino, L. Occurrence and Antimicrobial Susceptibility Profiles of Streptococcus equi subsp. zooepidemicus Strains Isolated from Mares with Fertility Problems. Antibiotics 2021, 11, 25. [Google Scholar] [CrossRef]
- Burgio, M.; Aiudi, G.G.; Yusuf, M.S.M.; Delgado Bermejo, J.V.; Rivas Lopez, C.; Carbonari, A.; Lucente, M.S.; Landi, V.; Corrente, M.; Tempesta, M.; et al. Prevalence of Streptococcus equi subsp. zooepidemicus in Martina Franca and Andalusian donkey population, EAVLD Congress 2024, 23-27/10/2024. Ital. J. Food Saf. 2024, 1 (Suppl. S1), 72. [Google Scholar]
- Haenni, M.; Lupo, A.; Madec, J. Antimicrobial Resistance in Streptococcus spp. Microbiol. Spectr. 2018, 6, 2017. [Google Scholar] [CrossRef]
- Warburton, P.; Roberts, A.P.; Allan, E.; Seville, L.; Lancaster, H.; Mullany, P. Characterization of tet(32) genes from the oral metagenome. Antimicrob. Agents Chemother. 2009, 53, 273–276. [Google Scholar] [CrossRef]
- Palmieri, C.; Magi, G.; Mingoia, M.; Bagnarelli, P.; Ripa, S.; Varaldo, P.E.; Facinelli, B. Characterization of a Streptococcus suis tet(O/W/32/O)-carrying element transferable to major streptococcal pathogens. Antimicrob. Agents Chemother. 2012, 56, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Animal Health and Welfare, (AHAW); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. Assessment of animal diseases caused by bacteria resistant to antimicrobials: Horses. EFSA J. 2021, 19, e07112. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Garbarino, C.; Luppi, A.; Russo, S.; Filippi, A.; Arrigoni, N.; Massella, E.; Ricchi, M. Comparison between broth microdilution and agar disk diffusion methods for antimicrobial susceptibility testing of bovine mastitis pathogens. J. Microbiol. Methods 2023, 212, 106796. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Sullivan, E.; Kovac, J. Comparative Analysis of Bacillus cereus Group Isolates’ Resistance Using Disk Diffusion and Broth Microdilution and the Correlation between Antimicrobial Resistance Phenotypes and Genotypes. Appl. Environ. Microbiol. 2022, 88, e0230221-21. [Google Scholar] [CrossRef]
- Bovo, F.; Lazzarotto, T.; Ambretti, S.; Gaibani, P. Comparison of Broth Microdilution, Disk Diffusion and Strip Test Methods for Cefiderocol Antimicrobial Susceptibility Testing on KPC-Producing Klebsiella pneumoniae. Antibiotics 2023, 12, 614. [Google Scholar] [CrossRef]
- van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef]
- McClure, S.R.; Koenig, R.; Hawkins, P.A. A randomized controlled field trial of a novel trimethoprim-sulfadiazine oral suspension for treatment of Streptococcus equi subsp zooepidemicus infection of the lower respiratory tract in horses. J. Am. Vet. Med. Assoc. 2015, 246, 1345–1353. [Google Scholar] [CrossRef]
- Pisello, L.; Rampacci, E.; Stefanetti, V.; Beccati, F.; Hyatt, D.R.; Coletti, M.; Passamonti, F. Temporal efficacy of antimicrobials against aerobic bacteria isolated from equine endometritis: An Italian retrospective analysis (2010–2017). Vet. Rec. 2019, 185, 598. [Google Scholar] [CrossRef]
- Mao, Y.; Shisler, J.L.; Nguyen, T.H. Enhanced detection for antibiotic resistance genes in wastewater samples using a CRISPR-enriched metagenomic method. Water Res. 2025, 274, 123056. [Google Scholar] [CrossRef]
- Kumavath, R.; Gupta, P.; Tatta, E.R.; Mohan, M.S.; Salim, S.A.; Busi, S. Unraveling the role of mobile genetic elements in antibiotic resistance transmission and defense strategies in bacteria. Front. Syst. Biol. 2025, 5, 1557413. [Google Scholar] [CrossRef]
| Species | Nasal Swabs | Genital Swabs | Total |
|---|---|---|---|
| HORSE | 37 | 12 | 49 |
| DONKEY | 11 | 0 | 11 |
| MULE | 1 | 0 | 1 |
| TOTAL | 49 | 12 | 61 |
| ID | Animal Swabs | Resistance Genes | STs | Virulence Genes | Phenotype Details | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | HORSE | NASAL | ant(6)-Ia | erm(B) | 536 | mf3 | DAP/ERY-SXT | ||
| 15 | HORSE | NASAL | lsa(C) | 147 | Fbp54, mf3 | AMP/PEN-CHL-DAP/CLI/ERY-LZD-SYN-TET/TGC-VAN | |||
| 47 | HORSE | NASAL | lsa(C) | 547 | Fbp54, mf3, hasC | AMP/PEN-CHL-CLI/ERY-LZD-SYN-TET/TGC-VAN | |||
| 48 | MULE | NASAL | mef(A) | cat(pC194) | 369 | Fbp54, mf3 | AMP/PEN-CHL-DAP/CLI/ERY-LZD-MXF-SYN-TET/TGC-SXT-VAN | ||
| 36 | HORSE | GENITAL | tet(32) | 364 | mf3 | MOX-TET | |||
| 41 | HORSE | NASAL | tet(O) | 470 | mf3 | CLI-TET | |||
| 54 | HORSE | NASAL | tet(W) | 61 | Fbp54, mf2, hasC | TET | |||
| 59 | HORSE | NASAL | tet(W) | 61 | Fbp54, mf2 | TET | |||
| 60 | HORSE | GENITAL | tet(W) | 72 | Fbp54, mf2 | TET | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessiani, A.; Baffoni, M.; Averaimo, D.; Cantelmi, M.C.; Coccaro, A.; Rulli, M.; Piersanti, V.; Pompilii, C.; Cito, F.; Chiaverini, A.; et al. Antimicrobial Resistance and Genetic Characterization of Streptococcus equi subsp. zooepidemicus in Equines from Central Italy: Insights from a One Health Perspective. Animals 2025, 15, 2713. https://doi.org/10.3390/ani15182713
Alessiani A, Baffoni M, Averaimo D, Cantelmi MC, Coccaro A, Rulli M, Piersanti V, Pompilii C, Cito F, Chiaverini A, et al. Antimicrobial Resistance and Genetic Characterization of Streptococcus equi subsp. zooepidemicus in Equines from Central Italy: Insights from a One Health Perspective. Animals. 2025; 15(18):2713. https://doi.org/10.3390/ani15182713
Chicago/Turabian StyleAlessiani, Alessandra, Marina Baffoni, Daniela Averaimo, Maria Chiara Cantelmi, Antonio Coccaro, Marco Rulli, Vanessa Piersanti, Cinzia Pompilii, Francesca Cito, Alexandra Chiaverini, and et al. 2025. "Antimicrobial Resistance and Genetic Characterization of Streptococcus equi subsp. zooepidemicus in Equines from Central Italy: Insights from a One Health Perspective" Animals 15, no. 18: 2713. https://doi.org/10.3390/ani15182713
APA StyleAlessiani, A., Baffoni, M., Averaimo, D., Cantelmi, M. C., Coccaro, A., Rulli, M., Piersanti, V., Pompilii, C., Cito, F., Chiaverini, A., & Petrini, A. (2025). Antimicrobial Resistance and Genetic Characterization of Streptococcus equi subsp. zooepidemicus in Equines from Central Italy: Insights from a One Health Perspective. Animals, 15(18), 2713. https://doi.org/10.3390/ani15182713





