Pituitary Abscess Syndrome in Ruminants: Nine Cases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fecteau, G.; Parent, J.; George, L.W. Neurologic examination of the ruminant. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 1–8. [Google Scholar] [CrossRef]
- Boileau, M.J.; Gilliam, J. Brainstem and cranial nerve disorders of ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 67–99. [Google Scholar] [CrossRef]
- Mayhew, I.G.J.; MacKay, R.J. Large Animal Neurology, 3rd ed.; Wiley=Blackwell: St. Louis, MO, USA, 2022; 608p. [Google Scholar] [CrossRef]
- Perdrizet, J.A.; Dinsmore, P. Pituitary abscess syndrome. Comp. Cont. Educ. Pract. Vet. 1986, 8, S311–S319. [Google Scholar]
- Fernandes, C.G.; Schild, A.L.; Riet-Correa, F.; Baialardi, C.E.G.; Stigger, A.L. Pituitary abscess in young calves associated with the use of a controlled suckling device. J. Vet. Diagn. Investig. 2000, 12, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Yeruham, I.; Orgad, U.; Avidar, Y.; Elad, D. Pituitary abscess and high urea concentration as causes of neurological signs in a cow. Revue Méd. Vét. 2002, 153, 829–831. [Google Scholar]
- Loretti, A.P.; Ilha, M.R.S.; Riet-Correa, G.; Driemeier, D.; Colodel, E.M.; Barros, C.S.L. Síndrome do abscesso pituitário em bezerros associada ao uso de tabuleta nasal para desmame interrompido. Pesq. Vet. Bras. 2003, 23, 39–46. [Google Scholar] [CrossRef]
- Müller, K.R.; Blutke, A.; Matiasek, K.; Wieland, M.J. Pituitary abscess syndrome in a Simmental heifer. Vet. Rec. Case Rep. 2014, 2, e000041. [Google Scholar] [CrossRef]
- Braun, U.; Malbon, A.; Kochan, M.; Riond, B.; Janett, F.; Iten, C.; Dennler, M. Computed tomographic findings and treatment of a bull with pituitary gland abscess. Acta Vet. Scand. 2017, 59, 8. [Google Scholar] [CrossRef]
- Konradt, G.; Bassuino, D.M.; Prates, K.S.; Bianchi, M.V.; Snel, G.G.M.; Sonne, L.; Driemeier, D.; Pavarini, S.P. Suppurative infectious diseases of the central nervous system in domestic ruminants. Pesq. Vet. Bras. 2017, 37, 820–828. [Google Scholar] [CrossRef]
- Stewart, J.L.; Bates, M.C.; Edwards, B.W.; Aldrigde, B.M. Hyponatremia as the presenting feature of a pituitary abscess in a calf. Vet. Sci. 2017, 4, 8. [Google Scholar] [CrossRef]
- Lomas, S.T.; Hazell, S.L. The isolation of Mycoplasma arginini from a pituitary abscess in a goat. Aust. Vet. J. 1983, 60, 281–282. [Google Scholar] [CrossRef]
- Allen, A.L.; Goupil, B.A.; Valentine, B.A. A retrospective study of brain lesions in goats submitted to three veterinary diagnostic laboratories. J. Vet. Diag. Investig. 2013, 25, 482–489. [Google Scholar] [CrossRef]
- Dabak, M.; Eroksuz, Y.; Baydar, E.; Eroksuz, H.; Cevik, A.; Muz, A. Decreased pituitary hormone levels in a sheep with pituitary abscess. J. Appl. Anim. Res. 2016, 44, 5–8. [Google Scholar] [CrossRef]
- Helman, S. A case of basilar empyema in a Texel ewe lamb. UK Vet. 2010, 15, 41–48. [Google Scholar] [CrossRef]
- Reilly, L.; Habecker, P.; Beech, J.; Johnston, J.; Sweeney, C.; Hamir, A. Pituitary abscess and basilar empyema in 4 horses. Equine Vet. J. 1994, 26, 424–426. [Google Scholar] [CrossRef]
- Morgan, R.E.; Fiske-Jackson, A.R.; Biggi, M. Pituitary gland abscess in a horse subsequent to head trauma. Equine Vet. Educ. 2020, 32, O36–O39. [Google Scholar] [CrossRef]
- Gao, L.; Guo, X.; Tian, R.; Wang, Q.; Feng, M.; Bao, X.; Deng, K.; Yao, Y.; Lian, W.; Wang, R.; et al. Pituitary abscess: Clinical manifestations, diagnosis and treatment of 66 cases from a large pituitary center over 23 years. Pituitary 2017, 20, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Stringer, F.; Foong, Y.C.; Tan, A.; Hayman, S.; Zajac, J.D.; Grossmann, M.; Zane, J.N.Y.; Zhu, J.; Ayyappan, S. Pituitary abscess: A case report and systematic review of 488 cases. Orphanet J. Rare Dis. 2023, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.R. Cerebrospinal fluid collection and analysis in suspected sheep neurological disease. Small Rumin. Res. 2010, 92, 96–103. [Google Scholar] [CrossRef]
- Scott, P.R. Diagnostic techniques and clinicopathologic findings in ruminant neurologic disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 215–230. [Google Scholar] [CrossRef]
- Stokol, T.; Divers, T.J.; Arrigan, J.W.; McDonough, S.P. Cerebrospinal fluid findings in cattle with central nervous system disorders: A retrospective study of 102 cases (1990–2008). Vet. Clin. Pathol. 2009, 38, 103–112. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Donnelly, W.J.; Leonard, F.C.; Fanning, S.; Maguire, D. (Eds.) Laboratory diagnosis of bacterial disease. In Veterinary Microbiology and Microbial Disease, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 143–149. [Google Scholar]
- Meyer, D.J.; Harvey, J.W. Veterinary Laboratory Medicine: Interpretation and Diagnosis, 2nd ed.; Saunders: Philadelphia, PA, USA, 2004; 351p. [Google Scholar]
- Kaneko, J.J.; Harvey, J.; Bruss, M. (Eds.) Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008; 928p. [Google Scholar]
- Morin, D.E. Brainstem and cranial nerve abnormalities: Listeriosis, otitis media/interna, and pituitary abscess syndrome. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 243–273. [Google Scholar] [CrossRef]
- Zguigal, H.; Ghoshal, N.G. Gross and histologic study of the rostral epidural rete mirabile and the cavernous sinus in one-humped camels. Am. J. Vet. Res. 1991, 52, 1173. [Google Scholar] [CrossRef]
- Nagy, D.W. Diagnostics and ancillary tests of neurologic dysfunction in the ruminant. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.L.; Sousa, D.E.; Ferreira-Junior, J.A.; Castro, M.B.; Fino, T.C.; Borges, J.R.J.; Soto-Blanco, B.; Câmara, A.C.L. Paralytic rabies in a goat. BMC Vet. Res. 2018, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Schöb, L.C.; Gerspach, C.; Stirn, M.; Hofmann-Lehmann, R.; Riond, B. Findings related to cerebrospinal fluid and central nervous system disorders in small ruminants—A retrospective study on sheep and goats. Animals 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Câmara, A.C.L.; Sousa, D.E.R.; Mâcedo, I.L.; Soares, K.L.; Borges, J.R.J.; Martins, C.F.; Mesquita, A.Q.; Dutra, V.; Castro, M.B. Suppurative meningoencephalitis by Pseudomonas aeruginosa from direct extension of chronic otitis in a Gir cow. Vet. Sci. 2023, 10, 398. [Google Scholar] [CrossRef]
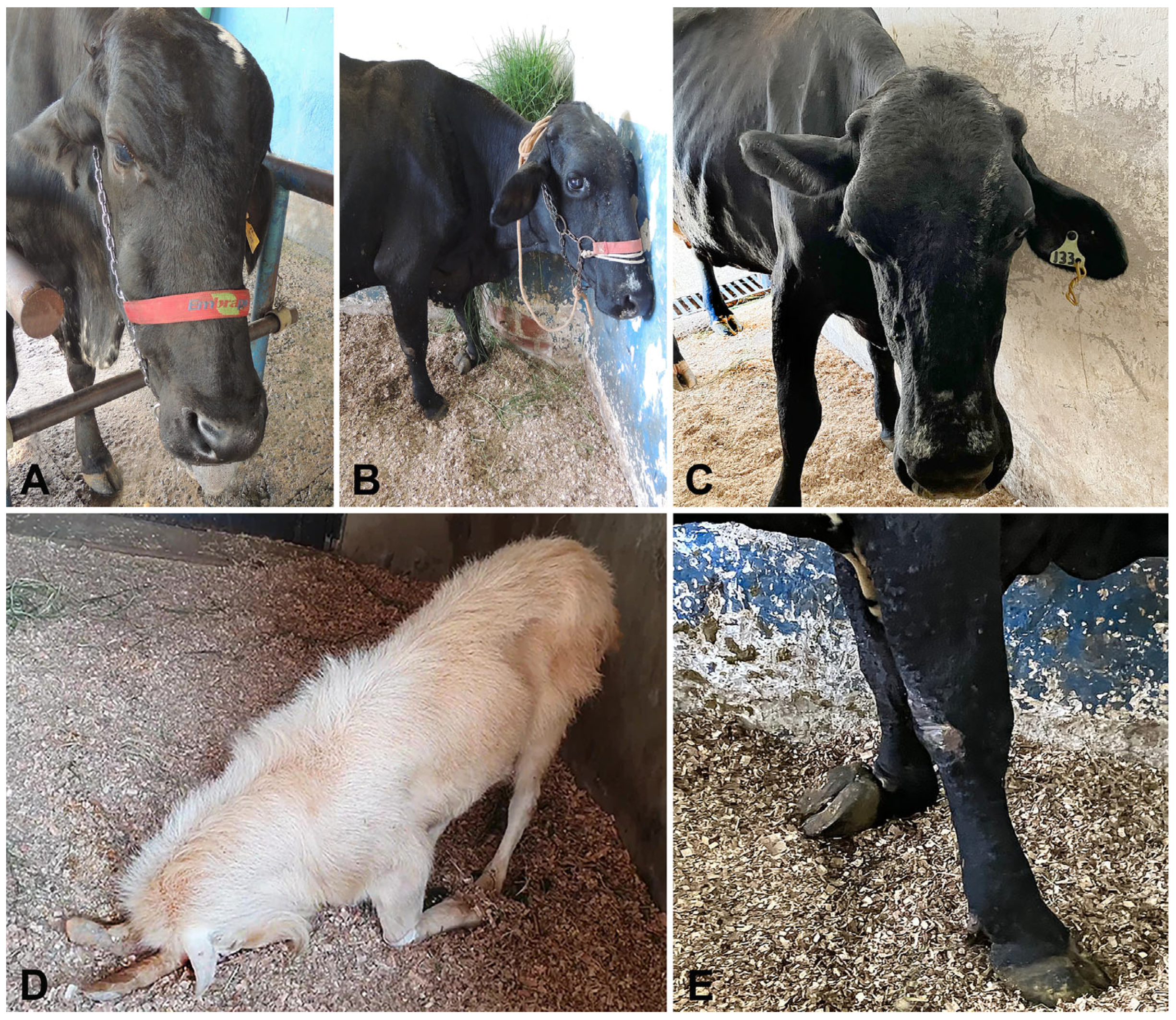
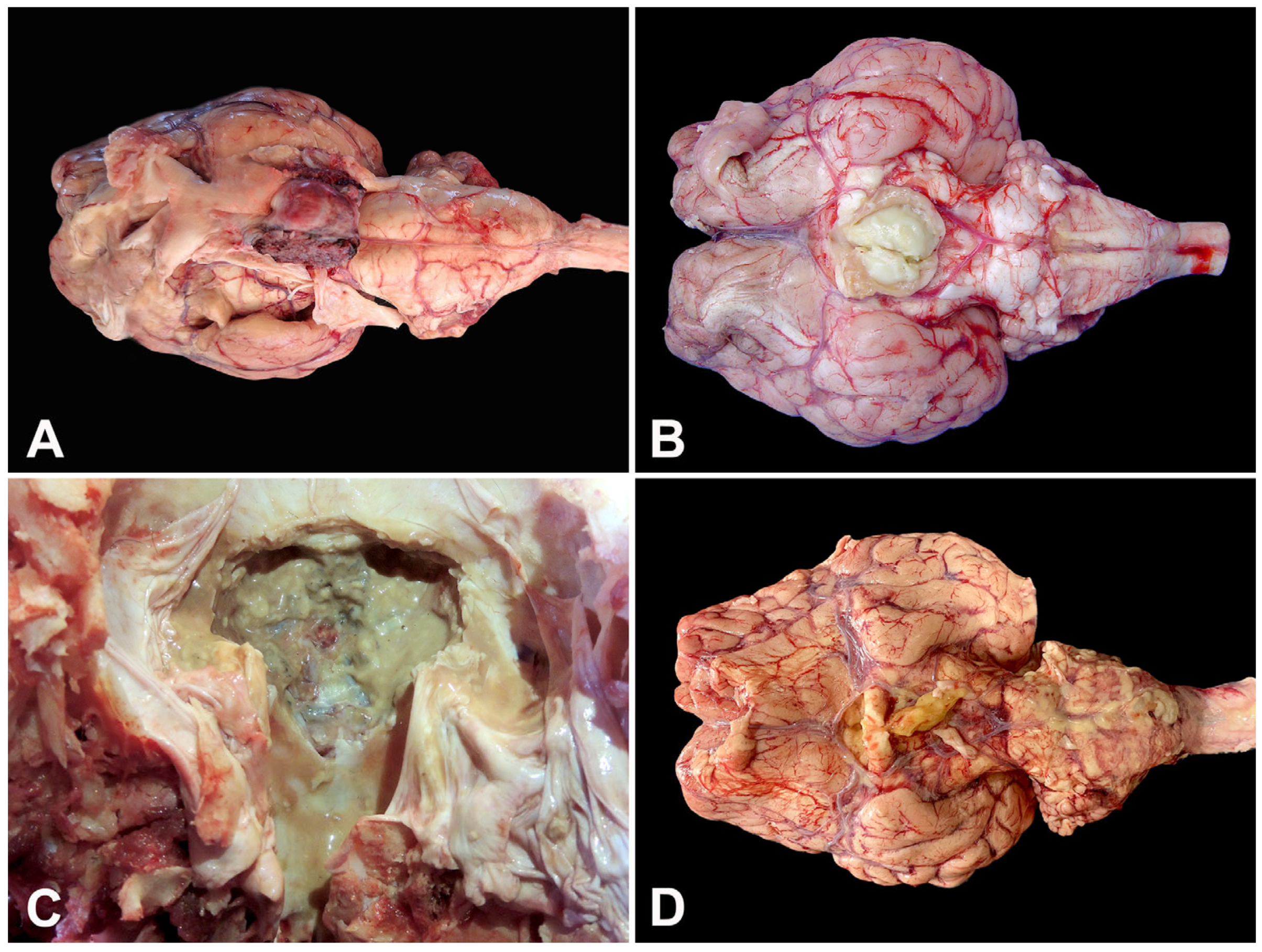
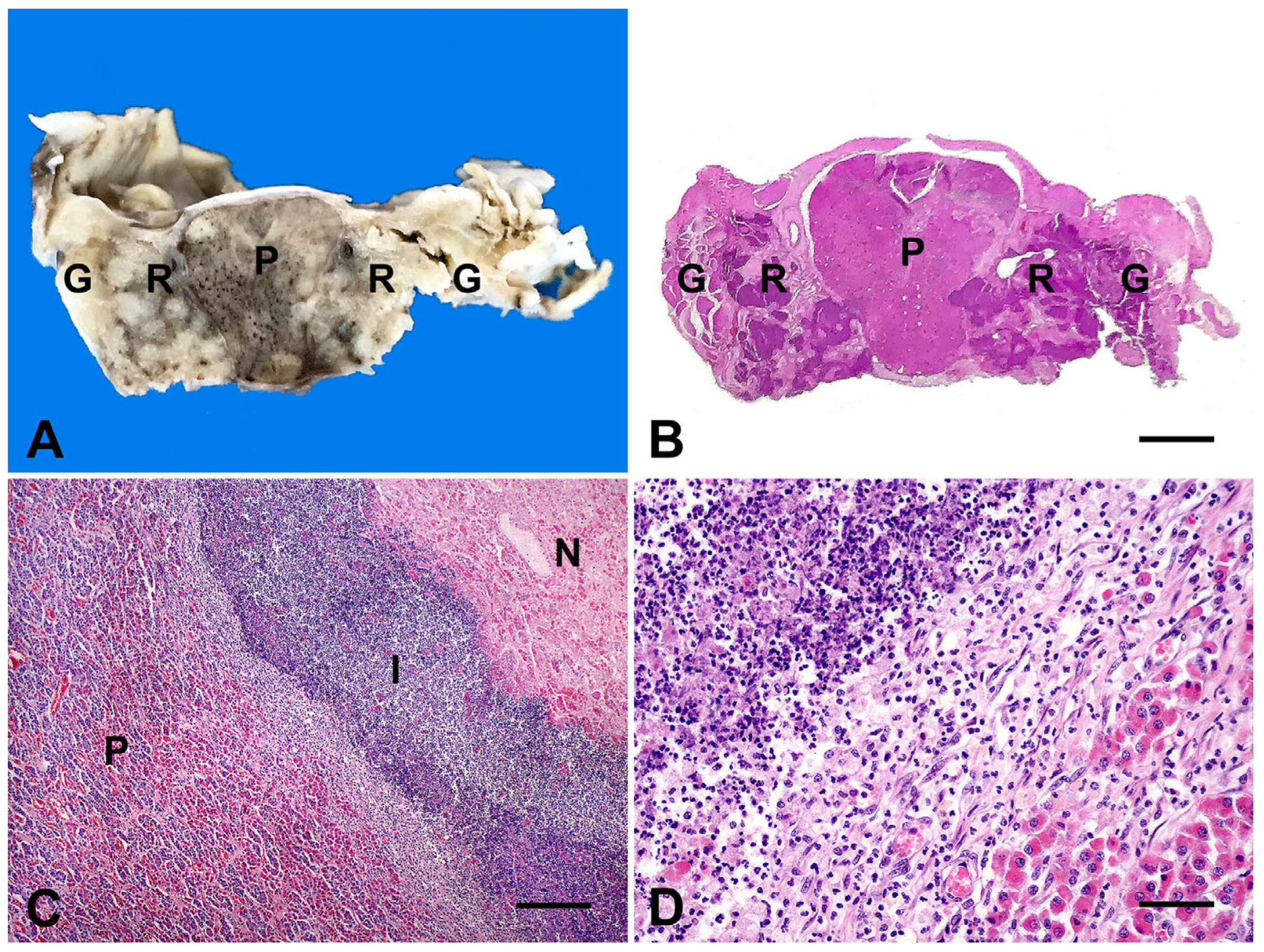
| Case | Species | Breed | Age | Month/Year | Clinical Evolution a | Risk Factors | Clinical Signs | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Cattle | Tabapuã | 10 months | 1/2005 | 20 days | Traumatic rhinitis (weaner nose ring) | Depression, fever, sialorrhea, purulent nasal discharge, head pressing | Penicillin (40,000 UI/kg) |
| 2 | Cattle | Holstein | 11 months | 8/2005 | 10 days | Bronchopneumonia | Depression, dehydration, hypotonic tongue, nystagmus, miosis, pedaling movements, opisthotonus, sialorrhea, pulmonary crackles | None |
| 3 | Cattle | Nelore | 4 years | 9/2011 | 3 days | Gingival fistula | Blindness, recumbency | Fluids and thiamine (10 mg/kg) |
| 4 | Cattle | Girolando | 3 years | 10/2016 | 4 days | NAD | Blindness, circling, sialorrhea, recumbency | NAD |
| 5 | Sheep | Crossbred | 4 years | 4/2019 | 24 h | None | Dehydration, nystagmus, opisthotonus, recumbency, pedaling movements | None |
| 6 | Cattle | Girolando | 3 years | 5/2019 | 20 days | Mandibular abscess | Face hypoalgesia, dropped jaw, tongue protrusion, sialorrhea | Sulphadimethoxine plus trimethopim (15 mg/kg), flunixin meglumine (2.2 mg/kg) |
| 7 | Sheep | Crossbred | 3 years | 6/2021 | 3 days | None | Depression, purulent nasal discharge, opisthotonus, recumbency | Fluids and meloxican (0.6 mg/kg) |
| 8 | Cattle | Girolando | 7 years | 5/2023 | 2 days | Gingival ulcer/mastitis | Depression, hypermetria, hypotonic tongue, proprioceptive deficits, head pressing, left auricular and palpebral ptosis | Florfenicol (20 mg/kg), dexamethasone (0.1 mg/kg) |
| 9 | Goat | Saanen | 8 years | 9/2024 | 3 days | Wounds in both horns | Hypermetria, ataxia, head tilt, head pressing, reduced left menace response | Penicillin (40,000 UI/kg), dexamethasone (0.5 mg/kg), thiamine (10 mg/kg) |
| Parameter/Case | 1 | 2 | 5 | 6 | 7 | 8 | 9 | RV (Cattle) * | RV (Sheep) * | RV (Goats) * |
|---|---|---|---|---|---|---|---|---|---|---|
| Hematocrit (%) | 33 | 33 | 39 | 29 | 24 | 39 | 33 | 24–46 | 24–50 | 19–38 |
| RBC (×106/µL) | 8.1 | 8.2 | 10.4 | 6.1 | 7.1 | 8.7 | 17.4 | 5–10 | 8–16 | 8–18 |
| Hemoglobin (g/dL) | 10.4 | 10.3 | 12.9 | 9.4 | 7.9 | 13.2 | 11.3 | 8–15 | 8–16 | 8–14 |
| Leucocytes (/µL) | 7300 | 23,700 | 9800 | 10,400 | 5250 | 4700 | 16,850 | 4000–12,000 | 4000–12,000 | 4000–13,000 |
| Segmented neutrophils (/µL) | 3212 | 15,705 | 4018 | 3120 | 4253 | 1128 | 15,165 | 600–4000 | 700–6000 | 1200–7200 |
| Bands (/µL) | 4065 | - | - | 104 | - | - | - | 0–100 | 0–100 | 0–100 |
| Lymphocytes (/µL) | 1825 | 6873 | 4704 | 3640 | 893 | 3337 | 843 | 2500–7500 | 2000–9000 | 2000–9000 |
| Monocytes (/µL) | 438 | 1185 | 98 | 624 | 105 | 141 | 843 | 25–840 | 0–750 | 0–650 |
| Eosinophils (/µL) | - | 237 | 980 | 2912 | - | 94 | - | 0–2400 | 0–1000 | 50–650 |
| Fibrinogen (mg/dL) | 1000 | 900 | 700 | 600 | 600 | 1000 | 600 | 200–600 | 100–500 | 100–400 |
| STP (g/dL) | 7.8 | 9.4 | 7 | 8 | 6.9 | 8.6 | 8.2 | 6.7–7.4 | 6–7.9 | 6.4–7 |
| Albumin (g/dL) | ND | ND | 2.1 | 1.7 | 0.9 | ND | 2.1 | 3–3.5 | 2.4–3 | 2.7–3.9 |
| Globulin (g/dL) | ND | ND | 4.9 | 6.3 | 6 | ND | 6.1 | 3–3.4 | 3.5–5.7 | 2.7–4.1 |
| Urea (mg/dL) | ND | ND | 52 | 44 | 57 | 31 | 30 | 42.8–64.2 | 17.1–42.8 | 21.4–42.8 |
| Creatinine (mg/dL) | ND | ND | 1.4 | 2.4 | 1.3 | 1.3 | 1.3 | 1–2 | 1.2–1.9 | 1.2–1.8 |
| AST (UI/L) | ND | ND | 94 | ND | 162 | 609 | 424 | 20–34 | 68–90 | 43–132 |
| GGT (UI/L) | ND | ND | 91 | ND | 84 | 23 | 45 | 6.1–17.4 | 20–52 | 20–56 |
| Case | Day | Aspect | Color | Density | pH | Proteins (mg/dL) | Pandy | Red Blood Cells (/µL) | Leucocytes (/µL) | Pleocytosis | Bacterial Culture |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Cloudy | Colorless | 1.014 | ND | 500 | ND | 72 | 50 | Neutrophilic | Trueperella pyogenes |
| 2 | 1 | Clear | Colorless | 1.008 | ND | 412 | ND | 11 | 17 | Monocytic | ND |
| 8 | 2 | Clear | Colorless | 1.002 | 8 | 34.6 | ND | 22 | 13 | Monocytic | No growth |
| 9 | Cloudy | Light yellow | 1.010 | 7 | 187.8 | Positive | 71 | 1871 | Neutrophilic | Corynebacterium sp. | |
| 9 | 2 | Clear | Colorless | 1.008 | 7 | 36.8 | Positive | 9 | 4 | Absent | ND |
| 5 | Clear | Colorless | 1.008 | 7 | 54.6 | Positive | 8 | 4 | Absent | ND | |
| RV * | - | Clear | Colorless | <1.010 | <67 | Negative | Rare | <10 | Absent | No growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroneze, G.; Cerqueira, L.d.A.; Borges, J.R.J.; Castro, M.B.d.; Câmara, A.C.L. Pituitary Abscess Syndrome in Ruminants: Nine Cases. Animals 2025, 15, 2692. https://doi.org/10.3390/ani15182692
Maroneze G, Cerqueira LdA, Borges JRJ, Castro MBd, Câmara ACL. Pituitary Abscess Syndrome in Ruminants: Nine Cases. Animals. 2025; 15(18):2692. https://doi.org/10.3390/ani15182692
Chicago/Turabian StyleMaroneze, Gabriele, Liz de Albuquerque Cerqueira, José Renato Junqueira Borges, Márcio Botelho de Castro, and Antonio Carlos Lopes Câmara. 2025. "Pituitary Abscess Syndrome in Ruminants: Nine Cases" Animals 15, no. 18: 2692. https://doi.org/10.3390/ani15182692
APA StyleMaroneze, G., Cerqueira, L. d. A., Borges, J. R. J., Castro, M. B. d., & Câmara, A. C. L. (2025). Pituitary Abscess Syndrome in Ruminants: Nine Cases. Animals, 15(18), 2692. https://doi.org/10.3390/ani15182692





