1. Introduction
Follicular development is a central process of female bird reproductive physiology, and its dynamic balance directly affects egg production performance. In avian ovaries, only a small number of primordial follicles develop into mature follicles and eventually enter the ovulation stage, while the majority of follicles degenerate due to atresia mechanism [
1]. This process is closely related to the proliferation, differentiation and apoptosis of granulosa cells (GCs). As the main executors of follicular function, GCs coordinate follicular development and follicular maturation through the synthesis of hormones, growth factors and regulation of signaling pathways [
2]. Studies have shown that abnormal apoptosis of GCs is considered to be one of the main causes of follicular atresia, which indirectly affects the avian egg-laying cycle [
3]. Therefore, it is worthy to analyze the underlying regulatory mechanisms of this process to enhance poultry reproductive performance.
In recent years, microRNAs (miRNAs) have been found to be widely involved in the regulation of ovarian development and follicular function [
4]. miRNAs modulate various cellular processes-including cell cycle progression, apoptosis, and differentiation, and bind to the 3′ untranslated region (3′UTR) of target mRNAs to suppress their expression [
5]. In mammals, numerous miRNAs play crucial roles in GC function during follicular development [
6]. The
miR-194 family comprises two mature sequences,
miR-194-5p and
miR-194-3p [
7], they highly express in tissues such as liver, intestine, and genitourinary system [
8,
9,
10]. These miRNAs are associated with process such as tumorigenesis [
11], fibrosis [
12], inflammatory [
13], and more.
miR-194 expression upregulated in GCs from polycystic ovary syndrome (PCOS) patients. Overexpression of
miR-194 suppressed the proliferation of Human ovarian granulosa-like tumor cell line KGN cells by targeting Heparin-binding epidermal growth factor-like growth factor (
HB-EGF) and promoted apoptosis [
14]. In prostate cancer cells,
miR-194 inhibited cell survival and tumor growth by targeting N-calmodulin. Gao et al. further demonstrated that
miR-194 significantly reduced the proliferative capacity of these cells and induced apoptosis [
15]. Collectively, these studies suggest that
miR-194 plays an inhibitory role in cell proliferation. However, there is still limited research on the role of
miR-194 in avian ovarian GCs, and a systematic study of its action and molecular mechanisms is needed.
Chromodomain helicase DNA-binding protein 4 (
CHD4) is the core ATPase subunit of the Nucleosome Remodeling and Deacetylase (NuRD) chromatin remodeling complex. This complex regulates chromatin architecture and gene transcription by promoting ATP-dependent nucleosome sliding and histone deacetylation, thereby maintaining genomic integrity [
16]. In mammalian ovaries,
CHD4 interacts with
MTA3 to form the NuRD complex, which regulates GC progression through the G2/M phase of the cell cycle, ultimately influencing
Cyclin B expression and promoting cell proliferation [
17]. To date, the role of
CHD4 or the NuRD complex in avian follicular development and GC proliferation or apoptosis has not been directly studied. However, transcriptomic analyses revealed differential gene expression patterns in chicken GCs at various developmental stages, suggesting that chromatin remodeling factors, including
CHD4, may contribute to the regulation of follicular development [
18]. The critical role of
CHD4 in chromatin structure remodeling and cell fate determination, requires further investigation into its mechanistic function in GCs.
In this study, we used GCs from Zhedong white geese to investigate whether miR-194-3 influences GC proliferation and apoptosis, and to explore potential molecular mediators underlying these processes. Guided by bioinformatic target prediction, we focused our analyses on CHD4 as a candidate target. To address these questions, we combined miRNA mimic/inhibitor experiments, siRNA-mediated gene knockdown, qRT-PCR, Western blotting, EdU incorporation, flow cytometry, and dual-luciferase reporter assays. These approaches were intended to clarify miR-194-3–dependent regulatory mechanisms that may contribute to follicular development in geese.
2. Materials and Methods
2.1. Laboratory Animals
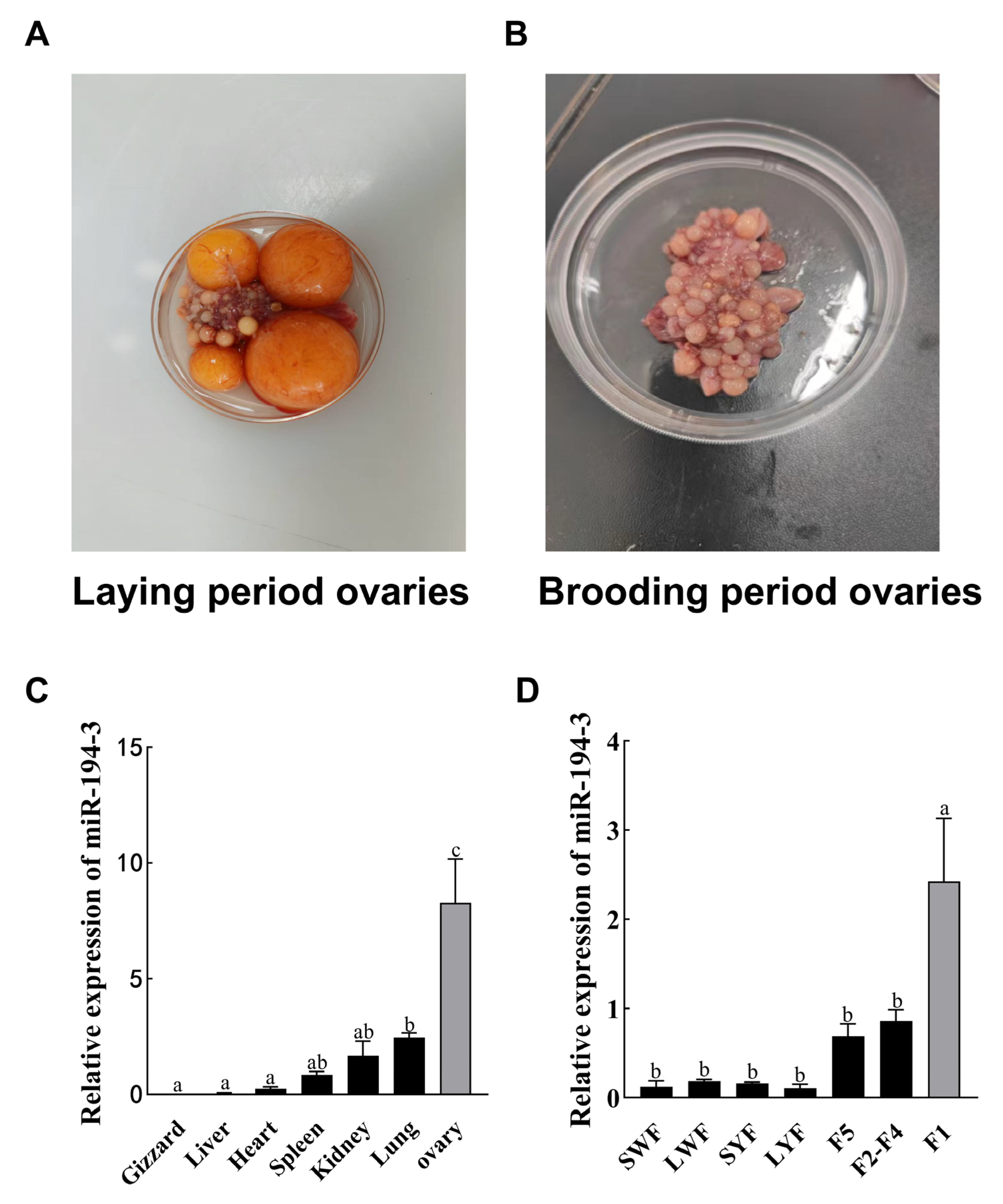
The 18-month-old laying female geese used in the experiment were purchased from Zhejiang Xiangshan Wenjie White Goose Co., Ltd. (Xiangshan, Zhejiang, China) and randomly assigned to the wire mesh panel pen (0.5 m2/feather) for centralized rearing. Geese were provided with ad libitum access to feed and water using disk-type feeders (≥16 cm/goose) and dropper-type drinking nipples. Lighting and ventilation conditions were consistent with the local natural environment, without additional heating or artificial light supplementation. Ten 18-month-old Zhedong white geese were sampled during the egg-laying period, and another 10 were sampled during the brooding (nest-holding) period. Geese were first anesthetized with isoflurane inhalation, followed by euthanasia via intravenous injection of sodium pentobarbital after loss of consciousness. Gizzard, hepatic, cardiac, splenic, renal, pulmonary, and ovarian tissues were collected from the laying period, and ovarian tissues were collected from the brooding period (n = 10). For ovarian tissue sampling, ovaries were quickly excised, visible large hierarchical follicles (diameter >10 mm) were carefully removed before sampling to ensure comparability between geese and physiological stages. Approximately 100 mg of cortical tissue per goose was collected. 10 individual biological replicates were included for each group (laying and brooding). All samples were immediately snap-frozen in liquid nitrogen for further analysis.
2.2. GCs Isolation and Culture
Follicles were harvested from geese during the laying stage and were first classified into preovulatory follicles (F1–F5) and pre-hierarchical follicles (0–10 mm), which were classified into small white follicles (SWF), large white follicles (LWF), small yellow follicles (SYF), and large yellow follicles (LYF) based on size and color. All samples were preserved in liquid nitrogen. For all in vitro experiments, GCs were individually isolated from F1 follicles of different geese using the method described by Gilbert [
19]. Cells from each goose were cultured and subjected to the same experimental procedures independently. The GCs were seeded into appropriate culture plates depending on the experimental requirements. Specifically, 6-well plates were used for transfection, RNA and protein extraction, and flow cytometry assays; 24-well plates were used for EdU cell proliferation assays; and 96-well plates were used for CCK-8 cell viability assays. Thus, unless otherwise stated, all experiments involving primary GCs were conducted using cells derived from F1-stage preovulatory follicles. All cells were maintained in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) and 1% penicillin–streptomycin (Gibco, Carlsbad, CA, USA), and incubated in a humidified atmosphere of 5% CO
2 at 37 °C. Cell passaging and subsequent experiments were carried out when stable growth was achieved. All experimental procedures were approved by the Laboratory Animal Welfare Ethics Committee of Northeast Agricultural University (Approval No. SRM-06), and adhered to the Guidelines for Ethical Review of Laboratory Animal Welfare [
20].
2.3. Cell Transfection
GCs were transfected when confluency reached approximately 80%. The
miR-194-3 mimic, inhibitor, and corresponding negative controls were designed based on the mature sequence of acyg-
miR-194-3 obtained from previous transcriptome analysis results [
21].
CHD4 targeting siRNA (si-
CHD4) and siRNA negative control (si-NC) were designed based on the coding sequence of goose
CHD4 and specifically silences goose
CHD4 mRNA. All oligonucleotide sequences were synthesized by Beijing Sevin Innovative Biotechnology Co., Ltd. (Beijing, China) and Sangon Biotech Co., Ltd. (Shanghai, China), respectively. The
CHD4 gene sequence used in this study was obtained from the NCBI RefSeq database (accession number XM_067004496.1), based on the
Anser cygnoides genome assembly GCF_000971095.1 (AnsCyg_PRJNA183603_v1.0). To investigate the targeting relationship between
miR-194-3 and
CHD4, wild-type (WT) and mutant (MUT) sequences (200 bp upstream and downstream of the predicted
miR-194-3 binding site within the
CHD4 3′UTR) were synthesized by Heilongjiang Genesoul Technology Co., Ltd. (Harbin, Heilongjiang, China). The sequences were listed in
Table 1. Transfection of mimic, inhibitor, and siRNA was performed using Lipofectamine
® 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. We screened the concentrations of
miR-194-3 mimic and
miR-194-3 inhibitor, the three concentrations designed for mimic were 25 nM, 50 nM and 75 nM, and the three concentrations designed for inhibitor were 100 nM, 200 nM and 300 nM, respectively. We tested and screened the most suitable concentrations for transfection experiments. The optimal expression levels of
miR-194-3 mimic and
miR-194-3 inhibitor were 50 nM and 300 nM, respectively. All experiments were repeated three times. si-
CHD4 was performed at the recommended concentration of 50 nM.
2.4. RNA Extraction, Reverse Transcription and Fluorescence Quantitative PCR
Total RNA was extracted from Zhedong White goose tissues, and we transfected follicular GCs using a commercial RNA extraction reagent (Takara, Shiga, Japan). cDNA was synthesized using the stem-loop method with the miRNA cDNA synthesis kit (Gene-better, Beijing, China). cDNA was generated using the All-in-One First Strand cDNA Synthesis Kit II with dsDNase (Seven, Beijing, China) to analysis mRNA. Quantitative real-time PCR (qRT-PCR) was performed using the FastStart Universal SYBR Green Master (ROX) kit (Roche, Basel, Switzerland) in a 10 μL reaction volume, containing 5 μL of 2× SYBR Green Master Mix, 0.3 μL each of forward and reverse primers (final concentration: 300 nM), 1 μL of diluted cDNA template (approximately 20 ng), and 3.4 μL of RNase-free water. Each reaction was run in triplicate. U6 small nuclear RNA was used as the internal control for miRNA quantification, while GAPDH served as the internal control for mRNA expression analysis. qRT-PCR reactions were run on a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA), and data were analyzed using QuantStudio™ Design and Analysis Software v1.5.1 (Thermo Fisher Scientific, Waltham, MA, USA). All reactions were performed in triplicates. Relative gene expression were calculated using the 2
–ΔΔCT method. The specific primer sequences used in this study are listed in
Table 2.
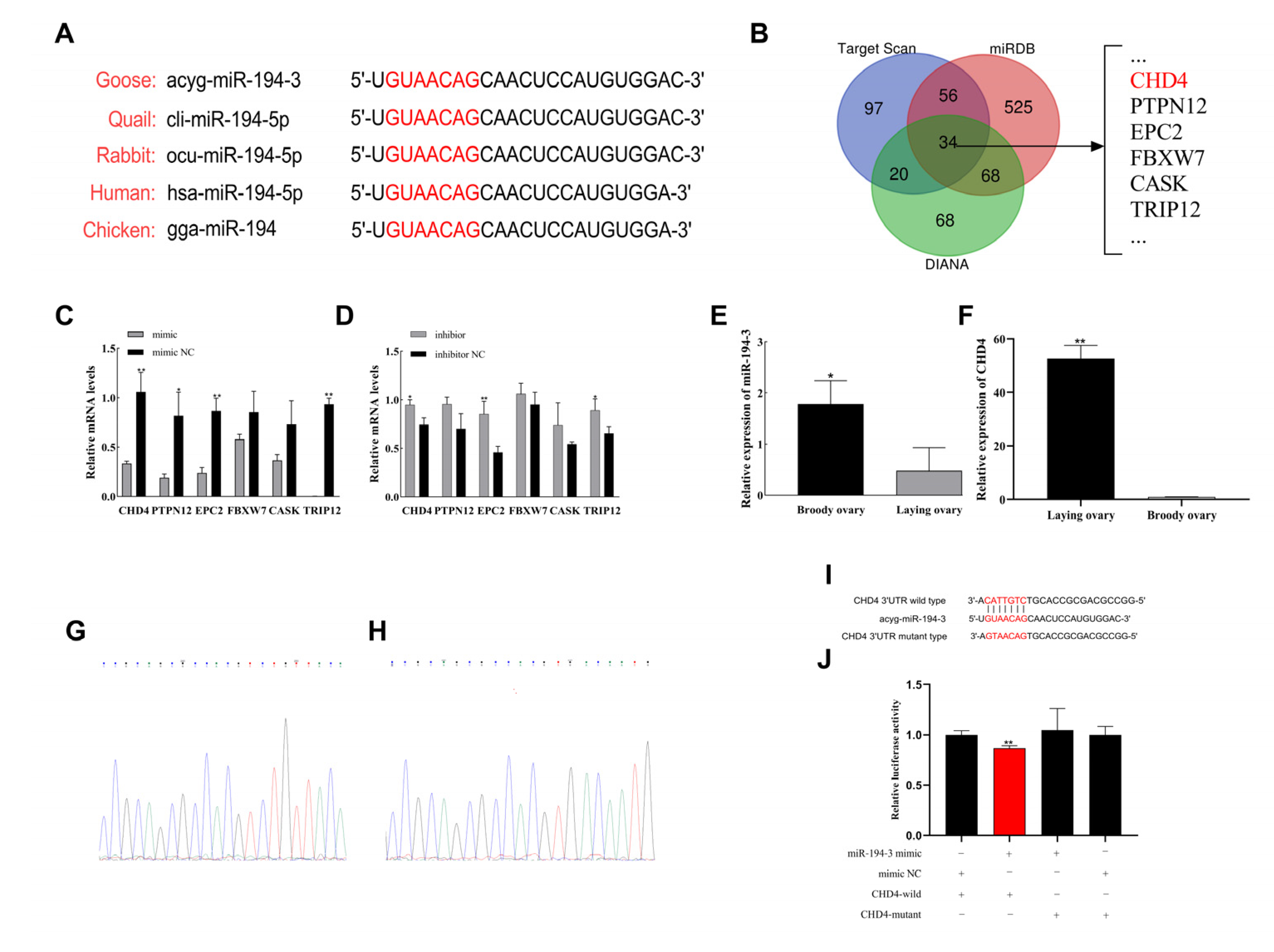
2.5. Target Gene Prediction
The target genes of
miR-194-3 were predicted using three bioinformatic databases: TargetScanHuman v8.0 (
https://www.targetscan.org/, accessed on 24 October 2024), miRDB (
http://mirdb.org/, accessed on 24 October 2024), and DIANA Tools (
http://www.microrna.gr/, accessed on 24 October 2024). Only genes predicted by at least two databases were considered for further analysis.
2.6. Flow Cytometry
2.6.1. Cell Cycle Analysis by Flow Cytometry
Cells were transfected with miR-194-3 mimic, miR-194-3 inhibitor, or si-CHD4 in six-well plates and incubated for 48 h. Cells used for each biological replicate were isolated from a different individual Zhedong white goose. Then, the cells were harvested and fixed in 70% pre-chilled ethanol at 4 °C for 24 h. Finally, the cells were centrifuged to remove ethanol, washed with PBS, and stained with propidium iodide (PI) solution containing 50 μg/mL PI and 100 μg/mL RNase A (Beyotime Biotechnology, Shanghai, China) for 30 min at 37 °C in the dark. Each group included three biological replicates, and each replicate was analyzed in triplicate as technical replicates. Cell cycle distribution was analyzed by flow cytometry using a BD FACSCalibur™ cytometer (BD Biosciences, San Jose, CA, USA). Data acquisition and analysis were performed with FlowJo v10.0.7 (Tree Star Inc., Ashland, OR, USA).
2.6.2. Flow Cytometry Analysis of Apoptosis
At 48 h post-transfection, cells were harvested and washed twice with cold PBS, then resuspended in 1× Binding Buffer (Meilun Biotechnology, Dalian, Liaoning, China) at a final concentration of 1 × 106 cells/mL. A 100 µL aliquot of the cell suspension was transferred into a 5 mL flow cytometry tube. Subsequently, 5 µL of Annexin V-FITC and 5 µL of propidium iodide (PI) were added. The samples were gently vortexed and incubated for 15 min at room temperature in the dark. Then, 400 µL of Binding Buffer was added to each tube. Apoptotic were analyzed using a BD FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA), and data were processed using FlowJo software v10.0.7 (Tree Star Inc., Ashland, OR, USA). Each group included three biological replicate. The apoptotic rate was calculated as the percentage of Annexin V+/PI− (early apoptosis) and Annexin V+/PI+ (late apoptosis) cells among the total cell population, based on quadrant analysis in flow cytometry.
2.7. Western Blot
Total protein was extracted from follicular GCs of Zhedong White geese 72 h post-transfection using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitors. Protein samples were collected at 72 h post-transfection, as protein-level changes were more stable and prominent at this time point based on preliminary experiments, compared to earlier time points. Protein concentrations were determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China), and equal amounts of protein samples (20 μg per lane) were mixed with 5× SDS loading buffer (Beyotime Biotechnology, Shanghai, China), boiled at 100 °C for 5 min, and stored at −20 °C until use. Proteins were separated by 12% SDS-PAGE and transferred onto PVDF membranes (Beyotime Biotechnology, Shanghai, China). Membranes were blocked with 5% non-fat milk in TBST for 2 h at room temperature, then incubated overnight at 4 °C with primary antibodies against PCNA (Catalog Number: AF6237, Affinity Biosciences, diluted 1:1000, Wuhan, China), CDK2 (Catalog Number: AF6237, Affinity Biosciences, diluted 1:1000, Wuhan, China), CCND1 (Catalog Number: AF0931, Affinity Biosciences, diluted 1:1000, Wuhan, China), Bcl-2 (Catalog Number: AF6139, Affinity Biosciences, diluted 1:1000, Wuhan, China), Caspase-3 (Catalog Number: WL04004, Wanleibio, diluted 1:500, Shenyang, China), Caspase-9 (Catalog Number: WL01551, Wanleibio, diluted 1:1000, Shenyang, China), and GAPDH (Catalog Number: AF7021, Affinity Biosciences, diluted 1:3000, Wuhan, China). After washing three times in TBST (8 min each), membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:5000; Proteintech Group, lnc.) for 2 h at room temperature. Signal detection was performed using enhanced chemiluminescence (ECL) reagents (Seven Biotrchnology, Beijing, China), and images were captured using a gel imaging system (Bio-Rad, Hercules, CA USA). Protein were quantified using ImageJ software (version 1.53; National Institutes of Health, Bethesda, MD, USA), with GAPDH as the loading control. Three biological replicates were included for each group.
2.8. Dual Luciferase Reporter Assay
Human embryonic kidney 293T (HEK293T) cells were used for the dual-luciferase reporter assay. Cells were co-transfected with the miR-194-3 mimic and WT CHD4 reporter plasmid, mimic NC and WT CHD4 plasmid, miR-194-3 mimic and MUT CHD4 plasmid, or mimic NC and MUT CHD4 plasmid in 6-well plates. After 48 h, cells were harvested, and luciferase activity was measured using a Dual-Luciferase® Reporter Assay System (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Each group was analyzed in three independent biological replicates, and each biological replicate was measured in triplicate as technical replicates.
2.9. CCK-8 Assay
GCs were seeded into 96-well plates and cultured for 24 h before transfection. Cell viability was assessed at 12, 24, 48 and 72 h post-transfection using the Cell Counting Kit-8 (CCK-8; Seven Biotechnology, Beijing, China). At each time point, 10 μL of CCK-8 reagent was added to each well 2 h prior to measurement. Absorbance was recorded at 450 nm using a microplate reader (BioTek Instruments, Winooski, VT, USA). Each group included three independent biological replicates, and each biological replicate was measured in triplicate as technical replicates.
2.10. EdU Assay
Zhedong White Goose follicular GCs were seeded into 24-well plates and cultured for 24 h before transfection. Then, cells were incubated with 500 μL of diluted EdU working solution per well for 2 h, following the instructions of the BeyoClick™ EdU-555 Cell Proliferation Detection Kit (Beyotime Biotechnology, Shanghai, China). The cells were then fixed, and the Click reaction solution—(comprising Click Reaction Buffer, CuSO4, Azide 555), and Click Additive Solution—was added and incubated for 30 min at room temperature in the dark. Subsequently, cell nuclei were stained with Hoechst 33342 (5 μg/mL) for 10 min at room temperature in the dark. After staining, cells were washed twice with PBS to remove excess dye prior to imaging. Fluorescence images were captured using a fluorescence microscope (Olympus IX73, Olympus, Tokyo, Japan). The proliferation rate was calculated by determining the ratio of EdU-positive cells to the total number of nuclei using ImageJ software (version 1.53; National Institutes of Health, Bethesda, MD, USA).
2.11. Data Analysis
All data were presented as mean ± SEM. Statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). For comparisons involving more than two groups, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used. For two-group comparisons (e.g., mimic vs. mimic NC or siRNA vs. si-NC), the unpaired two-tailed Student’s t-test was applied. All experiments were replicated a minimum of three times unless otherwise stated. Differences were considered statistically significant at p < 0.05 (* p < 0.05; ** p < 0.01).
4. Discussion
The precise balance between proliferation and apoptosis of ovarian GCs is essential for normal follicular development and is closely associated with the egg-laying performance of female birds [
22]. Therefore, it is important to elucidate the mechanisms that regulate GC proliferation and apoptosis during follicle selection. Numerous studies demonstrated that microRNAs (miRNAs) were involved in follicular development [
23]. In the present study,
miR-194-3 inhibited cell proliferation and promoted apoptosis, which was consistent with findings in various other cellular models. For example,
miR-194 suppresses proliferation and promoted apoptosis in esophageal squamous carcinoma cells by targeting KDM5B [
24]. Similarly,
miR-194 overexpression arrested cell cycle progression and induced apoptosis in non-small cell lung cancer [
25]. Moreover,
miR-194 promoted apoptosis by targeting
HB-EGF, thereby inhibiting proliferation in GCs derived from patients with polycystic ovary syndrome (PCOS) [
14]. These findings demonstrates that
miR-194-3 plays a conserved role in negatively regulating cell proliferation across different cell types. In our study, the expression of
miR-194-3 increased progressively during follicular development and was significantly upregulated at the F1 stage, suggesting that
miR-194-3 may play a key regulatory role in follicle maturation. Previous studies showed that miRNA expression was stage-specific during follicular development [
26]. Our findings further reveal that
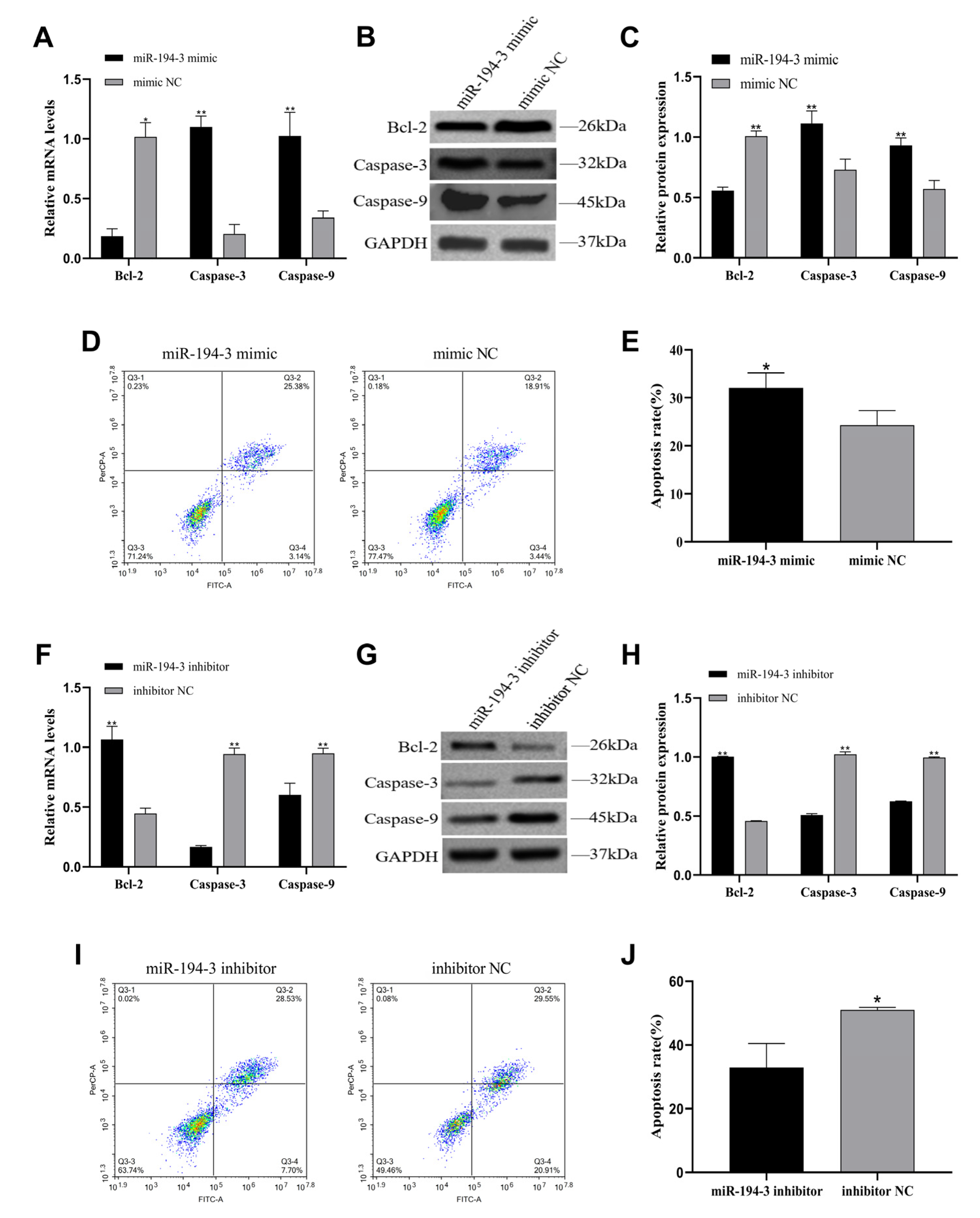
miR-194-3 exerts pro-apoptotic effects by upregulating
Caspase-3 and
Caspase-9, and downregulating
Bcl-2. In parallel, it suppresses proliferation by arresting the cell cycle, ultimately limiting follicular progression. The progressive increase in
miR-194-3 expression during follicular development suggests that it may be more relevant to later stages, rather than early follicle selection. It likely contributes to maintaining GC differentiation and tissue homeostasis post-dominance acquisition. However, the specific mechanism still needs further research.
A core ATPase subunit of the NuRD (nucleosome remodeling and deacetylase) chromatin remodeling complex is known to play a crucial role in DNA damage repair and cell cycle regulation [
27]. Multiple studies demonstrated that pro-proliferative function of
CHD4 in modulating cell proliferation and apoptosis. For instance, Lin et al. reported that
CHD4 promoted cell proliferation and suppressed apoptosis in lung adenocarcinoma [
28]. Similarly, D’Alesio et al. identified
CHD4 as a critical gene for breast cancer cell growth through RNA interference screening, with knockdown of
CHD4 markedly inhibiting tumor cell proliferation [
29]. In addition, Kwintkiewicz et al. found that MAT3 could assemble with
CHD4 to form the NuRD complex in mouse ovaries, jointly regulating granulosa cell progression through the G2/M phase. Deletion of
CHD4 significantly reduced the expression of Cyclin B1/B2 and impaired granulosa cell proliferation [
17], highlighting its important role in ovarian function. Our dual-luciferase reporter assay supports a direct interaction between
miR-194-3 and the
CHD4 3′UTR in a heterologous reporter system. These results are consistent with the hypothesis that
miR-194-3 may exert part of its biological effects via
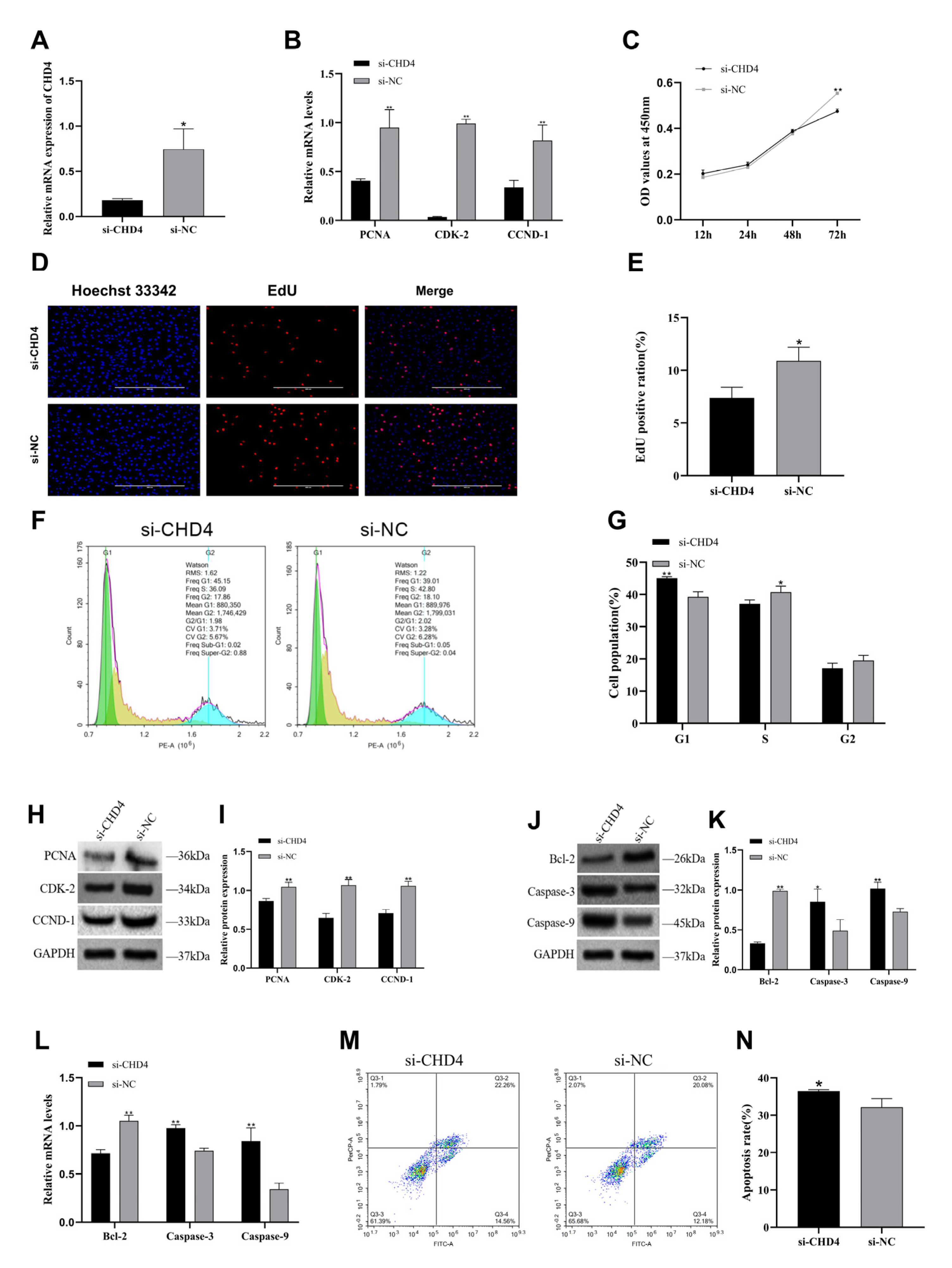
CHD4 suppression, but do not rule out contributions from additional targets or indirect downstream pathways. In the present study, knockdown of
CHD4 in goose GCs resulted in significant cell cycle arrest, along with the downregulation of proliferation-related genes and the upregulation of apoptosis-related genes. These findings reinforced the critical role of
CHD4 in promoting GC proliferation and were in strong agreement with previously reported results in mammalian GC models. Collectively, our findings suggest that
CHD4 promotes GCs proliferation and suppresses apoptosis in Zhedong white geese, at least in part by accelerating cell cycle progression. Furthermore, our in vivo expression data showed that
miR-194-3 was significantly upregulated in the ovaries during the brooding period, whereas
CHD4 expression was markedly decreased. This opposing expression pattern further supports a potential inverse regulatory relationship between the two molecules. The opposing expression patterns of
miR-194-3 and
CHD4 observed in vivo are consistent with a potential inverse regulatory relationship; together with the in vitro data, these observations suggest that the
miR-194-3–
CHD4 interaction may contribute to follicular development in physiological contexts, but further in vivo validation is required.
We hypothesized that miR-194-3 suppressed the expression of proliferation-associated proteins such as PCNA, CDK2, and CCND1, while it promoted the expression of pro-apoptotic factors including Caspase-3 and Caspase-9, by downregulating CHD4. However, the antibody used in this study targeted total Caspase-3 rather than the cleaved (active) form. Since cleaved Caspase-3 is a more specific marker of apoptosis activation, future studies will incorporate cleaved Caspase-3-specific antibodies to more precisely confirm the involvement of the apoptotic pathway. This downregulation may impair the NuRD complex-mediated chromatin remodeling and reduce transcriptional activity of proliferation-related genes, ultimately resulting in cell cycle arrest and the activation of apoptotic pathways. Furthermore, the increased expression of miR-194-3 observed in the ovaries of Zhedong White Geese during the clutching period may attenuate GC proliferation and promote follicular atresia via CHD4 inhibition. This, in turn, may contribute to the cessation of egg-laying. These findings provided novel molecular insights into the regulation of ovarian function associated with broodiness in Zhedong White Geese.
Egg-laying performance in geese is significantly influenced by the number of developing follicles and the ovulation rhythm, with GC proliferation and survival directly determining the availability of functional follicles [
30]. Our study demonstrated that inhibition of
miR-194-3 or upregulation of
CHD4 expression promoted GC proliferation and inhibited apoptosis, which may delay follicular atresia, thereby extending the laying period and enhancing egg production. In poultry production, it is conceivable that molecular regulation of
miR-194-3 expression could be used to optimize egg-laying performance and broodiness behavior in geese. Furthermore, given its high specificity in the ovary,
miR-194-3 may serve as a biomarker for predicting egg-laying potential. However, these applications require validation through extensive follow-up research. This study revealed regulatory pathway in the poultry reproductive system, enriching our understanding of non-coding RNA roles in follicle development. Future research should focus on identifying downstream effectors of this pathway and elucidate its connections with follicle selection, dominance, and other physiological processes, thereby laying a theoretical foundation for miRNA-based molecular breeding technologies.
Limitations and future directions: While our luciferase assay indicates a direct interaction between miR-194-3 and the CHD4 3′UTR, this assay was performed in HEK293T cells and reflects binding in a heterologous reporter context. Although CHD4 knockdown phenocopied several effects of miR-194-3 overexpression, these data do not prove that CHD4 is the sole mediator of miR-194-3′s actions in goose granulosa cells. Future experiments should include: (1) Rescue assays should be performed in primary granulosa cells by co-expressing a CHD4 open reading frame (ORF) lacking its 3′UTR, so that it can be determined whether restoration of CHD4 reverses the phenotypic effects induced by miR-194-3. (2) A minimum of two independent siRNAs targeting CHD4 should be employed, and knockdown efficiency should be documented at both the mRNA and protein levels. (3) Apoptotic activation should be confirmed through measurement of cleaved (active) apoptotic markers, for example, cleaved caspase-3, in order to verify execution of the apoptotic program. (4) Correlation analyses and functional assays conducted in vivo should be undertaken to reinforce the physiological relevance of the in vitro findings.