Dose-Dependent Effects of L-Serine Supplementation on Boar Sperm Quality During Chilled and Cryopreserved Storage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Semen Extender
2.3. Semen Collection and Preparation
2.4. Fresh Semen Evaluation
2.5. Experimental Design
2.6. Semen Cooling Storage
2.7. Semen Cryopreservation
2.8. Post-Thaw Semen Evaluation
2.8.1. Sperm Motility
2.8.2. Sperm Viability, Mitochondrial Function, and Acrosome Integrity
2.8.3. Lipid Peroxidation
2.8.4. Antioxidant Enzyme Activity
Total Antioxidant Capacity (T-AOC)
Glutathione Peroxidase (GPx)
Superoxide Dismutase (SOD)
Catalase (CAT)
Glutathione S Transferase (GST)
Glutathione Reductase Activity (GRD)
2.9. Statistical Analysis
3. Results
3.1. Fresh Semen Quality of Boars
3.2. Effect of L-Serine on Semen Quality During Chilled Storage
3.3. Lipid Peroxidation and Antioxidant Enzyme Activities During Chilled Storage
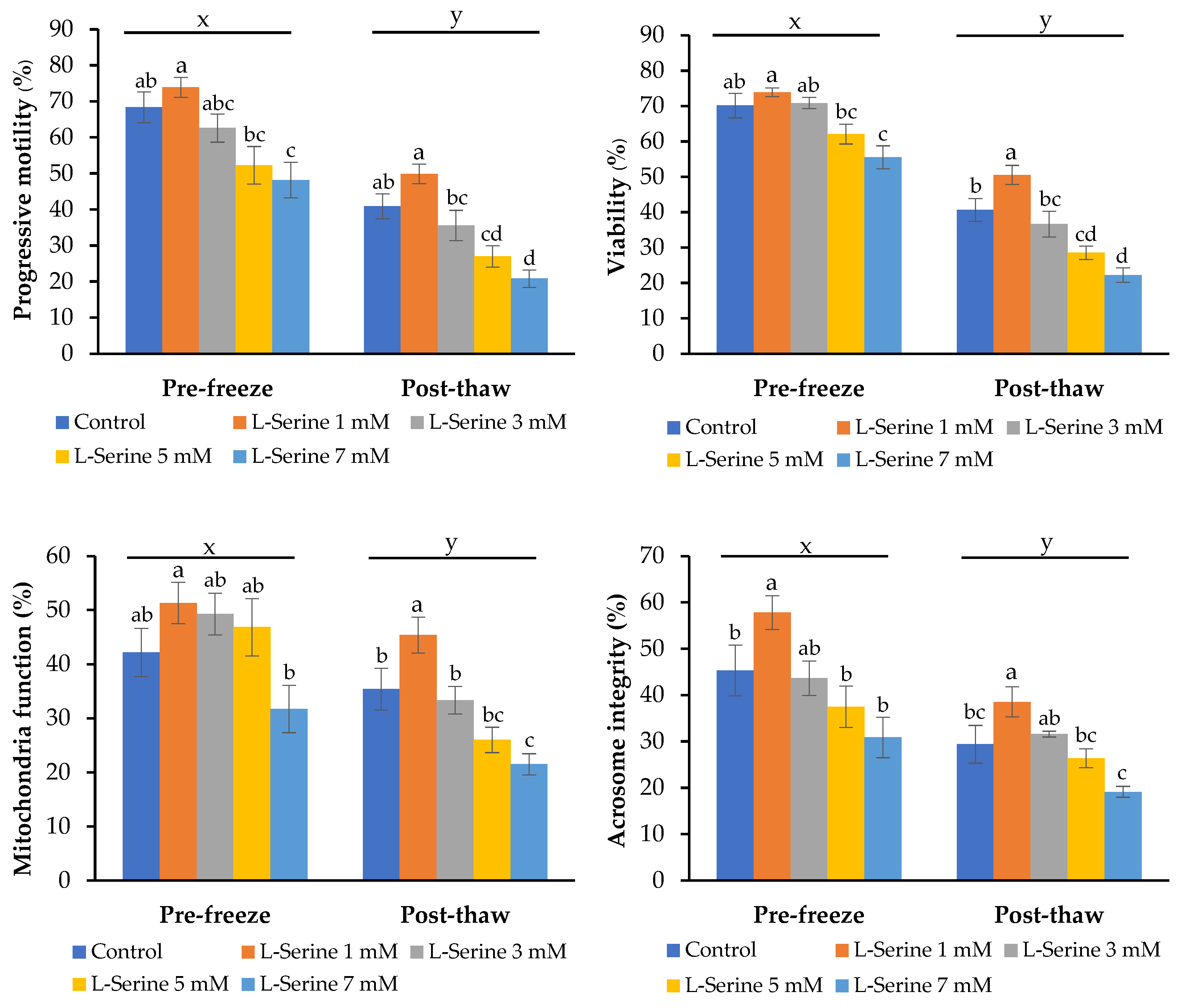
3.4. Effect of L-Serine on Sperm Quality After Cryopreservation
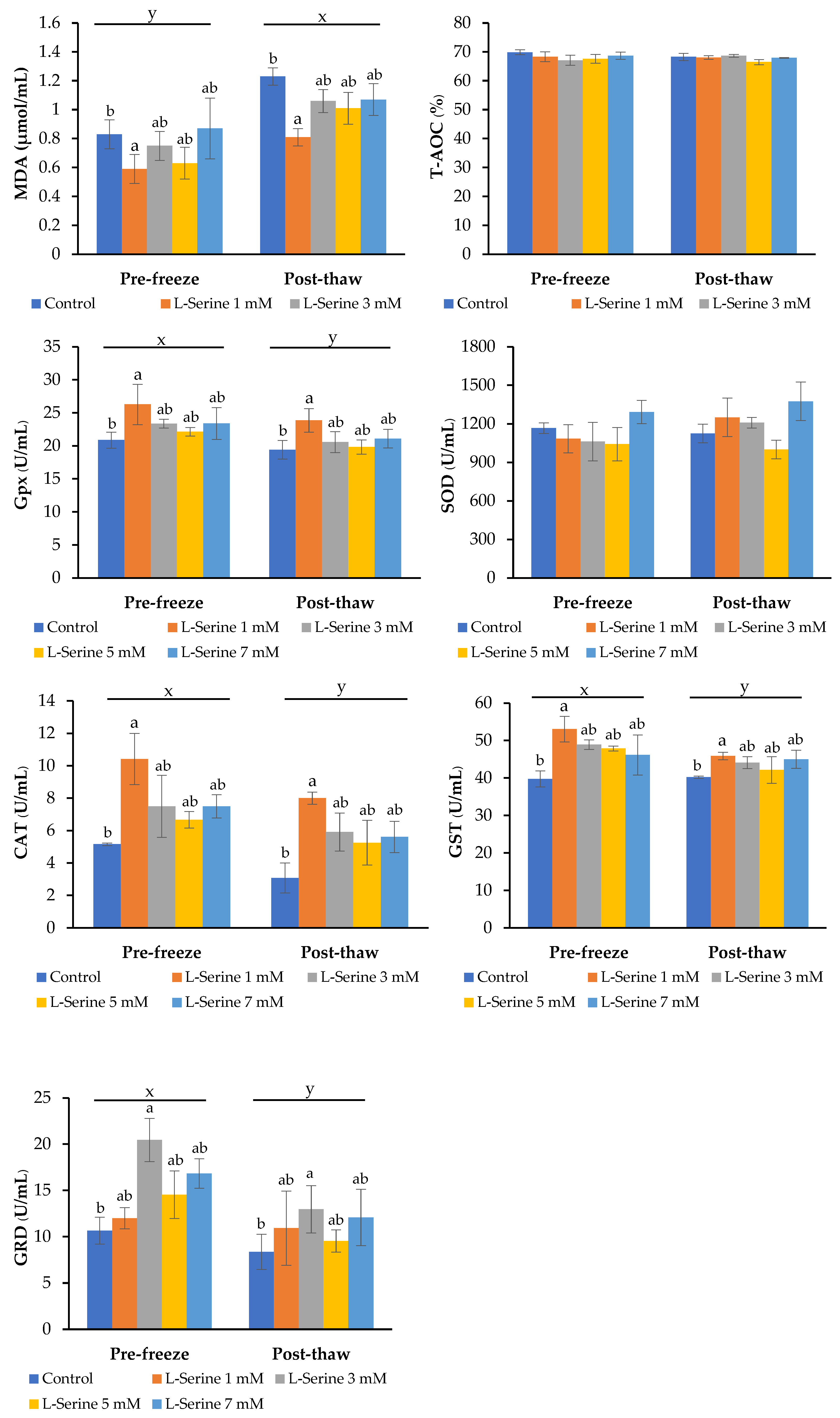
3.5. Lipid Peroxidation and Antioxidant Enzyme Activities After Cryopreservation
4. Discussion
4.1. Dose-Dependent Effects of L-Serine on Boar Semen Preservation
4.2. Antioxidant Mechanisms of L-Serine
4.3. Chilled Semen: Short-Term Preservation Benefits
4.4. Cryopreserved Semen: Antioxidant Enzyme Responses to Cryopreservation
4.5. The Antioxidant Paradox and Dose-Dependent Detrimental Effect Across Preservation Methods
4.6. Chilled and Cryopreserved Semen: Differential Dose Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanapiwat, P.; Kaeoket, K. Cryopreservation of boar semen: Where we are. Thai J. Vet. Med. 2020, 50, 283–295. [Google Scholar] [CrossRef]
- Bolarin, A.; Berndtson, J.; Tejerina, F.; Cobos, S.; Pomarino, C.; D’Alessio, F.; Blackburn, H.; Kaeoket, K. Boar semen cryopreservation: State of the art, and international trade vision. Anim. Reprod. Sci. 2024, 269, 107496. [Google Scholar] [CrossRef]
- Peña, F.J.; O’Flaherty, C.; Ortiz Rodríguez, J.M.; Martín Cano, F.E.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega Ferrusola, C. Redox Regulation and Oxidative Stress: The Particular Case of the Stallion Spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Buhr, M.M.; Curtis, E.F.; Kakuda, N.S. Composition and behavior of head membrane lipids of fresh and cryopreserved boar sperm. Cryobiology 1994, 31, 224–238. [Google Scholar] [CrossRef]
- Shabani Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Sim, W.C.; Yin, H.Q.; Choi, H.S.; Choi, Y.K.; Kim, S.K.; Lee, B.H. L-Serine Supplementation Attenuates Alcoholic Fatty Liver by Enhancing Homocysteine Metabolism in Mice and Rats. J. Nutr. 2015, 145, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef]
- Rabattoni, V.; Marchesani, F.; Murtas, G.; Sacchi, S.; Mozzarelli, A.; Bruno, S.; Peracchi, A.; Pollegioni, L.; Campanini, B. The human phosphorylated pathway: A multienzyme metabolic assembly for L-serine biosynthesis. FEBS J. 2023, 290, 843–861. [Google Scholar] [CrossRef]
- Thananurak, P.; Chuaychu-noo, N.; Thélie, A.; Vongpralub, T.; Blesbois, E. Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poult. Sci. 2020, 99, 1185–1198. [Google Scholar] [CrossRef]
- Kheawkanha, T.; Chankitisakul, V.; Thananurak, P.; Pimprasert, M.; Boonkum, W.; Vongpralub, T. Solid storage supplemented with serine of rooster semen enhances higher sperm quality and fertility potential during storage at 5 °C for up to 120 h. Poult. Sci. 2023, 102, 102648. [Google Scholar] [CrossRef]
- Kong, Y.; He, M.; Gao, J.; Xu, J.; Lu, N.; Wu, C.; Sun, L.; Dai, J. Comparative Analysis of Classic Semen Extenders for Frozen–Thawed Boar Semen. Animals 2025, 15, 1885. [Google Scholar] [CrossRef]
- de Oliveira, M.B.; Ferreira, H.N. Importância da adição de antioxidantes ao sêmen criopreservado de carneiros. Multidiscip. Rev. 2019, 2, e2019010. [Google Scholar] [CrossRef]
- Khoi, H.X.; Shimizu, K.; Yoneda, Y.; Minagawa, I.; Abe, Y.; Kuwabara, Y.; Sasanami, T.; Kohsaka, T. Monitoring the reactive oxygen species in spermatozoa during liquid storage of boar semen and its correlation with sperm motility, free thiol content and seasonality. Andrologia 2021, 53, e14237. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, I.; Morrell, J.M. Sperm quality and in vitro fertilizing ability of boar spermatozoa stored at 4 °C versus conventional storage for 1 week. Front. Vet. Sci. 2024, 11, 1444550. [Google Scholar] [CrossRef] [PubMed]
- Chankitisakul, V.; Boonkum, W.; Kaewkanha, T.; Pimprasert, M.; Ratchamak, R.; Authaida, S.; Thananurak, P. Fertilizing ability and survivability of rooster sperm diluted with a novel semen extender supplemented with serine for practical use on small holder farms. Poult. Sci. 2022, 101, 102188. [Google Scholar] [CrossRef] [PubMed]
- Ratchamak, R.; Authaida, S.; Koedkanmark, T.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Dietary supplementation with ginseng extract enhances testicular function, semen preservation, and fertility rate of mature and aging Thai native roosters. Theriogenology 2024, 227, 31–40. [Google Scholar] [CrossRef]
- Vongpralub, T.; Thananurak, P.; Sittikasamkit, C.; Chuawongboon, P.; Duangjinda, M.; Boonkum, W.; Chankitisakul, V. Comparison of Effects of Different Antioxidants Supplemented to Long-term Extender on Boar Semen Quality Following Storage at 17 °C. Thai J. Vet. Med. 2018, 46, 119–126. [Google Scholar] [CrossRef]
- Ratchamak, R.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Cryopreservation and quality assessment of boar semen collected from bulk samples. Vet. Med. 2019, 64, 209–216. [Google Scholar] [CrossRef]
- Ratchamak, R.; Ratsiri, T.; Kheawkanha, T.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Evaluation of cryopreserved boar semen after supplementation sericin form silkworm (Bombyx mori) in semen extender. Anim. Sci. J. 2020, 91, e13428. [Google Scholar] [CrossRef]
- Karirat, T.; Saengha, W.; Deeseenthum, S.; Ma, N.L.; Sutthi, N.; Wangkahart, E.; Luang-In, V. Data on exopolysaccharides produced by Bacillus spp. from cassava pulp with antioxidant and antimicrobial properties. Data Brief 2023, 50, 109474. [Google Scholar] [CrossRef] [PubMed]
- Fontagné-Dicharry, S.; Larroquet, L.; Dias, K.; Cluzeaud, M.; Heraud, C.; Corlay, D. Effects of dietary oxidized fish oil supplementation on oxidative stress and antioxidant defense system in juvenile rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 74, 43–51. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Hoagland, R.E.; Locke, M.A.; Hickey, W.J. Glutathione-S transferase activity and metabolism of glutathione conjugates by rhizosphere bacteria. Appl. Environ. Microbiol. 1995, 61, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Hammerstedt, R.H.; Graham, J.K.; Nolan, J.P. Cryopreservation of mammalian sperm: What we ask them to survive. J. Androl. 1990, 11, 73–88. [Google Scholar] [CrossRef]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Maralani, M.N.; Movahedian, A.; Javanmard, S.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res. Pharm. Sci. 2012, 7, 209–215. [Google Scholar]
- Zhou, X.; He, L.; Wu, C.; Zhang, Y.; Wu, X.; Yin, Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 2017, 61, 1700262. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wu, X.; Wan, D.; Yin, Y. Effects of Dietary Serine Supplementation on Intestinal Integrity, Inflammation and Oxidative Status in Early-Weaned Piglets. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Pizzi, F.; Gliozzi, T.M. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction 2001, 121, 395–401. [Google Scholar] [CrossRef][Green Version]
- Hu, R.; Yang, X.; Gong, J.; Lv, J.; Yuan, X.; Shi, M.; Fu, C.; Tan, B.; Fan, Z.; Chen, L.; et al. Patterns of alteration in boar semen quality from 9 to 37 months old and improvement by protocatechuic acid. J. Anim. Sci. Biotechnol. 2024, 15, 78. [Google Scholar] [CrossRef]
- Pollock, K.; Yu, G.; Moller-Trane, R.; Koran, M.; Dosa, P.I.; McKenna, D.H.; Hubel, A. Combinations of Osmolytes, Including Monosaccharides, Disaccharides, and Sugar Alcohols Act in Concert During Cryopreservation to Improve Mesenchymal Stromal Cell Survival. Tissue Eng. Part C Methods 2016, 22, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Menegat, M.B.; Mellagi, A.P.; Bortolin, R.C.; Menezes, T.A.; Vargas, A.R.; Bernardi, M.L.; Wentz, I.; Gelain, D.P.; Moreira, J.C.; Bortolozzo, F.P. Sperm quality and oxidative status as affected by homogenization of liquid-stored boar semen diluted in short- and long-term extenders. Anim. Reprod. Sci. 2017, 179, 67–79. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Zhu, J.; Ju, H.; Liang, M.; Wang, S.; Chen, S.; Ferreira-Dias, G.; Liu, Z. Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage. Vet. Sci. 2023, 10, 214. [Google Scholar] [CrossRef]
- Sakamoto, T.; Imai, H. Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Kutikhin, A.G. Inherited variations in the SOD and GPX gene families and cancer risk. Free Radic. Res. 2012, 46, 581–599. [Google Scholar] [CrossRef]
- Njoroge, W.E.; Zhu, Z.; Umehara, T.; Yamanaka, T.; Zeng, W.; Okazaki, T.; Shimada, M. Synthesis of functional enzymes involved in glutathione production during linear motility in boar sperm. Free Radic. Biol. Med. 2025, 228, 126–136. [Google Scholar] [CrossRef]
- Yeste, M.; Flores, E.; Estrada, E.; Bonet, S.; Rigau, T.; Rodríguez-Gil, J.E. Reduced glutathione and procaine hydrochloride protect the nucleoprotein structure of boar spermatozoa during freeze–thawing by stabilising disulfide bonds. Reprod. Fertil. Dev. 2013, 25, 1036–1050. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Evidence for Increased Lipid Peroxidative Damage and Loss of Superoxide Dismutase Activity as a Mode of Sublethal Cryodamage to Human Sperm During Cryopreservation. J. Androl. 1992, 13, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Placidi, M.; Barbonetti, A.; Tatone, C.; Emidio, G.D. Mechanisms underlying human sperm cryodamage: The role of reactive oxygen species (ROS) and antioxidants. RIVER J. 2024, 1, 3–9. [Google Scholar] [CrossRef]
- Sadeghi, N.; Boissonneault, G.; Tavalaee, M.; Nasr-Esfahani, M.H. Oxidative versus reductive stress: A delicate balance for sperm integrity. Syst. Biol. Reprod. Med. 2023, 69, 20–31. [Google Scholar] [CrossRef]
- Tvrdá, E.; Mackovich, A.; Greifová, H.; Lukáč, N. Lycopene offers protection against oxidative damage in frozen-thawed bovine semen. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 115–118. [Google Scholar]
- He, L.; Ding, Y.; Zhou, X.; Li, T.; Yin, Y. Serine signaling governs metabolic homeostasis and health. Trends Endocrinol. Metab. 2023, 34, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.L.; Lorenz, N.I.; Klann, K.; Münch, C.; Depner, C.; Steinbach, J.P.; Ronellenfitsch, M.W.; Luger, A.L. Serine-dependent redox homeostasis regulates glioblastoma cell survival. Br. J. Cancer 2020, 122, 1391–1398. [Google Scholar] [CrossRef]
- Funahashi, H.; Sano, T. Select antioxidants improve the function of extended boar semen stored at 10 °C. Theriogenology 2025, 63, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Kaeoket, K.; Chanapiwat, P. The beneficial effect of resveratrol on the quality of frozen-thawed boar sperm. Animals 2023, 13, 2829. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control | L-Serine | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| 1 mM | 3 mM | 5 mM | 7 mM | ||||
| Progressive motility (%) | |||||||
| Day 1 | 72.02 b | 74.60 b | 80.10 a | 75.10 b | 74.58 b | 1.12 | 0.0133 |
| Day 3 | 63.23 c | 68.84 b | 74.47 a | 65.37 bc | 61.99 c | 1.30 | <0.0001 |
| Day 5 | 59.03 b | 61.40 b | 70.93 a | 57.88 b | 57.73 b | 1.31 | <0.0001 |
| Viability (%) | |||||||
| Day 1 | 76.26 b | 76.63 b | 82.87 a | 74.57 bc | 70.90 c | 1.11 | <0.0001 |
| Day 3 | 68.41 b | 67.45 b | 76.47 a | 62.92 bc | 58.12 c | 1.46 | <0.0001 |
| Day 5 | 60.24 b | 63.16 b | 70.12 a | 57.98 bc | 53.77 c | 1.64 | <0.0001 |
| Mitochondria function (%) | |||||||
| Day 1 | 69.59 b | 70.08 b | 79.07 a | 69.28 b | 69.66 b | 1.12 | <0.0001 |
| Day 3 | 62.32 b | 62.77 ab | 69.74 a | 57.54 bc | 54.95 c | 1.20 | <0.0001 |
| Day 5 | 49.07 bc | 52.69 b | 59.94 a | 49.12 bc | 43.87 c | 1.22 | <0.0001 |
| Acrosome integrity (%) | |||||||
| Day 1 | 62.95 b | 63.81 b | 69.53 a | 60.63 b | 59.74 b | 0.88 | <0.0001 |
| Day 3 | 54.25 b | 57.90 b | 63.39 a | 56.57 b | 47.12 c | 1.13 | <0.0001 |
| Day 5 | 43.13 bc | 46.44 b | 53.05 a | 38.98 c | 32.59 d | 1.32 | <0.0001 |
| Treatments | Control | L-Serine | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| 1 mM | 3 mM | 5 mM | 7 mM | ||||
| MDA (µmol/mL) | |||||||
| Day 1 | 0.67 bc | 0.65 ab | 0.56 a | 0.75 bc | 0.76 c | 0.02 | <0.0001 |
| Day 3 | 0.95 ab | 0.99 ab | 0.85 a | 1.04 b | 1.08 b | 0.03 | 0.0007 |
| Day 5 | 1.10 b | 1.00 ab | 0.94 a | 1.00 ab | 1.13 b | 0.04 | 0.0070 |
| T-AOC (%) | |||||||
| Day 1 | 70.68 b | 71.67 ab | 72.07 ab | 71.50 ab | 72.24 b | 0.55 | 0.0500 |
| Day 3 | 70.18 b | 71.97 ab | 72.55 a | 72.40 ab | 71.79 ab | 0.56 | 0.0376 |
| Day 5 | 69.64 b | 70.83 ab | 72.05 a | 71.70 ab | 71.48 ab | 0.47 | 0.0249 |
| Gpx (U/mL) | |||||||
| Day 1 | 10.26 b | 11.65 ab | 12.87 a | 12.11 ab | 11.25 ab | 0.30 | 0.0422 |
| Day 3 | 9.92 b | 10.53 ab | 11.73 a | 11.69 a | 11.73 a | 0.37 | 0.0024 |
| Day 5 | 7.78 b | 11.05 ab | 12.31 a | 11.97 ab | 11.84 ab | 0.54 | 0.0320 |
| SOD (U/mL) | |||||||
| Day 1 | 813.33 b | 937.50 b | 918.33 b | 916.67 b | 1500.00 a | 73.87 | 0.0126 |
| Day 3 | 875.00 b | 937.50 b | 875.00 b | 843.75 b | 1500.00 a | 66.77 | 0.0005 |
| Day 5 | 791.67 b | 937.50 b | 906.25 b | 875.00 b | 1250.00 a | 43.17 | 0.0003 |
| CAT (U/mL) | |||||||
| Day 1 | 4.75 b | 6.67 ab | 9.83 a | 8.33 a | 2.75 b | 0.77 | 0.0189 |
| Day 3 | 3.06 b | 3.56 ab | 7.56 a | 6.81 ab | 4.81 ab | 0.54 | 0.0246 |
| Day 5 | 3.25 b | 4.06 ab | 7.50 a | 6.25 ab | 3.12 b | 0.55 | 0.0118 |
| GST (U/mL) | |||||||
| Day 1 | 25.71 | 24.73 | 25.95 | 21.78 | 26.54 | 0.59 | 0.0694 |
| Day 3 | 24.80 | 23.81 | 25.75 | 26.42 | 25.63 | 0.51 | 0.6413 |
| Day 5 | 25.29 | 24.38 | 23.45 | 25.87 | 23.95 | 0.68 | 0.8478 |
| GRD (U/mL) | |||||||
| Day 1 | 1.11 | 1.10 | 1.36 | 1.25 | 1.32 | 0.04 | 0.2875 |
| Day 3 | 1.14 | 1.11 | 1.28 | 1.23 | 1.26 | 0.03 | 0.3153 |
| Day 5 | 1.20 | 1.14 | 1.32 | 1.31 | 1.24 | 0.04 | 0.4975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chankitisakul, V.; Saiyamanon, H.; Boonkum, W.; Wangkahart, E.; Ratchamak, R. Dose-Dependent Effects of L-Serine Supplementation on Boar Sperm Quality During Chilled and Cryopreserved Storage. Animals 2025, 15, 2670. https://doi.org/10.3390/ani15182670
Chankitisakul V, Saiyamanon H, Boonkum W, Wangkahart E, Ratchamak R. Dose-Dependent Effects of L-Serine Supplementation on Boar Sperm Quality During Chilled and Cryopreserved Storage. Animals. 2025; 15(18):2670. https://doi.org/10.3390/ani15182670
Chicago/Turabian StyleChankitisakul, Vibuntita, Himalai Saiyamanon, Wuttigrai Boonkum, Eakapol Wangkahart, and Ruthaiporn Ratchamak. 2025. "Dose-Dependent Effects of L-Serine Supplementation on Boar Sperm Quality During Chilled and Cryopreserved Storage" Animals 15, no. 18: 2670. https://doi.org/10.3390/ani15182670
APA StyleChankitisakul, V., Saiyamanon, H., Boonkum, W., Wangkahart, E., & Ratchamak, R. (2025). Dose-Dependent Effects of L-Serine Supplementation on Boar Sperm Quality During Chilled and Cryopreserved Storage. Animals, 15(18), 2670. https://doi.org/10.3390/ani15182670







