1. Introduction
Due to global warming, heat stress has become increasingly prevalent in livestock production [
1]. Heat stress adversely affects animal nutritional metabolism, resulting in reduced body weight, impaired intake and feed efficiency, as well as diminished carcass quality, ultimately leading to substantial economic losses [
2]. Beef cattle are particularly susceptible to heat stress due to their rapid metabolic rate, rumen fermentation characteristics, impaired sweating capacity, and skin insulation properties [
3,
4]. Several reports have demonstrated that heat stress can significantly reduce beef quality. Surinder et al. [
5] demonstrated that beef cattle subjected to summer heat stress exhibited significantly higher shear force values in the muscles, leading to reduced tenderness and compromised meat quality. Heyok et al. [
6] reported that Korean cattle steers exposed to prolonged high-temperature and high-humidity stress showed a marked decline in post-slaughter muscle tenderness, ultimately resulting in substantial deterioration of beef quality attributes. The impact of heat stress on beef quality has emerged as a critical challenge for the high-quality development of the beef cattle industry.
Natural plant extracts contain a rich variety of active ingredients such as isoflavones and polysaccharides [
7]. These ingredients have the characteristics of antioxidation and anti-inflammation. Hence, reducing heat stress and improving beef quality by feeding with plant extract additives has been a research hotspot in recent years [
8,
9]. Soybean isoflavones are the secondary metabolites of polyphenol compounds in soybean products [
10]. Daidzein (7,4′-trihydroxyisoflavone) is one of the major soybean isoflavones [
11]. Liu et al. [
12] reported that supplementing daidzein in the diet significantly enhanced the growth performance, antioxidant capacity, and immune capacity of bull calves. Wang et al. [
13] demonstrated that dietary supplementation with isoflavones significantly upregulated both the expression levels and protein abundance of heat shock proteins (HSPs) in the serum of heat-stressed mice. Wang et al. [
14] elucidated that isoflavones attenuated heat stress hazards by upregulating HSP70 expression, thereby improving heat stress resilience in caprine species. These results indicate that daidzein can be used to alleviate heat stress in livestock.
Because the chemical structure of daidzein is similar to estrogens, some researchers have reported that daidzein could affect the meat tenderness of livestock. Zhao et al. [
15] demonstrated that supplementing daidzein in diet significantly improved beef tenderness by increasing the intramuscular fat content and marbling score in Xiangzhong black cattle. Rehfeldt et al. [
16] reported that adding daidzein to the diet significantly affected the meat tenderness of piglets by transforming the muscle fiber types. In our previous study, adding 1000 mg/kg daidzein to the diet significantly increased the fat thickness (1.8 cm vs. 1.4 cm,
p < 0.05) and significantly decreased the shear force (3.0 kg f vs. 3.5 kg f,
p < 0.05) of beef in Xianan steers [
17]. However, little attention has been paid to the effect of daidzein on meat quality in heat-stressed beef cattle.
This research was carried out to assess the impact of daidzein supplementation on production performance, serum biochemical indexes, meat quality, and the transcriptome of the longissimus dorsi (LM) muscle in heat-stressed Jinjiang cattle, so as to provide a theoretical basis for the application of daidzein in heat-stressed beef cattle production.
2. Materials and Methods
2.1. Animal Care
These experiments were conducted following Chinese guidelines for animal welfare. All the experimental procedures applied in this study were reviewed and approved by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University (JXAULL−20190015).
2.2. Animals, Diets, and Experimental Design
Daidzein (purity > 98%) was acquired from Ciyuan Biotechnology Limited Company (Baoji, Shanxi, China). The cattle were provided by Shenglong Cattle Industry Limited Company (Pingxiang, China). Twenty 20-month-old Jinjiang cattle (initial mean ± SE: 438 ± 34.6 kg of body weight) were randomly divided into two treatment groups (
n = 10 per treatment): control treatment and daidzein treatment (1000 mg/kg concentrate). The dosage of daidzein supplemented in the diet was based on our previous study, in which adding 1000 mg/kg daidzein to the diet significantly increased the fat thickness and significantly decreased the shear force of beef in Xianan steers [
17]. The composition and nutritional content of the basal diet are presented in
Table 1. All cattle were housed individually. Diets were fed twice daily at 07:00 and 14:00. Clean, fresh water was provided as needed. The 100-day feeding trial, conducted from July 20 to September 27, included a 10-day adaptation period followed by a 90-day experimental period under controlled environmental conditions in the cattle housing facility, with an averaging temperature of 30.68 °C, relative humidity of 68.05%, and a temperature–humidity index (THI) of 81.82.
2.3. Growth Performance and Serum Biochemical Parameters
The individual body weights of all cattle were recorded prior to morning feeding at both the start and end of the trial. Average daily gain (ADG) was calculated as ADG (kg/d) = (final body weight − initial body weight)/trial duration (days). Average daily dry matter intake (ADMI) was determined by ADMI (kg/d) = (feed offered − feed residues)/trial duration (days). Feed residues were collected and weighed daily before morning feeding. The feed conversion ratio (FCR) was derived as FCR = ADMI/ADG.
On the final day of the experiment, blood samples were collected from the caudal vein prior to morning feeding. After standing for 30 min, the blood samples were centrifuged at 3500 revolutions per minute for 15 min. Blood biochemical parameters, including glucose, free fatty acid (FFA), triglyceride (TG), total cholesterol (TC), total protein (TP), albumin, urea, glutamic-oxalacetic transaminase (AST), glutamic-pyruvic transaminase (ALT), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total antioxidant capacity (T-AOC), malondiadehyde (MDA), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-PX), growth hormone (GH), triiodothyronine (T3), tetraiodothyronine (T4), cortisol (COR), leptin, insulin, adiponectin, IgA, IgM, and IgG, were quantified using a Hitachi 3100 automatic biochemical analyzer (China).
2.4. Meat Quality
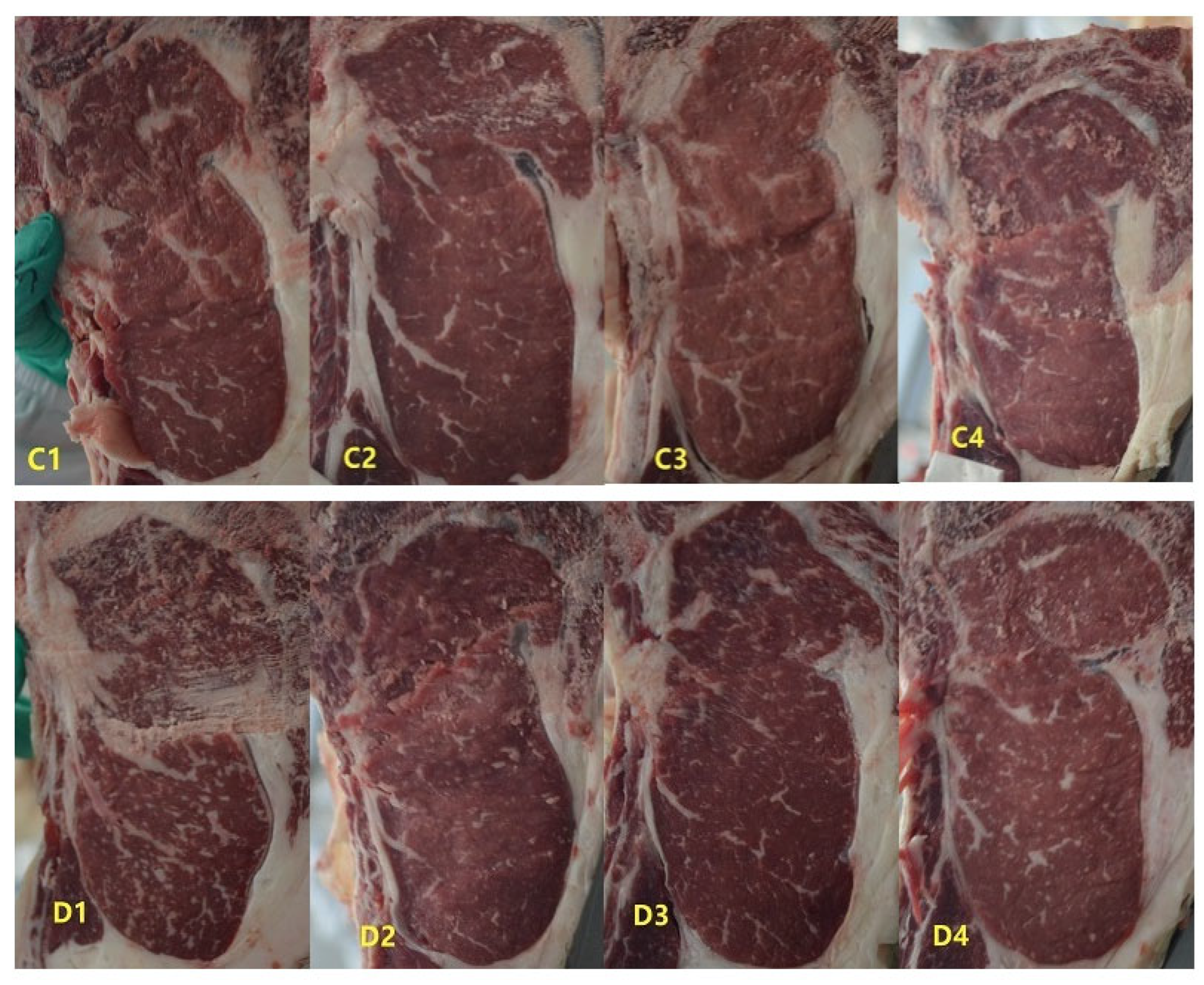
On the final experimental day, four bulls with medium body weight were selected from the control and daidzein groups, respectively. Those eight bulls were slaughtered following commercial slaughter protocols. The bulls were individually stunned in a 260 × 81 × 160 cm (length×width×height) steel-walled pen (stun box). Then, the stunned bulls were slaughtered using upright restraint. All carcasses were immediately chilled at 0 °C for a 7-day aging period. The meat quality was measured from the longissimus dorsi muscle excised from the right-side 12th−13th rib interface and carefully trimmed to exclude connective tissue and subcutaneous fat. The marbling score was evaluated using the Japanese Marbling Standard (JMBS) [
18]. The marbling standard consists of 12 grading levels (8–12 = Abundant, 5–7 = Moderate, 3–4 = Average, 2 = Slight, 1 = Trace). The pH value of the longissimus dorsi muscle was determined by a Delta 320 pH Meter (Mettler Toledo, Switzerland), with the probe inserted into the muscle core. Meat color values of L*, a*, and b* were measured by a colorimeter (WSC-S, Shanghai, China) with a D65 illuminant, 10° observer angle, and CIELAB color space.
To determine the cooking loss and shear force values, LM samples were standardized into 3 × 4 × 5 cm steaks, sealed within individual ziplock bags, and cooked in a temperature-controlled water bath (75 °C) until the core temperature reached 70 °C. Then, the cooked LM samples were cooled to room temperature naturally. The cooking losses were calculated as (raw weight–cooked weight)/raw weight ×100%. Six 3 × 1 × 1 cm muscle fiber-aligned subsamples were prepared from each LM and sheared perpendicular to fiber orientation using a C-LM4 device (Harbin, China) with a load cell of 15 kg and a crosshead speed of 200 mm/min. The shear force was expressed as mean peak force (kg·f) from the six replicates. To determine the drip losses, the valve-bag protected LM samples were stored at 4 °C for 48 h, and the fiber orientation was maintained parallel to gravitational force. Drip losses were calculated as (initial weight–refrigerated weight)/initial weight × 100%. The chemical compositions of the basal diet and LM were measured following the procedures of AOAC methods [
19].
2.5. RNA-Seq Library Preparation and Data Analysis
The total RNA was isolated from eight LM samples by TRIzol reagent (LC Science, Houston, TX, USA) according to the manufacturer’s instructions. The Agilent 2100 Bioanalyzer (Agilent, CA, USA) was used to assess the total RNA quality and concentration. Then, transcriptomic sequencing was performed by Illumina HiSeq™ 2000 (Novogene, Beijing, China).
To analyze gene expression, the number of unique-match reads was calculated and normalized to FPKM (fragment per kilo base of exon model per million mapped reads), which was used to indicate the condition of transcriptional expression. The amount of expression was calculated for each read of the eight sequenced samples using Cuffdiff [
20].
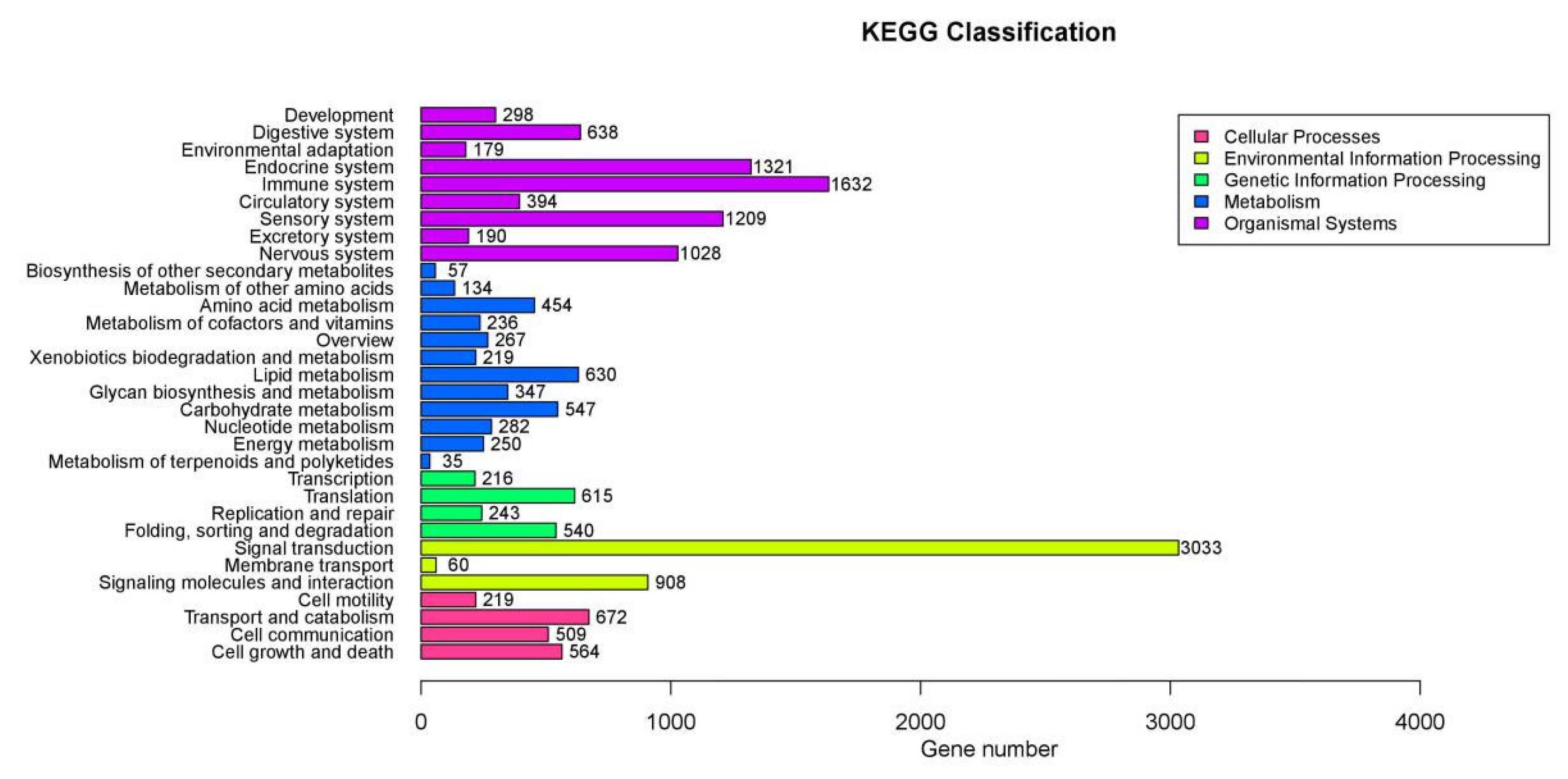
To determine the functional categories of differentially expressed genes (DEGs), all DEGs were subjected to GO and KEGG pathway analyses. GO enrichment analysis was used to map all DEGs to GO terms in GO database 2. The significance was calculated using a hypergeometric test, as suggested by Yang [
8].
To better understand the biological function of DEGs, all DEGs were annotated to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways.
2.6. Real-Time Quantitative PCR (qRT-PCR)
Microbial DNA was extracted from rumen fluid samples using an E.Z.N.A. stool DNA kit (Omega Bio-tek, Norcross, GA, United States) according to the manufacturer’s protocols. The 16S rDNA V3–V4 region of the eukaryotic ribosomal RNA gene was amplified by PCR ( 95 °C for 3 min, followed by 30 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, and a final extension at 72 °C for 10 min (until halted by user)) using primers 338F: ACTCCTACGGGAGGCAGCAG and 806R: GGACTACHVGGGTWTCTAAT. PCRs were performed in a triplicate 20 µL mixture containing 4 µL of 5×FastPfu buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of forward primer (5 µM), 0.8 µL of reverse primer (5 µM), 0.4 µL of FastPfu polymerase, 0.2 µL of BSA, and 10 ng of template DNA.
PCR products were purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using a Quantus™ Fluorometer (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar ratios and paired-end sequenced on an Illumina NovaSeq PE250 platform (Illumina, San Diego, CA, USA) according to the standard protocols of Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA884686).
2.7. Processing of Sequencing Data
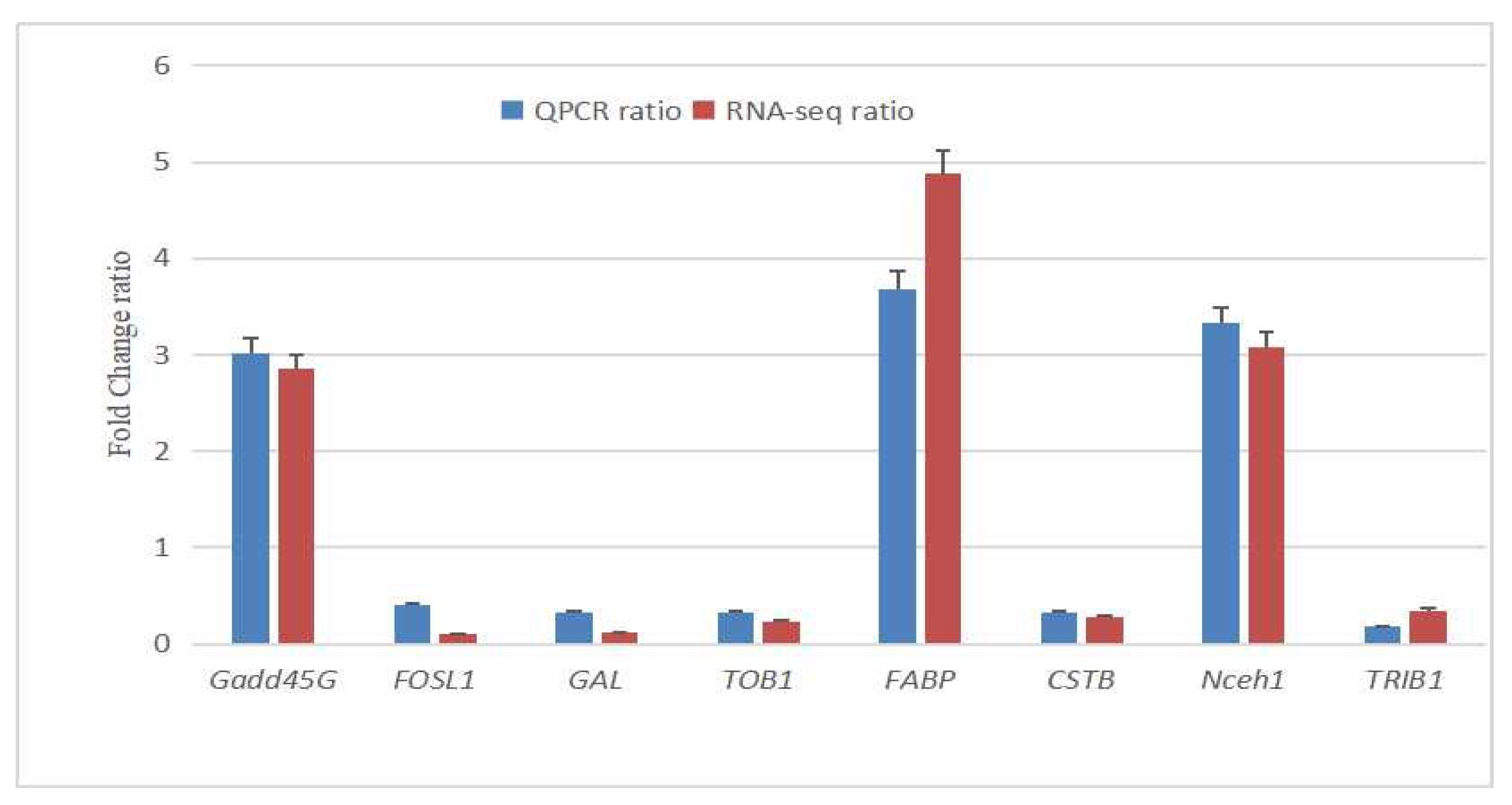
To confirm the reproducibility and repeatability of gene expression data obtained by RNA-Seq, eight DEGs in the longissimus dorsi muscle of heat-stressed Jinjiang cattle were selected for qRT-PCR validation. The TRIzol reagent (LC Science, Houston, TX, USA) was used to isolate total RNA. Gene-specific primers were designed according to the gene sequence using Primer 5.0 software (Premier Biosoft, Palo Alto, CA, USA) and synthesized by Takara Bioch (Dalian, China) (
Table 2). qRT-PCR was performed with the BioRad PCR machine with SYBR green master mix (TransGen Biotech, Beijing, China) following the manufacturer’s guidelines. The reaction mixtures were incubated in a 96-well plate at 95 °C for 20 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. All measurements were analyzed in triplicate. The relative mRNA expression of the eight DEGs was achieved after normalization of glyceraldehyde−3-phosphate dehydrogenase (GAPDH) reference using the 2
−ΔΔCt method [
21].
2.8. Statistical Analysis
The analysis of the experimental data was conducted using SPSS 26.0 (SPSS, Chicago, IL, USA), where independent sample t-tests were employed to assess the significance of production performance, serum biochemical indexes, and meat quality. An independent sample t-tests is an important parameter test method in statistics to compare the mean difference of two groups of independent samples. It verifies whether there is a significant mean difference between two non-interfering sample groups by analyzing the distribution characteristics of the two groups of data. The final results are presented as mean values, with differences regarded as showing a tendency when 0.05 < p < 0.10 and considered statistically significant at p < 0.05.
4. Discussion
In the present study, the average THI during the feeding trial was 81.82, indicating that the experimental Jinjiang cattle were exposed to heat stress, as defined by the criteria reported by Armstrong [
22]. Beef cattle primarily regulate body temperature through two key thermoregulatory mechanisms: evaporative cooling (panting and sweating) and conductive heat exchange. These physiological responses help reduce metabolic heat production by decreasing feed intake. Therefore, ADMI serves as a reliable indicator for assessing the health and performance of beef cattle under heat stress. Numerous studies have demonstrated that heat stress reduced dry matter intake in beef cattle, with feed intake showing a significantly negative correlation with environmental temperature [
23,
24]. In this study, daidzein supplementation significantly increased ADG in heat-stressed Jinjiang cattle, which was consistent with findings from previous research.
Leptin, a protein hormone predominantly secreted by adipose tissue, serves as a key regulator of energy homeostasis, neuroendocrine activity, and metabolic processes [
25]. Research has indicated that heat stress elevates leptin concentrations in beef cattle [
26]. The present study demonstrated that daidzein supplementation significantly decreased serum leptin levels compared to the control group. TC, a lipid metabolite in serum, is considered a fundamental metabolic parameter [
27]. TC levels can reflect alterations in metabolic function and the body’s adaptation to external environmental conditions [
28]. Studies have shown that heat stress significantly elevates serum TC levels in animals [
29]. The current results show that daidzein significantly decreased TC concentrations in heat-stressed Jinjiang cattle. These results indicate that daidzein has the potential to ameliorate heat stress-induced endocrine dysfunction in beef cattle.
When beef cattle are exposed to heat stress, meat quality is significantly compromised after slaughter, often resulting in the occurrence of DFD meat [
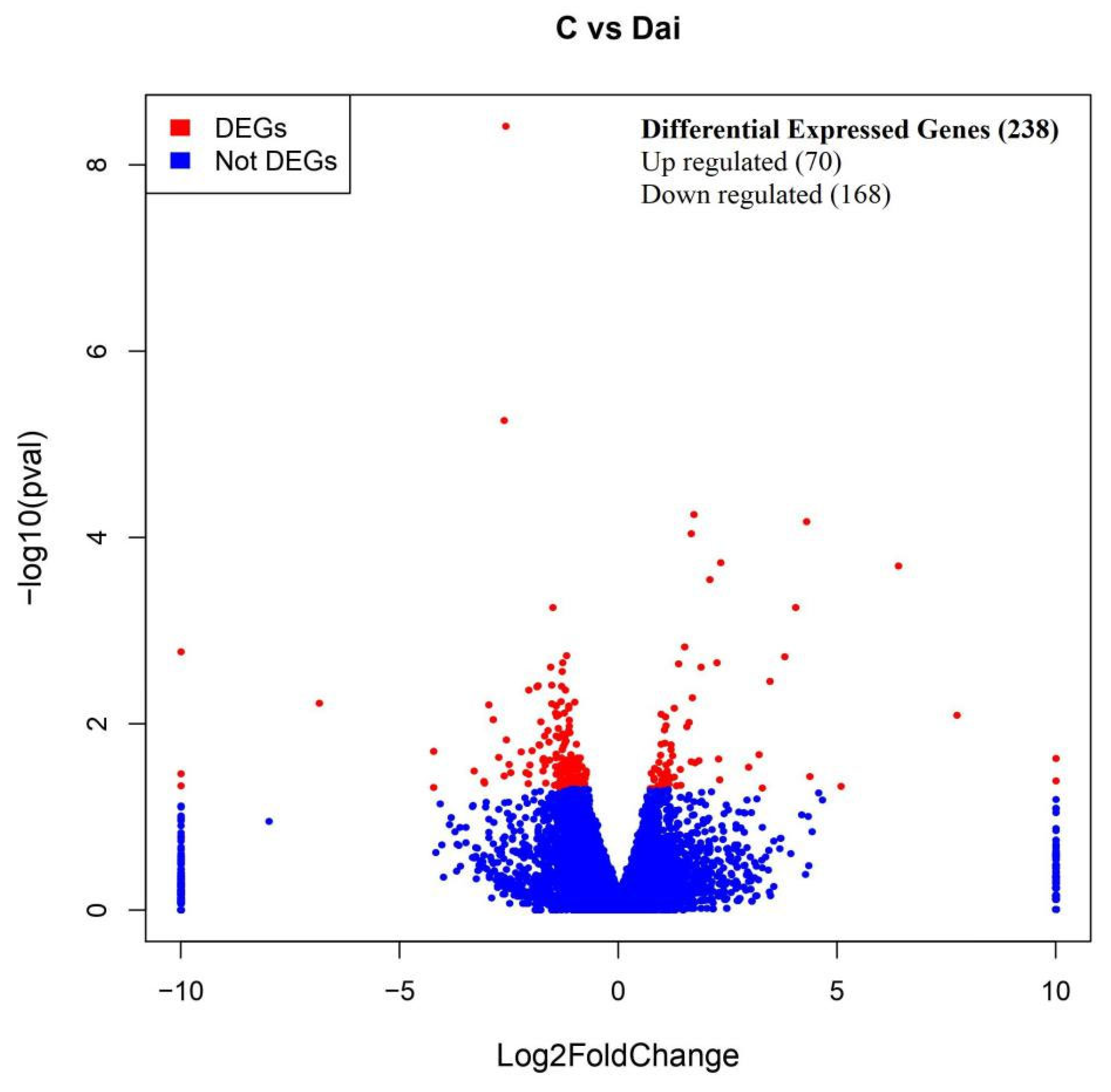
30]. In the current results, daidzein supplementation significantly decreased the L* value of LM in heat-stressed Jinjiang cattle, which suggests that daidzein can alleviate the effects of heat stress on beef meat color. Furthermore, the present findings indicate that adding daidzein to the diet significantly improved the marbling score and beef tenderness. However, daidzein did not significantly affect the IMF content of the longissimus dorsi muscle in heat-stressed Jinjiang cattle. Therefore, we hypothesized that daidzein might regulate the expression of genes related to differentiation of preadipocytes, thereby promoting a more uniform distribution of IMF in the longissimus dorsi muscle. This potential redistribution of fat may interfere with cross-linked collagen structures, ultimately enhancing beef tenderness. To explore this hypothesis, RNA-Seq was performed to elucidate the underlying mechanisms of daidzein on beef tenderness of the longissimus dorsi muscle in heat-stressed Jinjiang cattle. The results revealed that 238 genes were differentially expressed between the control and daidzein-treated groups (
p < 0.05). These DEGs were found to be significantly involved in both preadipocyte differentiation and connective tissue restructuring.
The FoxO signaling pathway plays an important role in regulating the adipogenic differentiation of preadipocytes. Nakae et al. [
31] reported that the FoxO gene acts as a negative regulatory factor, showing strong expression during the middle and late stages of preadipocyte differentiation, thereby inhibiting this process. Bastie [
32] found that the FoxO protein could inhibit the metabolism of glycolysis and fat synthesis, reducing triglyceride synthesis. Sakamoto et al. [
33] reported that the expression of the Gadd45 gene could be directly regulated by the FoxO signaling pathway and that the Gadd45 gene could promote preadipocyte differentiation by participating in cellular DNA methylation. In the present study, daidzein significantly downregulated the expression of PLK and TRAIL genes in the FoxO signaling pathway, thereby significantly inhibiting the FoxO signaling pathway’s activity, while significantly upregulating the expression of the Gadd45 gene. This suggests that daidzein promotes preadipocyte differentiation in the longissimus dorsi muscle of heat-stressed Jinjiang cattle through modulation of the FoxO signaling pathway.
The Notch signaling pathway plays a crucial role in regulating adipogenic differentiation [
34]. Inhibition of the Notch signaling pathway could promote the adipogenic differentiation of preadipocytes [
35]. The present results indicated that daidzein significantly downregulated the Notch gene expression, which was the key gene in the Notch signaling pathway, and significantly inhibited the Notch signaling pathway, thereby promoting the differentiation of preadipocytes in the longissimus dorsi muscle of heat-stressed Jinjiang cattle.
Daidzein significantly downregulated the expression of the
SEMA3 and
TOB genes in the present study. The
SEMA3 and
TOB genes were mainly responsible for the differentiation of MSCs [
36,
37]. The downregulated expression of the
SEMA3 and
TOB genes could inhibit the differentiation of MSCs towards fibroblasts, while promoting their differentiation into preadipocytes. This, in turn, may enhance the differentiation of preadipocytes in the longissimus dorsi muscle of heat-stressed Jinjiang cattle.
This experiment also indicated that daidzein significantly regulated the expression of several genes related to fat synthesis in the longissimus dorsi muscle of heat-stressed Jinjiang cattle. Specifically, daidzein significantly upregulated the expression of the
FABP8 and
PON3 genes, while significantly downregulating the expression of the
TRIB1,
DGKH, and
Nceh1 genes. These regulatory effects suggested that daidzein could promote triglyceride synthesis in the longissimus dorsi muscle. Considering that daidzein significantly improved the marbling score, but had no significant effect on the IMF content in the longissimus dorsi muscle of heat-stressed Jinjiang cattle, we hypothesized that the experimental fattening period of 90 days might have been insufficient to induce a measurable change in IMF content. Following adipogenic differentiation of preadipocytes, triglyceride synthesis and subsequent deposition within adipocytes lead to an increase in adipocyte size, which is a critical process in the development of intramuscular fat in the longissimus dorsi muscle [
38]. Therefore, the duration of this experiment may not have been long enough to observe a significant difference in IMF content.
Muscle fiber type, IMF content, and connective tissue structure were the main factors that affected beef tenderness [
8]. Based on the findings of the current study and previous research [
16], daidzein had no significant effect on the IMF content or muscle fiber types, but it significantly improved the marbling score and beef tenderness. Therefore, we hypothesized that daidzein might improve beef tenderness by changing the structure of connective tissue in the longissimus dorsi muscle of heat-stressed Jinjiang cattle.
To date, the molecular mechanism of fat deposits affecting beef tenderness is still unclear. Nishimura [
39] speculated that fat deposits could disrupt the structure of connective tissue in muscle and reduce the mechanical strength of connective tissue, thus improving the tenderness of meat. This hypothesis has gradually attracted more and more attention from scholars, but unfortunately, no in-depth research reports are currently available. The present study showed that daidzein significantly downregulated the expression of the
CSTB gene, which promoted the hydrolysis of cysteine by cysteine protease [
40]. There are two main forms of covalent cross-linking of collagen molecules: the pyridine cross-linking between lysine and hydroxylysine [
41], and the disulfide bonds based on cysteine [
42]. Thus, the hydrolysis of cysteine could destroy the cross-linking of collagen and improve beef tenderness.
As a myofiber cross-linking protein [
43], ACTN plays a role in maintaining muscle structure. The low expression of the
ACTN gene in muscle fibers would contribute to improved beef tenderness. The results of this experiment showed that daidzein significantly downregulated the expression of the
ACTN gene in the longissimus dorsi muscle of heat-stressed Jinjiang cattle. This downregulation may partially explain the observed improvement in beef tenderness following daidzein treatment.