What Need for Speed? Lizards from Islands Missing Predators Sprint Slower
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Island Characteristics
2.3. Animal Methods and Morphology
2.4. Sprint Trials
2.5. Statistical Analysis
3. Results
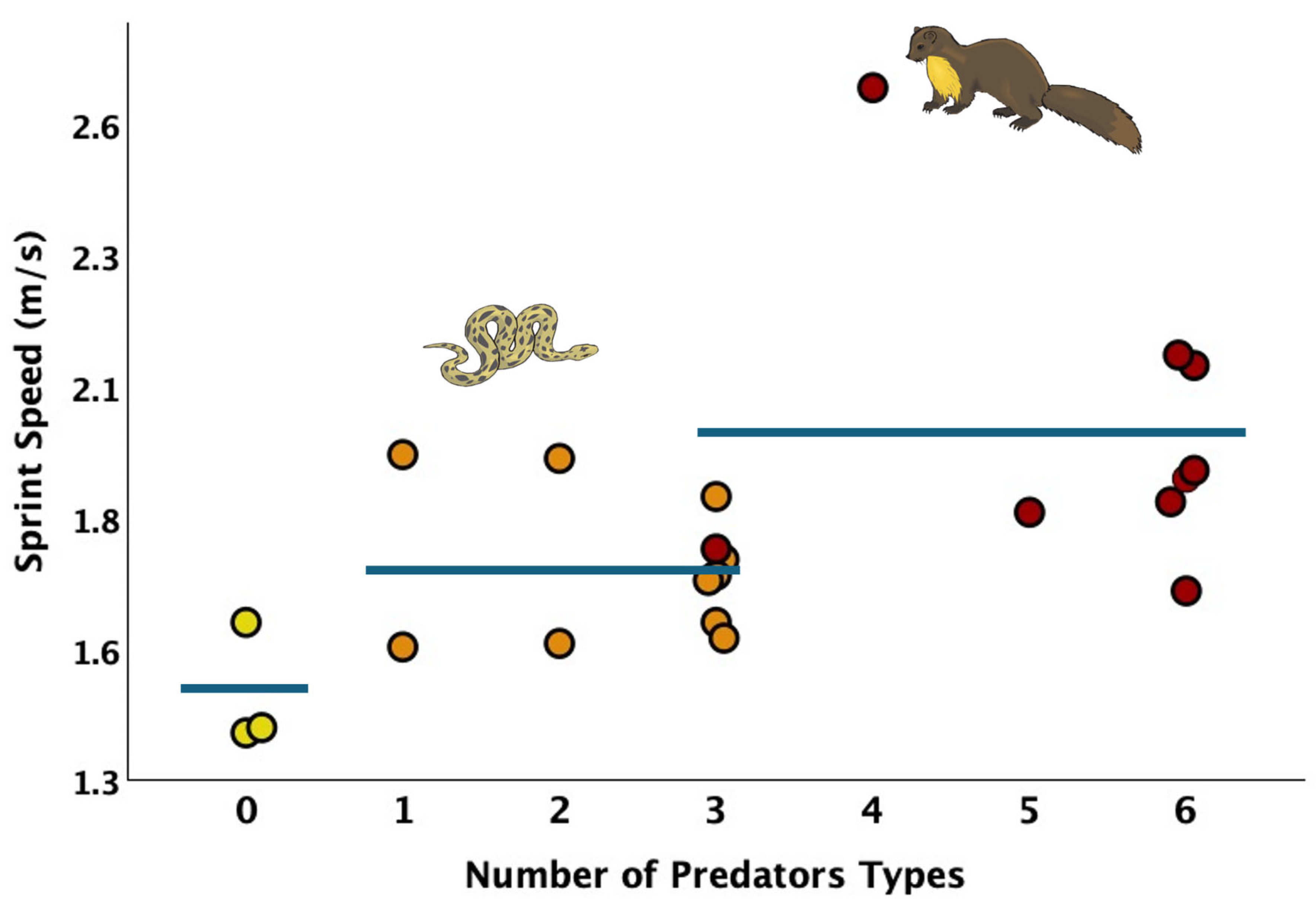
3.1. Variation and Simple Correlations
3.2. Model Selection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spatz, D.R.; Zilliacus, K.M.; Holmes, N.D.; Butchart, S.H.M.; Genovesi, P.; Cerballos, G.; Tershy, B.R.; Croll, D.A. Globally threatened vertebrates on islands with invasive species. Sci. Adv. 2017, 3, e1603080. [Google Scholar] [CrossRef]
- Sih, A.; Bolnick, D.I.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Pintor, L.M.; Preisser, E.; Rehage, J.S.; Vonesh, J.R. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 2010, 119, 610–621. [Google Scholar] [CrossRef]
- Brock, K.; Bednekoff, P.; Pafilis, P.; Foufopoulos, J. Evolution of antipredator defenses in island lizard species Podarcis erhardii (Reptilia: Lacertidae). The sum of all fears? Evolution 2015, 69, 216–231. [Google Scholar] [CrossRef]
- Martin, J.; Lopez, P. An experimental test of the costs of antipredatory refuge use in the wall lizard, Podarcis muralis. Oikos 1999, 84, 499–505. [Google Scholar] [CrossRef]
- Downes, S. Trading heat and food for safety: Costs of predator avoidance in a lizard. Ecology 1999, 82, 2870–2881. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Daniel, J.C. Isolation from mammalian predators differentially affects two congeners. Behav. Ecol. 2002, 13, 657–663. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Daniel, J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 2005, 272, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Alcover, J.A.; McMinn, M. Predators of vertebrates on islands. BioScience 1994, 44, 12–18. [Google Scholar] [CrossRef]
- Blazquez, M.C.; Rodriguez-Estrella, R.; Delibes, M. Escape behavior and predation risk of mainland and island Spiny-tailed iguanas (Ctenosaura hemilopha). Ethology 1997, 103, 990–998. [Google Scholar] [CrossRef]
- Berger, S.; Wikelski, M.; Romero, M.L.; Kalko, E.K.V.; Rödl, T. Behavioral and physiological adjustments to new predators in an endemic island species, the Galápagos marine iguana. Horm. Behav. 2007, 52, 653–663. [Google Scholar] [CrossRef]
- Cooper, W.E.; Pérez-Mellado, V. Historical influence of predation pressure on escape by Podarcis lizards in the Balearic Islands. Biol. J. Linn. Soc. 2012, 107, 254–268. [Google Scholar] [CrossRef]
- Novosolov, M.; Raia, P.; Meiri, S. The island syndrome in lizards. Glob. Ecol. Biogeogr. 2012, 22, 1466–8238. [Google Scholar] [CrossRef]
- Li, B.; Belasen, A.; Pafilis, P.; Bednekoff, P.; Foufopoulos, J. Effects of feral cats on the evolution of anti-predator behaviors in island reptiles: Insights from an ancient introduction. Proc. R. Soc. B 2014, 281, 20140339. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Rysman, J.F.; Leroy, B.; Claud, C.; Mace, G.M. A global picture of biological invasion threat on islands. Nat. Ecol. Evol. 2017, 1, 1862. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Cassey, P.; Duncan, R.P.; Evans, K.I.; Gaston, K.J. Avian extinction and mammalian introductions on oceanic islands. Science 2004, 305, 1955–1958. [Google Scholar] [CrossRef]
- Duncan, R.P.; Blackburn, T.M. Extinction and endemism in the New Zealand avifauna. Glob. Ecol. Biogeogr. 2004, 13, 1466–8238. [Google Scholar] [CrossRef]
- Nogales, M.; Martín, A.; Tershy, B.R.; Donlan, C.J.; Veitch, D.; Puerta, N.; Wood, B.; Alonso, J. A review of feral cat eradication on islands. Conserv. Biol. 2004, 18, 1523–1739. [Google Scholar] [CrossRef]
- Bauwens, D.; Garland, T., Jr.; Castilla, A.M.; Van Damme, R. Evolution of sprint speed in Lacertid lizards: Morphological, physiological and behavioral. Evolution 1995, 49, 848–863. [Google Scholar]
- Husak, J.F. Does survival depend on how fast you can run or how fast you do run? Funct. Ecol. 2006, 20, 1080–1086. [Google Scholar] [CrossRef]
- Husak, J.F.; Fox, S.F. Field use of maximal sprint speed by collared lizards (Crotaphytus collaris): Compensation and sexual selection. Evolution 2006, 60, 1888–1895. [Google Scholar] [PubMed]
- Losos, J.B. The evolution of form and function: Morphology and locomotor performance in West Indian Anolis lizards. Evolution 1990, 44, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Irschick, D.J.; Losos, J.B. Do lizards avoid habitats in which performance is submaximal? The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Am. Nat. 1999, 154, 293–305. [Google Scholar] [CrossRef]
- Husak, J.F.; Fox, S.F.; Lovern, M.B.; Van Den Bussche, R.A. Faster lizards sire more offspring sexual selection on whole-animal performance. Evolution 2006, 60, 2122–2130. [Google Scholar]
- van Damme, R.; Aerts, P.; Vanhooydonk, B. Variation in morphology, gait characteristics, and speed locomotion of two populations of lizards. Biol. J. Linn. Soc. 1998, 63, 409–427. [Google Scholar] [CrossRef]
- Vervust, B.; Grbac, I.; van Damme, R. Differences in morphology, performance and behaviour between recently diverged populations of Podarcis sicula mirror differences in predation pressure. Oikos 2007, 116, 1343–1352. [Google Scholar] [CrossRef]
- Pinch, F.C.; Claussen, D.L. Effects of temperature and slope on the sprint speed and stamina of the Eastern fence lizard, Sceloporus undulatus. J. Herpetol. 2003, 37, 671–679. [Google Scholar] [CrossRef]
- Brana, F. Morphological correlates of burst speed and field movement patterns: The behavioural adjustment of locomotion in wall lizards (Podarcis muralis). Biol. J. Linn. Soc. 2003, 80, 135–146. [Google Scholar] [CrossRef]
- Martin, J.; Avery, R.A. Effects of tail loss on the movement patterns of the lizard, Psammodromus algirus. Funct. Ecol. 1998, 12, 794–802. [Google Scholar] [CrossRef]
- Avery, R.A.; Mueller, C.F.; Jones, S.M.; Smith, J.A.; Bond, D.J. Speeds and movement patterns of European lacertid lizards: A comparative study. J. Herpetol. 1987, 21, 324–329. [Google Scholar] [CrossRef]
- Irschick, D.J. Effects of behaviour and ontogeny on the locomotor performance of a West Indian lizard, Anolis lineatopus. Funct. Ecol. 2000, 14, 1365–2435. [Google Scholar] [CrossRef]
- Parker, S.E.; McBrayer, L.D. The effects of multiple obstacles on the locomotor behavior and performance of a terrestrial lizard. J. Exp. Biol. 2016, 219, 1004–1013. [Google Scholar] [CrossRef]
- Losos, J.B.; Creer, D.A.; Schulte, J.A., 2nd. Cautionary comments on the measurement of maximum locomotor capabilities. J. Zool. Lond. 2002, 258, 57–61. [Google Scholar] [CrossRef]
- Pafilis, P.; Foufopoulos, J.; Poulakakis, N.; Lymberakis, P.; Valakos, E.D. Tail shedding in island lizards [Lacertidae, Reptilia]: Decline in antipredator defenses in relaxed predation environments. Evolution 2009, 63, 1262–1278. [Google Scholar] [CrossRef]
- Foufopoulos, J.; Ives, A.R. Reptile extinctions on land-bridge islands: Life-history attributes and vulnerability to extinction. Am. Nat. 1999, 153, 1–25. [Google Scholar] [CrossRef]
- Valakos, E.D.; Pafilis, P.; Sotiropoulos, K.; Lymberakis, P.; Maragou, P. The Amphibians and Reptiles of Greece; Frankfurt Contributions to Natural History; Edition Chimaira: Frankfurt, Germany, 2008. [Google Scholar]
- Fielding, J.; Turland, N. Flowers of Crete, 2nd ed.; Royal Botanic Gardens, Kew: London, UK, 2008. [Google Scholar]
- Brock, K.; Donihue, C.M.; Pafilis, P. New records of frugivory and ovophagy in Podarcis (Lacertidae) lizards from East Mediterranean Islands. North-West. J. Zool. 2014, 10, 223–225. [Google Scholar]
- Itescu, Y.; Schwarz, R.; Donihue, C.M.; Slavenko, A.; Roussos, S.A.; Sagonas, K.; Valakos, E.D.; Foufopoulos, J.; Pafilis, P.; Meiri, S. Inconsistent patterns in body size evolution in co-occurring island reptiles. Glob. Ecol. Biogeogr. 2018, 27, 538–550. [Google Scholar] [CrossRef]
- Adamopoulou, C.; Linardaki, E.; Thanou, E. Revisiting Spanopoula islet: Podarcis erhardii (Bedriaga, 1886) population thriving 45 years after first recorded. Herpetozoa 2025, 38, 93–95. [Google Scholar] [CrossRef]
- Santonastaso, T.; Lighten, J.; van Oosterhout, C.; Jones, K.L.; Foufopoulos, J.; Anthony, N.M. The effects of historical fragmentation on major histocompatibility complex class II β and microsatellite variation in the Aegean island reptile, Podarcis erhardii. Ecol. Evol. 2017, 7, 4568–4581. [Google Scholar] [CrossRef]
- Hurston, H.; Voith, L.; Bonanno, J.; Foufopoulos, J.; Pafilis, P.; Valakos, E.; Anthony, N. Effects of fragmentation on genetic diversity in island populations of the Aegean wall lizard Podarcis erhardii (Lacertidae, Reptilia). Mol. Phylogenetics Evol. 2009, 52, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Poulakakis, N.; Lymberakis, P.; Antoniou, A.; Chalkia, D.; Zouros, E.; Mylonas, M.; Valakos, E.D. Molecular phylogeny and biogeography of the wall-lizard Podarcis erhardii (Squamata: Lacertidae). Mol. Phylogenetics Evol. 2003, 28, 38–46. [Google Scholar] [CrossRef]
- Simou, C.; Pafilis, P.; Skella, A.; Kourkouli, A.; Valakos, E.D. Physiology of original and regenerated tails in Aegean wall lizard (Podarcis erhardii). Copeia 2008, 3, 504–509. [Google Scholar] [CrossRef]
- Peck, D.R.; Faulquier, L.; Pinet, P.; Jaquemet, S.; Le Corre, M. Feral cat diet and impact on sooty terns at Juan de Nova Island, Mosambique Channel. Anim. Conserv. 2008, 11, 65–74. [Google Scholar] [CrossRef]
- Fuller, P.O.; Higham, T.E.; Clark, A.J. Posture, speed, and habitat structure: Three-dimensional hindlimb kinematics of two species of padless geckos. Zoology 2011, 114, 104–112. [Google Scholar] [CrossRef]
- Gifford MEHerrel, A.; Mahler, D.L. The evolution of locomotor morphology, performance, and anti-predator behaviour among populations of Leiocephalus lizards from the Dominican Republic. Biol. J. Linn. Soc. 2008, 93, 445–456. [Google Scholar] [CrossRef][Green Version]
- Donihue, C.M. Aegean wall lizards switch foraging modes, diet, and morphology in a human-built environment. Ecol. Evol. 2016, 6, 7433–7442. [Google Scholar] [CrossRef] [PubMed]
- Donihue, C.M. Microgeographic variation in locomotor traits among lizards in a human-built environment. PeerJ 2016, 4, e1776. [Google Scholar] [CrossRef]
- Garland, T., Jr. Physiological correlates of locomotory performance in a lizard: An allometric approach. Am. J. Physiol. 1984, 247, R806–R815. [Google Scholar] [CrossRef]
- Avery, R.A.; Mueller, C.F.; Jones, S.M.; Smith, J.A.; Bond, D.J. The movement patterns of lacertid lizards: Speed, gait, and pauses in Lacerta vivipara. J. Zool. 1987, 211, 47–63. [Google Scholar] [CrossRef]
- Smith, S. A study of prey-attack behavior in young loggerhead shrikes, Lanius ludovicianus. Behavior 1976, 44, 113–141. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Information and likelihood theory: A basis for model selection and inference. In Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 3rd ed.; Springer: New York, NY, USA, 2004; pp. 60–77. [Google Scholar]
- Keller, L.F. Inbreeding and its fitness effects in an insular population of song sparrows (Melospiza melodia). Evolution 1998, 52, 240–250. [Google Scholar]
- Garland, T., Jr.; Bennet, A.F.; Daniels, C.B. Heritability of locomotor performance and its correlates in a natural population. Experientia 1990, 46, 530–533. [Google Scholar] [CrossRef]
- Manenti, T.; Pertoldi, C.; Nasiri, N.; Fristrup Schou, M.; Kjærsgaard, A.; Cavicche, S.; Loeschcke, V. Inbreeding affects locomotor activity in Drosophilia melanogaster at different ages. Behav. Genet. 2015, 45, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Stadler, S.R.; Brock, K.M.; Bednekoff, P.A.; Foufopoulos, J. More and bigger lizards reside on islands with more resources. J. Zool. 2023, 319, 163–174. [Google Scholar] [CrossRef]
- Rödl, T.; Berger, S.; Romero, L.; Wikelski, M. Tameness and stress physiology in a predator-naive island species confronted with novel predation threat. Proc. R. Soc. Lond. B 2007, 247, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.M.; Schumann, N.; Hoskins, A.J.; Daugherty, C.H.; Towns, D.R.; Chapple, D.G. Predictors of translocation success of captive-reared lizards: Implications for their captive management. Anim. Conserv. 2020, 23, 320–329. [Google Scholar] [CrossRef]
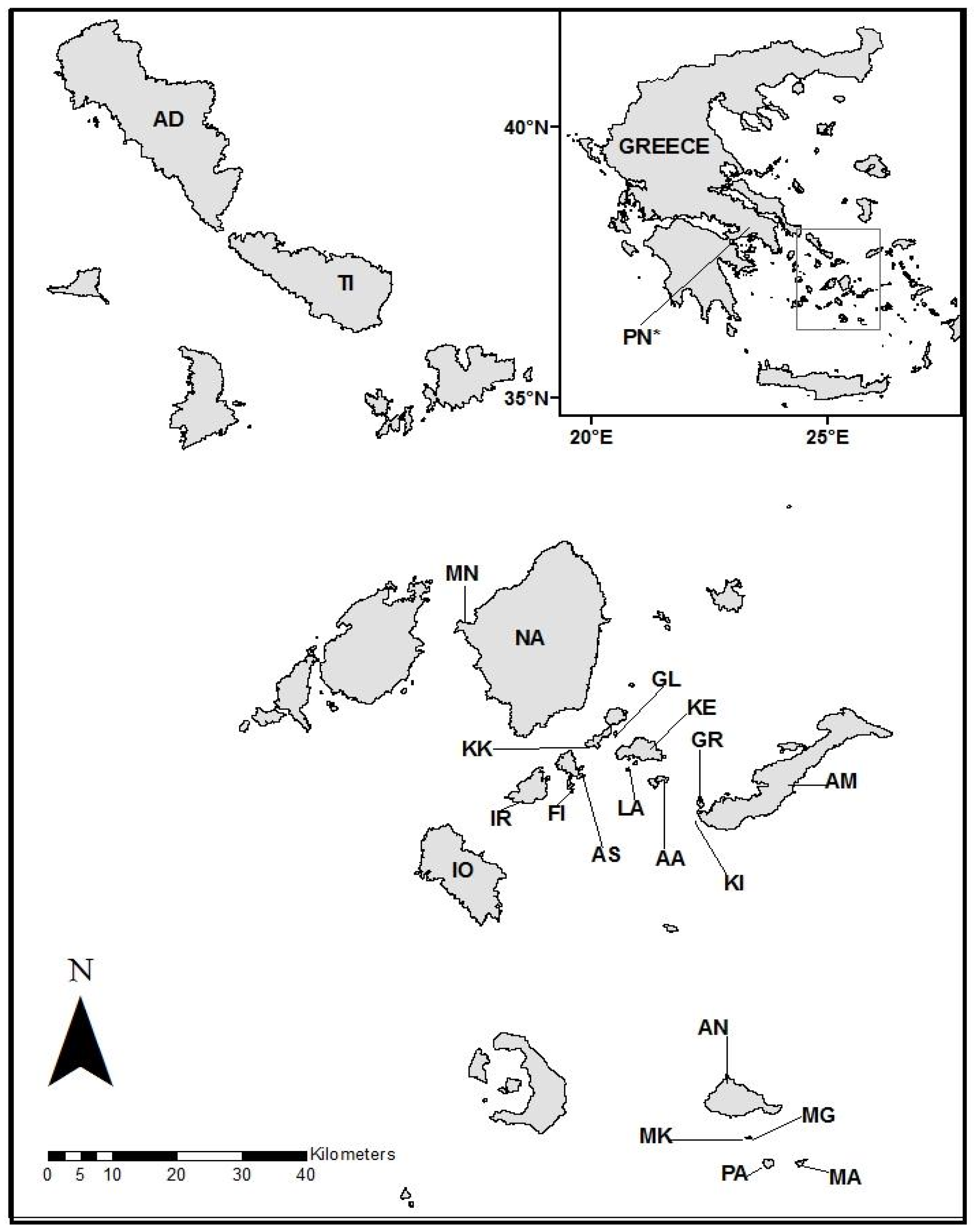

| Island | Number of Types of Predators | Types of Predators Present | Mean Sprint Speed (m/s) | Isolation Period (Years) | Island Area (km2) | Mean SVL (mm) | Mean Hindspan (mm) | Percent Openness | Lizard Density Ind/100 m |
|---|---|---|---|---|---|---|---|---|---|
| Lazaros (LA) | 0 | None | 1.39 | 9100 | 0.01 | 68.75 | 73.43 | 61 | 13 |
| Megalo Fteno (MG) | 0 | None | 1.40 | 9580 | 0.06 | 59.91 | 62.13 | 57 | 20 |
| Mikro Fteno (MK) | 0 | None | 1.60 | 5000 | 0.03 | 54.12 | 59.41 | 70 | 4 |
| Aspronissi (AS) | 1 | r | 1.55 | 5450 | 0.04 | 60.84 | 66.41 | 68 | 8 |
| Pachia (PA) | 1 | r | 1.92 | 11,850 | 1.36 | 55.92 | 58.71 | 61 | 7 |
| Kisiri (KI) | 2 | r, b | 1.56 | 5700 | 0.01 | 56.31 | 61.69 | 83 | 2 |
| Glaronissi (GL) | 2 | r, b | 1.91 | 5650 | 0.16 | 60.41 | 65.23 | 88 | 3 |
| Ano Antikeros (AA) | 3 | r, sb, b | 1.57 | 15,150 | 1.05 | 59.16 | 62.50 | 61 | 1 |
| Keros (KE) | 3 | r, sb, b | 1.60 | 9150 | 15.05 | 56.52 | 60.45 | 47 | 5 |
| Makria (MA) | 3 | r, sb, b | 1.68 | 13,500 | 0.5 | 56.49 | 63.22 | 67 | 5 |
| Gramvoussa (GR) | 3 | r, sb, b | 1.69 | 6700 | 0.76 | 59.60 | 63.90 | 60 | 1 |
| Fidussa (FI) | 3 | r, sb, b | 1.72 | 600 | 0.63 | 60.01 | 65.83 | 77 | 6 |
| Anafi (AN) | 3 | r, b, M | 1.74 | 3,600,000 | 49 | 57.30 | 61.99 | 85 | 4 |
| Kato Koufonissi (KK) | 3 | r, sb, b | 1.84 | 9100 | 4.30 | 58.46 | 67.17 | 67 | 5 |
| Mando (MN) | 4 | r, sb, b, M | 2.62 | 7 | 0.025 | 62.53 | 70.41 | 71 | 2 |
| Amorgos (AM) | 5 | r, sb, b, os, M | 1.81 | 200,000 | 123 | 61.91 | 69.63 | 47 | 4 |
| Iraklia (IR) | 6 | r, sb, v, b, os, M | 1.66 | 9800 | 18.08 | 59.40 | 63.71 | 70 | 4 |
| Ios (IO) | 6 | r, sb, v, b, os, M | 1.83 | 11,750 | 109.03 | 64.40 | 71.61 | 80 | 2 |
| Naxos (NA) | 6 | r, sb, v, b, os, M | 1.87 | 8700 | 448 | 60.87 | 69.22 | 74 | 6 |
| Andros (AD) | 6 | r, sb, v, b, os, M | 1.89 | 5800 | 379.95 | 68.49 | 77.60 | 71 | 2 |
| Parnitha (PN*) (* Mainland) | 6 | r, sb, v, b, os, M | 2.09 | 0 | 1000 | 65.22 | 82.86 | 67 | 4 |
| Tinos (TI) | 6 | r, sb, v, b, os, M | 2.11 | 5800 | 194.5 | 67.36 | 77.69 | 87 | 1 |
| Sprint Speed (Log10-Transformed) | |||
|---|---|---|---|
| Morphology | Pearson r | p-Value | n |
| Body Temperature | 0.239 | <0.0001 | 526 |
| SVL (Log10-transformed) | 0.087 | 0.045 | 526 |
| Hindlimb (Log10-transformed) | 0.234 | <0.0001 | 519 |
| Hindspan (Log10-transformed) | 0.195 | <0.0001 | 526 |
| Island Characteristics | |||
| Island Age (Log10-transformed) | −0.494 | 0.019 | 22 |
| Island Area (Log10-transformed) | 0.454 | 0.034 | 22 |
| Lizard Density (Log10-transformed) | −0.467 | 0.028 | 22 |
| Percent Openness | 0.331 | 0.132 | 22 |
| Number of Predator Types | 0.559 | 0.007 | 22 |
| Base Model | Model plus Island Age | |||
| Model Parameters | ΔAICc | Akaike Weight | ΔAICc | Akaike Weight |
| Mammals | 0 | 0.228 | 1.737 | 0.096 |
| Mammals + Zero Predation | 0.641 | 0.165 | 1.399 | 0.113 |
| Zero Predation | 1.357 | 0.116 | 2.896 | 0.054 |
| Birds + Zero Predation | 1.416 | 0.112 | 7.989 | 0.004 |
| Sum of All Fears | 3.231 | 0.045 | 6.654 | 0.008 |
| Mammals + Birds | 4.444 | 0.025 | 6.531 | 0.009 |
| Birds | 4.808 | 0.021 | 7.551 | 0.005 |
| R+SB+V+B+OS+M | 16.616 | 5.61 × 10−5 | 17.296 | 3.99 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semegen, S.L.; Fornberg, J.L.; Bednekoff, P.A.; Foufopoulos, J. What Need for Speed? Lizards from Islands Missing Predators Sprint Slower. Animals 2025, 15, 2651. https://doi.org/10.3390/ani15182651
Semegen SL, Fornberg JL, Bednekoff PA, Foufopoulos J. What Need for Speed? Lizards from Islands Missing Predators Sprint Slower. Animals. 2025; 15(18):2651. https://doi.org/10.3390/ani15182651
Chicago/Turabian StyleSemegen, Sarah L., Johanna L. Fornberg, Peter A. Bednekoff, and Johannes Foufopoulos. 2025. "What Need for Speed? Lizards from Islands Missing Predators Sprint Slower" Animals 15, no. 18: 2651. https://doi.org/10.3390/ani15182651
APA StyleSemegen, S. L., Fornberg, J. L., Bednekoff, P. A., & Foufopoulos, J. (2025). What Need for Speed? Lizards from Islands Missing Predators Sprint Slower. Animals, 15(18), 2651. https://doi.org/10.3390/ani15182651






