Histopathological and Immunohistochemical Study of Neoplastic Cell Heterogeneity in Early and Advanced Ovine Pulmonary Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Immunohistochemistry General Procedure
2.3. Confirmation of JSRV Infection and Investigations of OPA Tumor Histopathology, Growth Patterns, Mitotic Cell Count, Invasion, and Ki67 Labeling
2.4. Markers for Tumor Cell Assessment
2.5. Anterior Grade Protein 2 (AGR2) Assessment
2.6. Statistical Methodology
3. Results
3.1. JSRV-ENV Immunohistochemistry
3.2. Histopathology, Mitotic Count, and Ki67 Index of the OPA Tumors
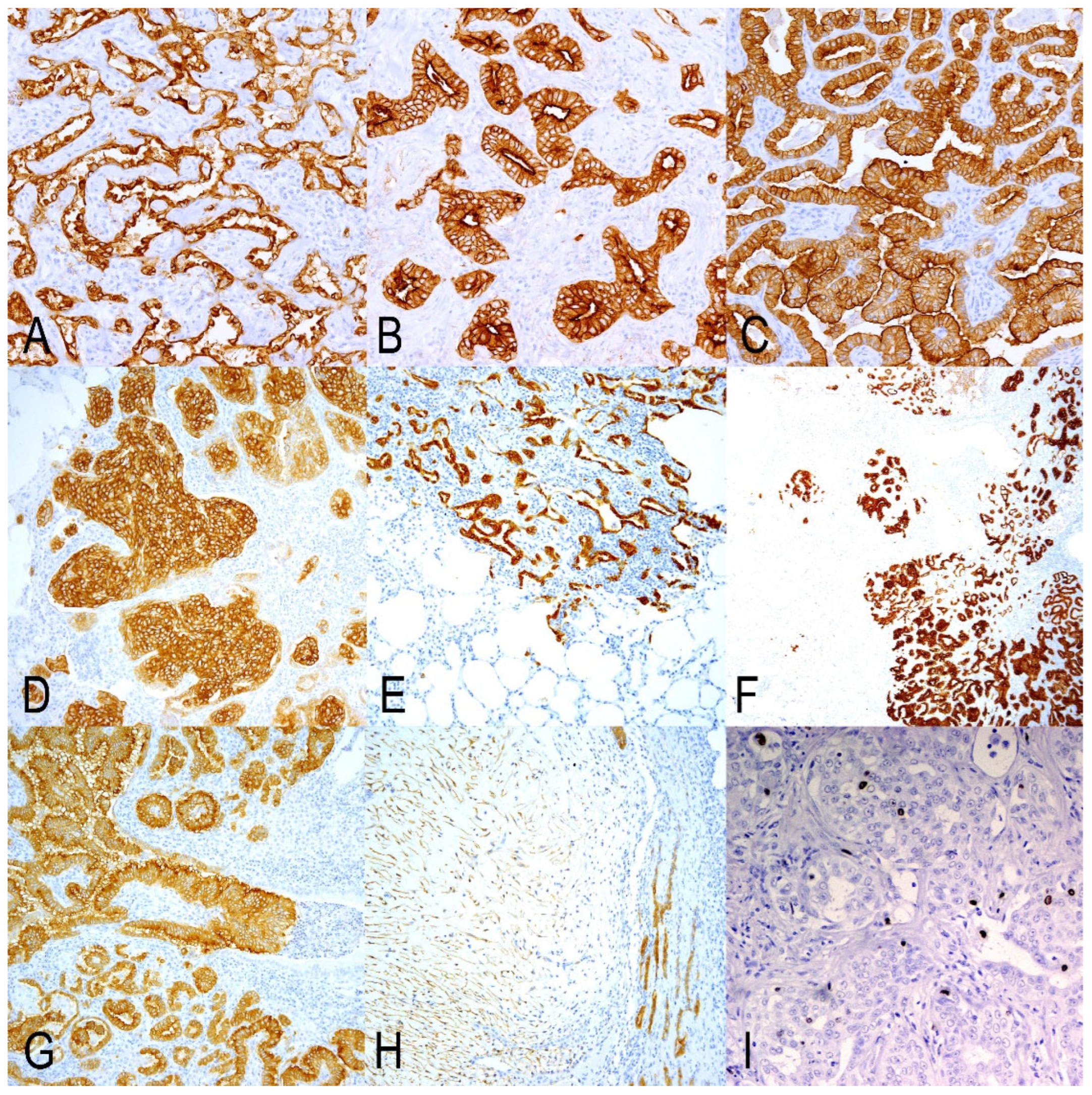
3.3. Markers of Secretory Cells of Terminal Bronchioloalveolar Respiratory Epithelium
3.4. Markers of Undifferentiated/Progenitor Cells in Lung Repair
3.5. Upregulated Genes with Particular Interest in OPA Tumors
4. Discussion
4.1. JSRV Infections of Target Cells in Close Lung Areas Are a Key Factor in OPA Progression
4.2. Several Histological Changes Can Be Observed in OPA Tumors as Indicators of Tumor Complexity
4.3. Cancer Cell Heterogeneity Does Not Influence the Gross Variations in OPA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPA | Ovine pulmonary adenocarcinoma |
| JSRV | Jaagsiekte sheep retrovirus |
| ATII | Alveolar type II pneumocytes |
| CC | Club cells |
| GA | Group A |
| GB | Group B |
| GC | Group C |
| IHC | Immunohistochemistry |
| AGR2 | Anterior grade protein 2 |
| JSRV-ENV | Jaagsiekte sheep retrovirus envelope protein |
| ENV | Envelope structural protein |
| Ca | Capsid |
| RT | Room temperature |
| HE | Hematoxylin-Eosin |
| DC-LAMP | CD208/dendritic cell–Lysosomal associated membrane protein |
| SPC | prosurfactant Protein C |
| CC10-1 | Club cell proteins 1 |
| CC10-2 | Club cell proteins 2 |
| K5 | keratin 5 |
| p63 | tumor protein 63 |
| CD44 | cell surface glycoprotein CD44/Lymphocyte adhesion receptor |
| SPs | Surfactant proteins |
| HLC | Human lung cancer |
References
- Sharp, J.M.; DeMartini, J.C. Natural history of JSRV in sheep. Curr. Top. Microbiol. Immunol. 2003, 275, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J.; Martineau, H.M.; Cousens, C. Pathology and pathogenesis of ovine pulmonary adenocarcinoma. J. Comp. Pathol. 2010, 142, 260–283. [Google Scholar] [CrossRef] [PubMed]
- Ortín, A.; De las Heras, M.; Borobia, M.; Ramo, M.A.; Ortega, M.; Ruíz de Arcaute, M. Ovine pulmonary adenocarcinoma: A transmissible lung cancer of sheep, difficult to control. Small Rumin. Res. 2019, 176, 37–41. [Google Scholar] [CrossRef]
- De las Heras, M.; González, L.; Sharp, J.M. Pathology of ovine pulmonary adenocarcinoma. Curr. Top. Microbiol. Immunol. 2003, 275, 25–54. [Google Scholar] [CrossRef]
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [CrossRef]
- Fan, H.; Palmarini, M.; DeMartini, J.C. Transformation and oncogenesis by jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 2003, 275, 139–177. [Google Scholar] [CrossRef]
- Hofacre, A.; Fan, H. Jaagsiekte sheep retrovirus biology and oncogenesis. Viruses 2010, 2, 2618–2648. [Google Scholar] [CrossRef]
- Mornex, J.F.; Thivolet, F.; De las Heras, M.; Leroux, C. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Curr. Top. Microbiol. Immunol. 2003, 275, 225–248. [Google Scholar] [CrossRef]
- Youssef, G.; Wallace, W.A.H.; Dagleish, M.P.; Cousens, C.; Griffiths, D.J. Ovine pulmonary adenocarcinoma: A large animal model for human lung cancer. ILAR J. 2015, 56, 99–115. [Google Scholar] [CrossRef]
- Gray, M.E.; Sullivan, P.; Marland, J.R.K.; Greenhalgh, S.N.; Meehan, J.; Gregson, R.; Clutton, R.E.; Cousens, C.; Griffiths, D.J.; Murray, A.; et al. A novel translational ovine pulmonary adenocarcinoma model for human lung cancer. Front. Oncol. 2019, 9, 534. [Google Scholar] [CrossRef]
- Gray, M.E.; Meehan, J.; Sullivan, P.; Marland, J.R.K.; Greenhalgh, S.N.; Gregson, R.; Clutton, R.E.; Ward, C.; Cousens, C.; Griffiths, D.J.; et al. Ovine pulmonary adenocarcinoma: A unique model to improve lung cancer research. Front. Oncol. 2019, 9, 335. [Google Scholar] [CrossRef]
- De Las Heras Guillamón, M.; Borderías Clau, L. The sheep as a large animal experimental model in respiratory diseases research. Arch. Bronconeumol. Engl. Ed. 2010, 46, 499–501. [Google Scholar] [CrossRef]
- Monot, M.; Archer, F.; Gomes, M.; Mornex, J.F.; Leroux, C. Advances in the study of transmissible respiratory tumours in small ruminants. Vet. Microbiol. 2015, 181, 170–177. [Google Scholar] [CrossRef]
- Karagianni, A.E.; Vasoya, D.; Finlayson, J.; Martineau, H.M.; Wood, A.R.; Cousens, C.; Dagleish, M.P.; Watson, M.; Griffiths, D.J. Transcriptional Response of Ovine Lung to Infection with Jaagsiekte Sheep Retrovirus. J. Virol. 2019, 93, e00876-19. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef]
- García-Goti, M.; González, L.; Cousens, C.; Cortabarría, N.; Extramiana, A.B.; Minguijón, E.; Ortín, A.; De las Heras, M.; Sharp, J.M. Sheep pulmonary adenomatosis: Characterization of two pathological forms associated with jaagsiekte retrovirus. J. Comp. Pathol. 2000, 122, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Corpa, J.M.; Castillo, D.; Murphy, B.G. Pathological spectrum of ovine pulmonary adenocarcinoma in small ruminants: A focus on the mixed form. Animals 2023, 13, 2828. [Google Scholar] [CrossRef]
- Wootton, S.K.; Metzger, M.J.; Hudkins, K.L.; Alpers, C.E.; York, D.; DeMartini, J.C.; Miller, A.D. Lung cancer induced in mice by the envelope protein of jaagsiekte sheep retrovirus (JSRV) closely resembles lung cancer in sheep infected with JSRV. Retrovirology 2006, 3, 94. [Google Scholar] [CrossRef]
- Caporale, M.; Cousens, C.; Centorame, P.; Pinoni, C.; De las Heras, M.; Palmarini, M. Expression of the jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep. J. Virol. 2006, 80, 8030–8037. [Google Scholar] [CrossRef]
- De las Heras, M.; de Martino, A.; Borobia, M.; Ortín, A.; Álvarez, R.; Borderías, L.; Giménez-Más, J.A. Solitary tumours associated with Jaagsiekte retrovirus in sheep are heterogeneous and contain cells expressing markers identifying progenitor cells in lung repair. J. Comp. Pathol. 2014, 150, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Cuba-Caparo, A.; De La Vega, E.; Copaira, M. Pulmonary adenomatosis of sheep--metastasizing bronchiolar tumors. Am. J. Vet. Res. 1961, 22, 673–682. [Google Scholar] [PubMed]
- Wilson, D.W. Tumors of the respiratory tract. In Tumors in Domestic Animals; Meuten, D.J., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 467–498. ISBN 9780813821795. [Google Scholar]
- Meuten, D.J.; Moore, F.M.; Donovan, T.A.; Bertram, C.A.; Klopfleisch, R.; Foster, R.A.; Smedley, R.C.; Dark, M.J.; Milovancev, M.; Stromberg, P.; et al. International guidelines for veterinary tumor pathology: A call to action. Vet. Pathol. 2021, 58, 766–794. [Google Scholar] [CrossRef] [PubMed]
- Salaun, B.; de Saint-Vis, B.; Pacheco, N.; Pacheco, Y.; Riesler, A.; Isaac, S.; Leroux, C.; Clair-Moninot, V.; Pin, J.-J.; Griffith, J.; et al. CD208/dendritic cell-lysosomal associated membrane protein is a marker of normal and transformed type II pneumocytes. Am. J. Pathol. 2004, 164, 861–871. [Google Scholar] [CrossRef]
- Platt, J.A.; Kraipowich, N.; Villafane, F.; DeMartini, J.C. Alveolar type II cells expressing jaagsiekte sheep retrovirus capsid protein and surfactant proteins are the predominant neoplastic cell type in ovine pulmonary adenocarcinoma. Vet. Pathol. 2002, 39, 341–352. [Google Scholar] [CrossRef]
- Phelps, D.S.; Floros, J. Localization of pulmonary surfactant proteins using immunohistochemistry and tissue in situ hybridization. Exp. Lung Res. 1991, 17, 985–995. [Google Scholar] [CrossRef]
- Kalina, M.; Mason, R.J.; Shannon, J.M. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am. J. Respir. Cell Mol. Biol. 1992, 6, 594–600. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.-A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 2015, 517, 616–620. [Google Scholar] [CrossRef]
- Dekker, S.; van Geemen, D.; van den Bogaerdt, A.J.; Driessen-Mol, A.; Aikawa, E.; Smits, A.I.P.M. Sheep-Specific Immunohistochemical Panel for the Evaluation of Regenerative and Inflammatory Processes in Tissue-Engineered Heart Valves. Front. Cardiovasc. Med. 2018, 5, 105. [Google Scholar] [CrossRef]
- Alavi, M.; Mah, V.; Maresh, E.L.; Bagryanova, L.; Horvath, S.; Chia, D.; Goodglick, L.; Liu, A.Y. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer 2015, 15, 655. [Google Scholar] [CrossRef]
- Cousens, C.; Meehan, J.; Collie, D.; Wright, S.; Chang, Z.; Todd, H.; Moore, J.; Grant, L.; Daniel, C.R.; Tennant, P.; et al. Tracking ovine pulmonary adenocarcinoma development using an experimental jaagsiekte sheep retrovirus infection model. Genes 2024, 15, 1019. [Google Scholar] [CrossRef]
- Liu, S.L.; Miller, A.D. Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein. Oncogene 2007, 26, 789–801. [Google Scholar] [CrossRef]
- Minguijón, E.; González, L.; De las Heras, M.; Gómez, N.; García-Goti, M.; Juste, R.A.; Moreno, B. Pathological and aetiological studies in sheep exhibiting extrathoracic metastasis of ovine pulmonary adenocarcinoma (Jaagsiekte). J. Comp. Pathol. 2013, 148, 139–147. [Google Scholar] [CrossRef]
- Cousens, C.; Bishop, J.V.; Philbey, A.W.; Gill, C.A.; Palmarini, M.; Carlson, J.O.; DeMartini, J.C.; Sharp, J.M. Analysis of integration sites of Jaagsiekte sheep retrovirus in ovine pulmonary adenocarcinoma. J. Virol. 2004, 78, 8506–8512. [Google Scholar] [CrossRef] [PubMed]
- Tavernari, D.; Battistello, E.; Dheilly, E.; Petruzzella, A.S.; Mina, M.; Sordet-Dessimoz, J.; Peters, S.; Krueger, T.; Gfeller, D.; Riggi, N.; et al. Nongenetic evolution drives lung adenocarcinoma spatial heterogeneity and progression. Cancer Discov. 2021, 11, 1490–1507. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO classification of lung tumors: Impact of advances since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Sekine, S.; Sato, T. Decoding the basis of histological variation in human cancer. Nat. Rev. Cancer 2024, 24, 141–158. [Google Scholar] [CrossRef]
- Martineau, H.M.; Cousens, C.; Imlach, S.; Dagleish, M.P.; Griffiths, D.J. Jaagsiekte sheep retrovirus infects multiple cell types in the ovine lung. J. Virol. 2011, 85, 3341–3355. [Google Scholar] [CrossRef]
- Murgia, C.; Caporale, M.; Ceesay, O.; Di Francesco, G.; Ferri, N.; Varasano, V.; de las Heras, M.; Palmarini, M. Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair. PLoS Pathog. 2011, 7, e1002014. [Google Scholar] [CrossRef]
- Flecknoe, S.J.; Wallace, M.J.; Cock, M.L.; Harding, R.; Hooper, S.B. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L664–L670. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y. Heterogeneous groups of alveolar type II cells in lung homeostasis and repair. Am. J. Physiol.-Cell Physiol. 2020, 319, C991–C996. [Google Scholar] [CrossRef]
- Blackburn, J.B.; Li, N.F.; Bartlett, N.W.; Richmond, B.W. An update in club cell biology and its potential relevance to chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L652–L665. [Google Scholar] [CrossRef]
- Napoli, M.; Wu, S.J.; Gore, B.L.; Abbas, H.A.; Lee, K.; Checker, R.; Dhar, S.; Rajapakshe, K.; Tan, A.C.; Lee, M.G.; et al. ΔNp63 regulates a common landscape of enhancer associated genes in non-small cell lung cancer. Nat. Commun. 2022, 13, 614. [Google Scholar] [CrossRef]
- De Las Heras, M.; Ortín, A.; Benito, A.; Summers, C.; Ferrer, L.M.; Sharp, J.M. In-situ demonstration of mitogen-activated protein kinase Erk 1/2 signalling pathway in contagious respiratory tumours of sheep and goats. J. Comp. Pathol. 2006, 135, 1–10. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Yin, L.; Yao, Z.; Tong, R.; Xue, J.; Lu, Y. Lung cancer stem cell markers as therapeutic targets: An update on signaling pathways and therapies. Front. Oncol. 2022, 12, 873994. [Google Scholar] [CrossRef]
- Firan, M.; Dhillon, S.; Estess, P.; Siegelman, M.H. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood 2006, 107, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Brychtova, V.; Mohtar, A.; Vojtesek, B.; Hupp, T.R. Mechanisms of anterior gradient-2 regulation and function in cancer. Semin. Cancer Biol. 2015, 33, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wootton, S.K.; Halbert, C.L.; Miller, A.D. Sheep retrovirus structural protein induces lung tumours. Nature 2005, 434, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Martineau, H.; De las Heras, M.; Murgia, C.; Huang, R.; Centorame, P.; Di Francesco, G.; Di Gialleonardo, L.; Spencer, T.E.; Griffiths, D.J.; et al. Host species barriers to Jaagsiekte sheep retrovirus replication and carcinogenesis. J. Virol. 2013, 87, 10752–10762. [Google Scholar] [CrossRef]
- Sharp, J.M.; Angus, K.W.; Jassim, F.A.; Scott, F.M. Experimental transmission of sheep pulmonary adenomatosis to a goat. Vet. Rec. 1986, 119, 245. [Google Scholar] [CrossRef]
- Tustin, R.C.; Williamson, A.L.; York, D.F.; Verwoerd, D.W. Experimental transmission of jaagsiekte (ovine pulmonary adenomatosis) to goats. Onderstepoort J. Vet. Res. 1988, 55, 27–32. [Google Scholar]
- Devi, V.R.; Manasa, B.B.; Samatha, V.; Mahesh, M.; Srikanth, K.V.; Rao, T.Y.; Satheesh, K.; Kishore, K.V.S.N. Pathology of natural cases of ovine pulmonary adenocarcinoma (Jaagsiekte) in goats. Braz. J. Vet. Pathol. 2016, 9, 108–112. [Google Scholar]
- Sanna, M.P.; Sanna, E.; De Las Heras, M.; Leoni, A.; Nieddu, A.M.; Pirino, S.; Sharp, J.M.; Palmarini, M. Association of jaagsiekte sheep retrovirus with pulmonary carcinoma in Sardinian moufflon (Ovis musimon). J. Comp. Pathol. 2001, 125, 145–152. [Google Scholar] [CrossRef]


| Antibody | Species | Source | Catalog Number | Dilution |
|---|---|---|---|---|
| AGR2 | Rabbit (P) | Abcam | Ab227584 | 1:300 |
| CD44 | Rabbit (P) | Abcam | Ab24505 | 1:1000 |
| CC10-1 | Rabbit (P) | Biovendor Lab Med Inc | RD181022220 | 1:2000 |
| CC10-2 | Rabbit (P) | Claudio Murgia | Not commercial | 1:15,000 |
| DC-LAMP | Rabbit (P) | Immunoytech | PNIM3448 | 1:2500 |
| JSRV-ENV | Mouse (M) | Dusty Miller | Not commercial | 1:500 |
| K5 | Rabbit (P) | Biorbyt | Orb-128270 | 1:8000 |
| Ki67 | Rabbit (P) | ThermoFischer Sci. | RM-1906 | 1:200 |
| SPC | Rabbit (P) | Abcam | Ab28744 | 1:10,000 |
| p63 | Mouse (M) | Novus Biologicals | NB-100-691 | 1:200 |
| Groups | Histopathology Tumor Patterns (%) | Local Invasion | Bronchial Lesion/Section | Mitoses/2.73 mm2 | Ki67 Index | Myxoid Nodules | |||
|---|---|---|---|---|---|---|---|---|---|
| Lepidic | Acinar | Papillary | Solid | ||||||
| A | 32.1 | 9.5 | 51.2 | 7.2 | 8/10 | 1.30 | 0.088 | 2.96 | 2/10 |
| B | 3.3 | 24.0 | 72.9 | 0 | 10/10 | 0.22 | 0.166 | 0.63 | 0/10 |
| C | 3.9 | 5.5 | 90.6 | 0 | 10/10 | 2.50 | 0.077 | 1.76 | 1/10 |
| Marker | Group | Median | Interval (25–75) | Significance (p) | |||
|---|---|---|---|---|---|---|---|
| Groups | GA vs. GB | GA vs. GC | GB vs. GC | ||||
| SPC | A | 0.066 | 0.0000–0.9571 | 0.046 | 0.229 | 0.017 | 0.151 |
| B | 0.318 | 0.0279–1.0000 | |||||
| C | 0.852 | 0.1612–1.0000 | |||||
| DC LAMP | A | 0.009 | 0.0000–0.9932 | 0.030 | 0.071 | 0.019 | 0.154 |
| B | 0.386 | 0.0646–0.9437 | |||||
| C | 0.869 | 0.0886–0.9943 | |||||
| CC10-1 | A | 0.126 | 0.0000–0.9931 | <0.001 | 0.003 | <0.001 | 0.251 |
| B | 0.000 | 0.0000–0.1102 | |||||
| C | 0.000 | 0.0000–0.0199 | |||||
| CC10-2 | A | 0.087 | 0.0235–0.7966 | <0.001 | 0.003 | <0.001 | 0.002 |
| B | 0.009 | 0.0000–0.1058 | |||||
| C | 0.000 | 0.0000–0.0000 | |||||
| K5 | A | 0.000 | 0.0000–0.0307 | 0.004 | 0.030 | 0.003 | 0.379 |
| B | 0.000 | 0.0000–0.0000 | |||||
| C | 0.000 | 0.0000–0.0000 | |||||
| p63 | A | 0.041 | 0.0000–0.0239 | 0.001 | <0.001 | 0.088 | 0.024 |
| B | 0.003 | 0.0000–0.0088 | |||||
| C | 0.012 | 0.0000–0.0646 | |||||
| AGR2 | A | 0.850 | 0.7317–0.9151 | 0.251 | 0.095 | 0.322 | 0.554 |
| B | 0.715 | 0.4789–0.8958 | |||||
| C | 0.765 | 0.5706–0.9267 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reséndiz-Pozos, R.A.; González-Saínz, J.M.; Ortín, A.; Asin, J.; Climent, M.; Borderías, L.; De las Heras, M. Histopathological and Immunohistochemical Study of Neoplastic Cell Heterogeneity in Early and Advanced Ovine Pulmonary Adenocarcinoma. Animals 2025, 15, 2632. https://doi.org/10.3390/ani15172632
Reséndiz-Pozos RA, González-Saínz JM, Ortín A, Asin J, Climent M, Borderías L, De las Heras M. Histopathological and Immunohistochemical Study of Neoplastic Cell Heterogeneity in Early and Advanced Ovine Pulmonary Adenocarcinoma. Animals. 2025; 15(17):2632. https://doi.org/10.3390/ani15172632
Chicago/Turabian StyleReséndiz-Pozos, Raúl A., Jose María González-Saínz, Aurora Ortín, Javier Asin, María Climent, Luis Borderías, and Marcelo De las Heras. 2025. "Histopathological and Immunohistochemical Study of Neoplastic Cell Heterogeneity in Early and Advanced Ovine Pulmonary Adenocarcinoma" Animals 15, no. 17: 2632. https://doi.org/10.3390/ani15172632
APA StyleReséndiz-Pozos, R. A., González-Saínz, J. M., Ortín, A., Asin, J., Climent, M., Borderías, L., & De las Heras, M. (2025). Histopathological and Immunohistochemical Study of Neoplastic Cell Heterogeneity in Early and Advanced Ovine Pulmonary Adenocarcinoma. Animals, 15(17), 2632. https://doi.org/10.3390/ani15172632





