Simple Summary
Land molluscs are one of the main contributors to global animal diversity, particularly to European fauna. Among Italian land gastropods, Trochoidea caroni (Deshayes, 1832) and T. elegans (Gmelin, 1791) are some of the most biogeographically interesting species. In fact, they belong to a group of species (“elegans group”) whose systematic position remains to be fully defined, despite being considered valid by most authors. T. caroni is currently an endemic species of the Italian peninsula and Sicily, while T. elegans has a western circum–Mediterranean distribution, but it was introduced to Great Britain and is rare in Belgium. It is not yet possible to draw certain conclusions on the unusual biogeography of these taxa, but our karyological data improve current knowledge on the phylogeography of T. caroni, and on the possible origin and evolution of these two taxa. We propose an evolutionary scenario for the chromosome rearrangements that occurred during the specific diversification of Trochoidea. Furthermore, the presence of NOR loci on the 16th pair of chromosomes in both Trochoideini and Cernuellini tribes suggests their derivation from a common ancestor.
Abstract
Trochoidea caroni (Gastropoda, Geomitridae) is a land snail previously found only in Sicily and Capri (Naples), but it has recently been found in other regions of the Italian peninsula. In this study, we performed karyological and molecular analysis on T. caroni from different sites across this regional range. Karyological analysis was performed on specimens from Palermo (Sicily), Capri (Campania), and Terracina (Lazio) using standard staining and NOR-FISH methods; the latter method was also performed on samples of T. elegans from Rome (Lazio). All T. caroni specimens had 2n = 48 chromosomes, but the 8th and 17th pairs differed morphologically between specimens from Capri and Terracina and those from Sicily. The mitochondrial 16S rRNA analysis grouped Sicilian and non-Sicilian populations of T. caroni in distinct subclades. Superimposing karyological data on their phylogenetic tree suggests that possible chromosomal rearrangements occurred during the diversification of Trochoidea. Our findings provide karyological and molecular evidence for a diversification between Sicilian and non-Sicilian populations of T. caroni. Furthermore, NOR-FISH revealed hybridisation signals on the 16th chromosome pair in both T. caroni and T. elegans (tribe Trochoideini). Similar NOR localisation has also been identified in Cernuella virgata (tribe Cernuellini), suggesting that it was inherited from the common ancestor of Trochoideini and Cernuellini.
1. Introduction
Geomitridae C. R. Boettger, 1909, is a widespread family of small- to medium-sized terrestrial snails distributed in northern Eurasia, the eastern Atlantic islands, North Africa, Madeira, the Azores, and the Canary Islands (Schileyko, 2006). The family Geomitridae family recently separated from the Hygromiidae (Razkin et al., 2015), includes numerous species (about 400) divided into 51 genera and 2 subfamilies—Geomitrinae C. R. Boettger, 1909, and Helicellinae Ihering, 1909—both of which consist of various tribes. The Geomitrinae family consists of the Cochlicellini Schileyko, 1972; Geomitrini C. Boettger, 1909; and Ponentinini Schileyko, 1991, tribes. Helicellinae Ihering, 1909, includes the following tribes: Cernuellini Schileyko, 1991; Helicellini Ihering, 1909; Helicopsini H. Nordsieck, 1987; Plentuisini Razkin, Gómez-Moliner, Prieto, Martínez-Ortí, Arrébola, Muñoz, Chueca and Madeira, 2015; and Trochoideini H. Nordsieck, 1987 [1].
Trochoidea Brown, 1827, is an interesting genus of the latter tribe, comprising both narrowly distributed species (also endemics) and forms with relatively wide distribution. According to Molluscabase [1], the following species are attributed to this genus: Trochoidea elegans (Gmelin, 1791), which is distributed in several European countries and North Africa (Spain, France, Italy, and Greece, and introduced in Great Britain, Tunisia, and Algeria); and T. pyramidata (Draparnaud, 1805) and T. trochoides (Poiret, 1789), which are widespread around the Mediterranean sea. The endemic taxa are as follows: T. cucullus (E. von Martens, 1875) and T. spratti (L. Pfeiffer, 1846), present in Malta; T. cumiae (Calcara, 1847), and T. liebetruti (Albers, 1852), living, respectively, in Lampedusa Island and Cyprus; T. hipponensis (Morelet, 1858), present in Algeria; T. hesperidum (Morelet, 1880) in Morocco; and T. caroni (Deshayes, 1832) and T. tarentina (L. Pfeiffer, 1848), endemic in Italy.
In Italy, the distribution area of the various taxa of Trochoidea is not clearly defined, except for that of T. caroni, locus typicus Sicily [2], previously reported as living in central-western Sicily and Capri [3,4,5]. New reports of living T. caroni specimens or their shells have recently been found in Latium, Tuscany (Museum specimen), and Campania, documenting a wider distribution of this taxon, unlike that previously hypothesised [6]. According to Bodon et al. [5], T. caroni has been introduced to peninsular Italy. Furthermore, the taxonomic status of various forms of Trochoidea is debated, particularly the ‘elegans’ species group, which includes T. elegans, T. caroni, T. spratti, and T. cumiae [7]. Molecular and karyological analyses are also largely unexplored in Trochoidea, providing relationships and karyotypes only of T. elegans, T. pyramidata, and T. trochoides [8,9,10,11].
Cytogenetic inferences, particularly when coupled with molecular data, have proven to be useful tools for identifying plesio- and apomorphic states and the presence of different evolutionary lineages and reproductive barriers, and for reconstructing the evolutionary trends of the studied taxa, including Gastropoda [12,13,14,15,16,17].
This paper presents the results of a karyological study conducted on T. caroni from different sites within its range, including Sicily (Palermo and Trapani) and sites outside of Sicily (Capri in Campania; Terracina in Lazio). Our findings were compared with those available in the literature concerning phylogenetically closely related species. The presence and distribution of primitive and derived chromosomal features are also discussed, and a proposal for the chromosomal diversification of Trochoidea is made. Preliminarily, to detect possible molecular diversification between Sicilian and non-Sicilian T. caroni populations, we also analysed a mitochondrial 16S rRNA gene fragment and performed a phylogenetic analysis that included other closely related Trochoidea species [8,9,10,18].
2. Materials and Methods
For karyological analysis, we used tissue samples of live T. caroni individuals: three samples from Capri (Naples, Campania, Italy), two samples from Terracina (Latina, Latium, Italy), four samples from Capaci (Palermo, Sicily, Italy), and two samples from Macari (Trapani, Sicily, Italy). All samples had already been analysed by Maio et al. [6], who discriminated them according to Giusti et al. [7], whose article details the origin of the specimens studied herein. For NOR-FISH staining, we also used chromosome samples of T. elegans from Santa Severa (Rome, Italy) that had already been used in our previous study [11]. No animals were sacrificed for this study.
2.1. Chromosome Analysis
Chromosomes were obtained as described in [11,14]. Briefly, gonads were incubated for three hours in 1 mL of calf serum containing 3 μg of a colcemid solution (10 μg/mL) and incubated for 30 min in hypotonic solution (0.35% sodium citrate solution + 0.28% KCl solution). Then, the gonads were fixed in 3:1 methyl alcohol + acetic acid for 15 min and scraped on a 100-mesh sieve. Cell suspensions were centrifuged at 1000 rpm and, after fixative refreshment, stored at −20 °C. If necessary, the cell suspensions were left at room temperature, and aliquots of 20 μL were dropped onto slides, air-dried, and stained for 5 min with 5% Giemsa solution at pH 7. Karyotypes were obtained from 5 enlarged metaphase plates, and the relative morphometric parameters of chromosomes were independently calculated by five authors (AP, NM, RC, FMG, and GO) using the public domain software Image J 1.45 (https://imagej.net/ij/, accessed on 1 June 2025). For chromosome nomenclature, we followed the work of Levan et al. [19].
NOR-FISH staining was performed as described by Petraccioli et al. [13] using the 18S rRNA biotin gene sequence units of the pectinid Adamussium colbecki (E. A. Smith, 1902) as a probe. After being dropped onto slides, the cells were aged for one day at room temperature, allowed to stand for two hours at 60 °C, and then incubated for 30 min in RNase at 100 μg/mL in Tris-HCl 10 mM at pH 6.5. Finally, the cells were dehydrated in alcohol series and air-dried. The chromosomes and probe were denatured for three min at 72 °C in the hybridisation mixture (10 ng/mL biotinylated 16 dUTP probe + 0.1 mg/mL shared E. coli DNA in 2× SSC with 50% formamide). The hybridisation was carried out at 40 °C for 20 h. Washes were performed in 1× SSC at 72 °C for 5 min and at RT for 2 min.
Probe detection was performed using monoclonal anti-biotin antibodies (Sigma cod. B7653) diluted 1:500 in PTB (1 mL PTB = 5 μL Tween 20% + 0.01 g dry milk in 1 mL 0.2 M PBS). After one hour, the slides were washed in 1 × PBS and incubated for 30 min in FITC-conjugated anti-anti-biotin antibodies (Sigma, Kawasaki, Japan) diluted to 1:50 in PTB. After washing in 1× PBS, chromosomes were counterstained with 5 μg/mL propidium iodide (PI) in 1× PBS for 15 min at RT and mounted with anti-fade (DABCO, Sigma). The hybridisation signals were detected and recorded under an epifluorescence microscope (Leica DM) equipped with a digital camera.
2.2. Molecular Analysis
DNA was extracted from the foot of two specimens for each studied population, according to Sokolov [20]. In brief, a foot piece (3–5 mm) from a specimen of each studied population was sliced with forceps. Tissue fragments were transferred to a 2 mL plastic tube containing 1 mL of the lysis buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 mM EDTA, 1% sodium dodecyl sulphate (SDS), 0.2 mg/mL Proteinase K) and incubated at 55 °C until complete digestion. Successively, 100 μL of saturated KCl solution was added, incubated on ice for 5 min, and centrifugated at 12,000 rpm. The supernatant was extracted twice with an equal volume of chloroform/isoamyl alcohol (24:1) mixture. Finally, DNA was extracted by adding to the supernatant 2 volumes of absolute alcohol, washed in 70% alcohol, centrifugated a 6000 rpm, air-dried and, dissolved in an adequate volume of TE buffer (10 mM Tris-HCl, 1 mM EDTA) to finish.
The mitochondrial 16S rDNA fragment was amplified via polymerase chain reaction (PCR) using the same primer pairs and PCR conditions as used by Sá-Pinto et al. [21]. After purification using the QIAEX gel purification kit (Quiagen, Hilden, Germany), the PCR products were sequenced using the BigDye Terminator Cycle sequencing protocol on an ABI Prism 310 automated sequencer (Applied Biosystems, Waltham, MA, USA), and all sequences were submitted to GenBank (accession number PX119029-36). Sequence chromatograms were edited with ChromasLite© 2.6.4, aligned using ClustalW [22] in BioEdit 7.0.5.3 [23], and blasted in GenBank. A neighbour joining phylogenetic tree of 16S rDNA was inferred from the alignment using MEGA11 v11.0.13 [24] with 1000 replicates using the sequence of Trochoidea retrieved from GenBank and using the 16S rDNA of Albinaria caerulea (A,N: NC_001761) as an outgroup. The 16S rDNA sequences of Xerocrassa (formerly Trochoidea) available in GenBank were not included because they were shorter than the other examined sequences.
3. Results
3.1. Karyological Analysis
Metaphase plates suitable for chromosome analysis were obtained from specimens from Capri, Terracina, and Palermo, which showed a karyotype of 2n = 48 chromosomes, with the first three pairs distinctly longer than the others, and which progressively decreased in length (Figure 1).
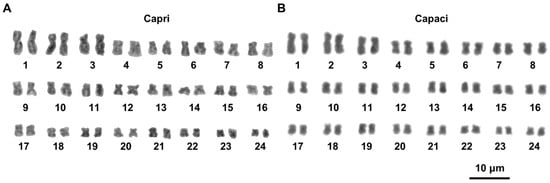
Figure 1.
Giemsa-stained karyotypes of T. caroni specimens from Capri (A) and Palermo (B). The scale bar applies to both images.
Specimens from Capri and Terracina had very similar karyotypes, with the metacentric pairs 1–2, 4–6, 9–11, 13, and 15–24; submetacentric pairs 3, 7, 12, and 14; and the subtelocentric pair 8. The karyotype of Palermo specimens differed from that of Capri/Terracina in showing metacentric chromosomes in the 8th pair and submetacentric elements in the 17th pair (Figure 1; Table 1).

Table 1.
Morphometric parameters of the chromosomes of T. caroni samples from Capri and Palermo. R.L. = relative length (chromosome length/total chromosome length × 100); C.I. = centromeric index (short arm/chromosome length × 100); Sh (chromosome shape; M = metacentric, sM = submetacentric, sT = subtelocentric).
In T. caroni, NOR-FISH was performed on metaphase plates, while in T. elegans, only diakinetic meiotic bivalents were suitable for the analysis. In the former, clear signals of hybridization were identified close to the centromeres of the short arms of 16th chromosome pair. In T. elegans, hybridization signals were detected on a bivalent presumed to be the 16th pair (Figure 2A,B).
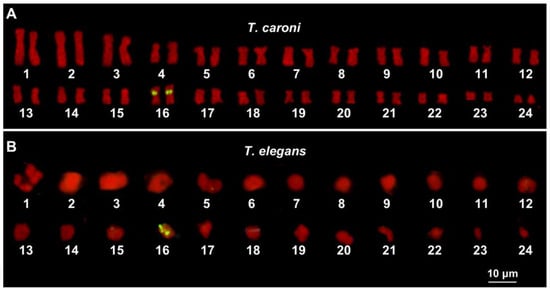
Figure 2.
Karyotypes from the gonadic metaphase (A) and diakinetic meiotic plates (B) of T. caroni (A) and T. elegans (B). The scale bar applies to both images.
3.2. Molecular Analysis
The selected fragments of 16S were successfully amplified in the study samples, and the newly generated sequences were deposited in GenBank (accession numbers PX119029 to PX119036). The Nj and ML phylogenetic analysis of the T. caroni sequences obtained herein, and those of other Trochoidea species segments deposited in GenBank, produced similar trees (Figure 3). Trochoidea samples were grouped into two main clades: one included T. pyramidata and T. trochoides, each of which was grouped in turn in two distinct subclades; the other including the subclades T. elegans and T. caroni, and the distant subclade T. spratti. In T. caroni, the samples from Palermo were external to the subclade, including samples from Trapani, Capri, and Terracina, with the last two samples showing a unique haplotype (Figure 3).
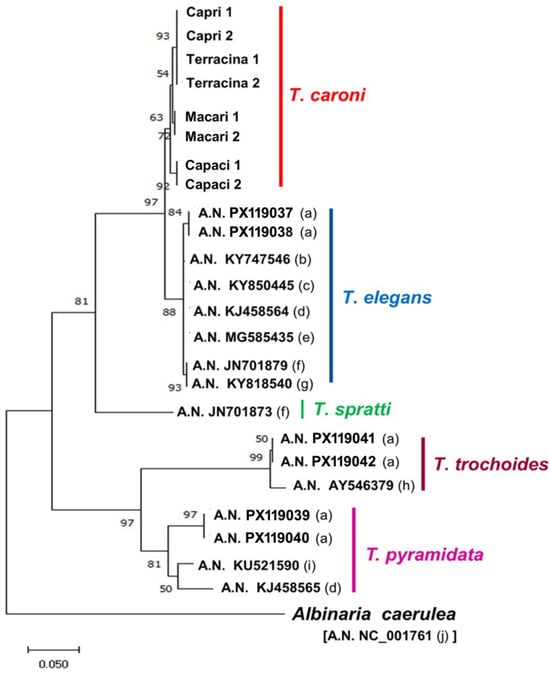
Figure 3.
16S rDNA neighbour joining phylogenetic tree showing the relationships between the studied samples of T. caroni and the deposited homologous GenBank segments of other Trochoidea species. a = present study; b = Ezzine et al. [25]; c = Chueca et al. [26]; d = Razkin et al. [10]; e = Caro et al. [27]; f = Sauer and Hausdorf [28]; g = Neiber et al. [29]; h = Steinke et al. [9]; i = Boeckers et al. [30]; j = Hatzoglou et al. [31].
4. Discussion
T. caroni is known to occur in central-western Sicily and Capri, and because of this, Bodon et al. [5] proposed that the population on Capri was a relict population of a wider peninsular species distribution, while its presence on the island, or even in the Italian peninsula, was due to human activity [6,32].
New discoveries of live specimens or their shells in continental Italy support a wider distribution of T. caroni than originally thought [6]. The results obtained in this study show that the continental and Sicilian populations are karyologically differentiated due to the different morphology of pairs 8 (subtelo- vs. metacentric) and 17 (metacentric vs. submetacentric) in the Capri and Palermo populations, respectively (see Figure 1 and Table 1). We put forward the hypothesis that the chromosomal rearrangements involved were two inversions or centromeric shifts [33] (see Figure 4). Other mechanisms, such as translocations or heterochromatin addition/deletion, could be involved. Chromosome painting could detect translocation events, while we maintain that the occurrence of heterochromatin addition/deletion is improbable, as two populations have a very scarce centromeric heterochromatin (authors’ observations; see also [11,14]).
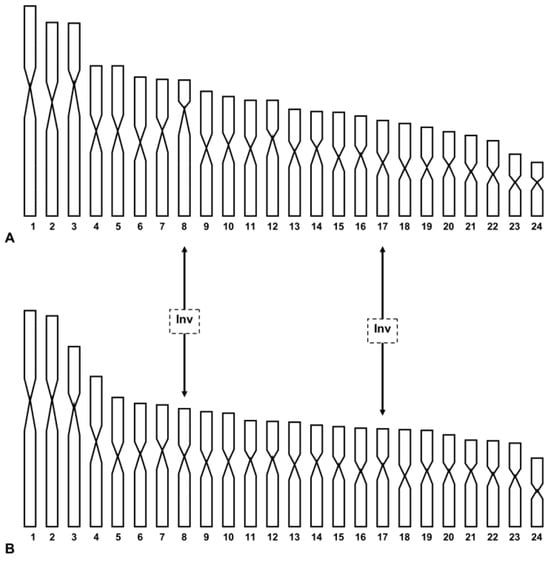
Figure 4.
Haploid karyograms of T. caroni from Capri (A) and Palermo (B), showing the origin of chromosomal differences between them on the basis of the inversion hypothesis. Inv = inversion.
Chromosomal polymorphism due to inversions and their role in the speciation process are matters of debate [34]. However, accumulating evidence shows that chromosomal polymorphism (such as inversions, chromosome fusions or fissions, and translocations), by reducing recombination between favourable combinations of alleles, is an important driving force in local adaptation, speciation processes, and sex chromosome evolution in both animals and plants [35,36].
Regarding the chromosomal diversification between the two populations of T. caroni studied, our preliminary molecular analysis shows T. caroni to be closely related to T. elegans and T. spratti as its sister species. Furthermore, 16S rDNA analysis shows that the populations of T. caroni from Capri and Terracina have a unique haplotype, separate from the Sicilian populations (p-distance 0.12), which in contrast display a discrete degree of diversification (p-distance 0.12). On the other hand, diversification among mainland Italy and Sicilian populations is well known and documented for different taxa, both vegetables (e.g., oak populations) [37]) and animals (e.g., Pulmonata gastropods of the genus Marmorana, anurans of the genus Bufotes, rabbits of the genus Oryctolagus, and butterflies of genera Pieris, Iphiclides, and Anthocharis) [38,39,40,41].
In the Italian peninsula and Sicily, a combination of geological–paleoclimatic events occurred (the salinity crisis in the Miocene; the glacial and interglacial periods in the Pliocene; and in the Pleistocene), resulting in population isolation and differentiation, dispersal/vicariance phenomena, introgression, hybridization, extinction, and other speciation processes with a high level of endemism [42,43,44,45,46]. However, further karyological and molecular studies are needed to provide insights into the degree of diversification between the continental and Sicilian populations of T. caroni.
It should be noted that species of the “elegans” group (T. elegans, T. caroni, T. spratti, and T. cumiae) to which T. caroni belongs have very similar internal anatomy (genitalia), and their distinction is mainly based on geographic distribution and their remarkable shell morphology [47,48]. According to Giusti et al. [7], the specific validity of “elegans” group members requires confirmation.
The karyotypes of T. caroni here described differ from those of T. elegans and of T. pyramidata and T. trochoides, the other species in the genus [11]. In fact, all Trochoidea so far studied have 2n = 48, but with a different chromosomal formula: T. caroni has 19M, 4sM, 1sT (from Capri), and 19M, 5 sM (from Palermo); T. elegans has 16M, 6sM, 2T; T. pyramidata has 20M, 4sM (from Capri); and T. trochoides has 16M, 7sM, 1T [11]. For the chromosome formula of T. pyramidata, we report the origin (Capri) and GenBank accession number (MZ504248-50) of the samples studied by [11], because the studied Capri samples were genetically different to the specimens from Tunisia (AN: KY747545); Siena, Italy (AN: AY741444); Italy (AN: KU521590); and Spain (AN KJ458565, AN: AY546377) [10,18,30,49], highlighting that T. pyramidata populations require taxonomic revision.
The Trochoidea clade appears to be karyologically uniform in terms of numbers of chromosomes (2n = 48). This could also apply to the NOR loci that are located on medium-sized chromosome pairs in both T. caroni and T. elegans (see Figure 2).
However, similar localisation of the NORs is displayed from the closely related species Cernuella virgata, of the tribe Cernuellini [11], suggesting that the NOR loci on a medium-sized chromosome pair (tentatively, the 16th pair) were inherited from the common ancestor of Trochoideini + Cernuellini.
The karyological data of the Trochoideini, i.e., of the genus Xerocrassa, concern only the number of chromosomes (2n = 52 in X. cretica, and 2n = 50 in X. geyer) [50,51]. Among the Cernuellini species, a chromosomal formula was described in C. virgata, which conserved the presumed primitive Geomitridae karyotype of 2n = 52 with all metacentric chromosomes [11].
On this basis, the 2n = 48 primitive karyotype of the common ancestor of Trochoidea could have originated from the translocation of two pairs of chromosomes of the primitive Geomitridae complement of 2n = 52 chromosomes. In Figure 4, we advance a hypothesis of the chromosome evolution that occurred during the specific diversification of Trochoidea. The hypothesis deserves confirmation from further chromosomal analysis performed by banding and molecular methods.
Note that the presumed primitive karyotype of the common ancestor of Trochoidea was derived by considering the most common form of chromosomes between the karyotypes of T. caroni from Capri and Palermo, presented here, and those of T. elegans, T. pyramidata, and T. trochoides [11], whose relationships place T. elegans sister to T. pyramidata + T. trochoides clade, according to Razkin et al. [10]. It should be emphasised that in representing the presumed primitive karyotype of Trochoidea we have not retained the chromosome order assigned by Petraccioli et al. [11] in the karyotype of T. elegans, T. pyramidata, and T. trochoides. In fact, to respect the chromosome shape, in some cases we shifted the original chromosome order to a nearby position, but always considering the standard deviation values. This was done to minimise rearrangements, then using parsimony. In our hypothetical scenario (Figure 5), we assume that the primitive karyotype of Trochoidea is derived from the translocation of two pairs of chromosomes, tentatively, pairs 23 and 26 on pairs 5 and 6 of the primitive karyotype of Geomitridae of 2n = 52 (filled in brown), giving rise to the submetacentric pairs 1 and 3 of 2n = 48 of the karyotypes of the common Trochoidea. As a result of translocations, the formation of neo-centromeres also occurred [52,53], as well as two inversions, which involved the original metacentric pairs 7 and 12 of the primitive Geomitridae karyotype, and transformed them into submetacentric pairs in the common karyotype of Trochoidea (filled in yellow). Several chromosome inversions occurred in this primitive karyotype during the specific diversification of the genus. In particular, in the T. pyramidata + T. trochoides clade there was first an inversion that led to the submetacentric shape of the original chromosome 5 of their common ancestor (filled in yellow in Figure 5), then a further inversion occurred in both the karyotype of T. pyramidata and T. trochoides, involving, respectively, pair 7 and the last pair, making them metacentric in T. pyramidata and telocentric in T. trochoides, highlighted in yellow and green in the relative karyotypes in Figure 5.
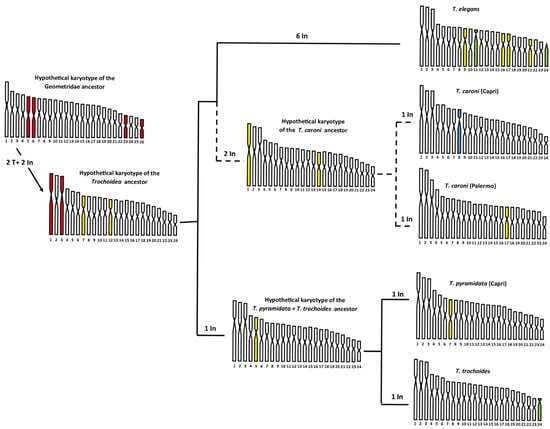
Figure 5.
Hypothetical chromosome evolution of the Trochoidea. Phylogenetic relationships between T. elegans, T. pyramidata, and T. trochoides are represented according to Razkin et al. [10] (branch lengths do not respect genetic distance). The relationships between T. elegans and T. caroni take into consideration that the species belong to the T. elegans group. The chromosomes involved in the rearrangements are highlighted in various colours (see the test for further explanation). T = translocation; In = inversion.
In the T. elegans + T. caroni clade, the first two inversions involved pairs 1 (submetacentric) and 14 (metacentric) of the common progenitor of Trochoidea, and these pairs were, respectively, metacentric and submetacentric in the common progenitor karyotype of the Capri and Palermo populations of T. caroni. In the last two populations, a further inversion occurred, with the chromosomes of pair 8 of the Capri shaped as subtelocentric and the chromosomes of pair 17 of the Palermo shaped as submetacentric (highlighted, respectively, in light blue and yellow in Figure 5).
Finally, the karyotype of T. elegans is derived from that of the common ancestor of T. trochoides by six inversions. Four of them formed as submetracentric chromosomes, pairs 9, 16, 17, and 21 (highlighted in yellow in the relative karyotype of Figure 5). The other two inversions formed as telocentric, pairs 11 and 24 (highlighted in green in the relative karyotype of Figure 5).
5. Conclusions
In conclusion, we first described the karyotype of the land snail T. caroni. It has 2n = 48 mostly bi-armed chromosomes, but Sicilian populations karyologically differ from the Capri and Terracina populations in the morphology of two pairs of chromosomes, probably originating from inversions. Preliminary molecular analysis also evidences a discrete diversification between the Sicilian population and those outside of Sicily. Comparison with data available in the literature on T. caroni relatives (T. elegans, T pyramidata and T. trochoides) allowed us to define the probable karyotype of the common ancestor of the Trochoidea (2n = 48, all biarmed chromosomes), which in turn was derived from the 2n = 52 all-metacentric primitive karyotype of Geomitridae by means of two translocations and two inversions. In addition, a hypothesis has also been put forward suggesting that during the specific diversification of Trochoidea, chromosomal evolution occurred through intrachromosomal rearrangements, and that NOR loci were conserved on chromosomes of a medium-sized pair in both Trochoideini and Cernuellini snails.
Author Contributions
Conceptualization, A.P., F.M.G., G.O. and N.M.; methodology, A.P. and G.O.; validation, formal analysis, investigation, data curation: A.P., F.M.G., G.O., I.S., N.M., P.C. and R.C.; writing—original draft preparation, F.M.G., G.O. and N.M.; writing—review, A.P., F.M.G., G.O., I.S., N.M., P.C. and R.C.; visualization, supervision, F.M.G., G.O. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animals used in our study are non-cephalopod mollusks and as such do not fall under the law in force in Italy for the protection of animals used for scientific purposes (legislative decree N. 26, 04/03/2014) which, in turn, incorporates the European Directive 2010/63 EU L276. Samples from Latium were sampled under authorization released by Ente Parco Regionale Riviera di Ulisse n. 44, del 07/01/2009 (Gaeta).
Informed Consent Statement
Not applicable.
Data Availability Statement
Newly generated cytogenetic data are available within this manuscript.
Acknowledgments
We are grateful to Ivano Niero for having provided anatomical description of some specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- MolluscaBase (Ed.) MolluscaBase. Geomitridae C. R. Boettger, 1909. World Register of Marine Species. 2025. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=994707 (accessed on 15 June 2025).
- Deshayes, G.P.; De Lamarck, B.E. Histoire naturelle des Vers. In Encyclopédie Méthodique (Dictionaire Encyclopédique Méthodique), ou par Ordre de Matieres, 2nd ed.; Part 2; Agasse: Paris, France, 1832; Volume 1–7, pp. 185–271. [Google Scholar]
- Alzona, C. Malacofauna Italica. Catalogo e bibliografia dei molluschi viventi, terrestri e d’acqua dolce. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1971, 111, 1–433. [Google Scholar]
- Cossignani, T.; Cossignani, V. Atlante Delle Conchiglie Terrestri e Dulciacquicole Italiane; L’Informatore Piceno: Ancona, Italy, 1995; p. 208. [Google Scholar]
- Bodon, M.; Cianfanelli, S.; Nardi, G. Mollusca (terrestrial and inland water species). In Checklist of the Italian Fauna; Version, 1.0; Bologna, M.A., Zapparoli, M., Oliverio, M., Minelli, A., Bonato, L., Cianferoni, F., Stoch, F., Eds.; LifeWatch: Lecce, Italy, 2021; Available online: https://www.lifewatchitaly.eu/en/initiatives/checklist-fauna-italia-en/checklist/ (accessed on 30 October 2024).
- Maio, N.; Petraccioli, A.; Crovato, P.; Amor, N.; Odierna, G. New faunistic data on Trochoidea (Trochoidea) caroni (Deshayes, 1832) (Gastropoda Pulmonata Hygromiidae). Biodivers. J. 2013, 4, 483–500. [Google Scholar]
- Giusti, F.; Manganelli, G.; Schembri, P.J. The Nonmarine Molluscs of the Maltese Islands; Museo Regionale di Scienze Naturali: Torino, Italy, 1995; Volume 15, pp. 1–607.
- Schileyko, A.A. Treatise on recent terrestrial pulmonate molluscs. Part 14. Helicodontidae, Ciliellidae, Hygromiidae. Ruthenica 2006, 2, 1907–2047. [Google Scholar]
- Steinke, D.; Albrecht, C.; Pfenninger, M. Molecular phylogeny and character evolution in the Western Palaearctic Helicidae s.l. (Gastropoda: Stylommatophora). Mol. Phylogenet. Evol. 2004, 32, 724–734. [Google Scholar] [CrossRef]
- Razkin, O.; Gómez-Moliner, B.J.; Prieto, C.E.; Martínez-Ortí, A.; Arrébola, J.R.; Muñoz, B.; Chueca, L.J.; Madeira, M.J. Molecular phylogeny of the western Palaearctic Helicoidea (Gastropoda, Stylommatophora). Mol. Phylogenet. Evol. 2015, 83, 99–117. [Google Scholar] [CrossRef]
- Petraccioli, A.; Crovato, P.; Guarino, F.M.; Mezzasalma, M.; Odierna, G.; Picariello, O.; Maio, N. Chromosome Diversity and Evolution in Helicoidea (Gastropoda: Stylommatophora): A Synthesis from Original and Literature Data. Animals 2021, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Petraccioli, A.; Guarino, F.M.; Maio, N.; Odierna, G. Molecular cytogenetic study of three common Mediterranean limpets, Patella caerulea, P. rustica and P. ulyssiponensis (Archaeogastropoda, Mollusca). Genetica 2010, 138, 219–225. [Google Scholar] [CrossRef][Green Version]
- Petraccioli, A.; Maio, N.; Odierna, G. Chromosomes of Lepidochitona caprearum (Scacchi, 1836) (Polyplacophora, Acanthochitonina, Tonicellidae) provide insights into Acanthochitonina karyological evolution. Comp. Cytogenet. 2012, 6, 397–407. [Google Scholar] [CrossRef][Green Version]
- Petraccioli, A.; Capriglione, T.; Colomba, M.; Crovato, P.; Odierna, G.; Sparacio, I.; Maio, N. Comparative cytogenetic study in four Alopiinae door snails (Gastropoda, Clausiliidae). Malacologia 2015, 58, 225–232. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Guarino, F.M.; Odierna, G.; Petraccioli, A.; Picariello, O. Changes in heterochromatin content and ancient chromosome fusion in the endemic Malagasy boids Sanzinia and Acrantophis. Salamandra 2019, 55, 140–144. [Google Scholar]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. First insights on the karyotype diversification of the endemic Malagasy leaf-toed geckos (Squamata: Gekkonidae: Uroplatus). Animals 2022, 12, 2054. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. Comparative cytogenetics of Hemorrhois hippocrepis and Malpolon monspessulanus highlights divergent karyotypes in Colubridae and Psammophiidae (Squamata: Serpentes). Eur. Zool. J. 2023, 90, 201–210. [Google Scholar] [CrossRef]
- Manganelli, G.; Salomone, N.; Giusti, F. A molecular approach to the phylogenetic relationships of the western Palaearctic Helicoidea (Gastropoda, Stylommatophora). Biol. J. Linn. Soc. 2005, 85, 501–512. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Sokolov, E.P. An improved method for DNA isolation from mucopolysaccharide-rich molluscan tissues. J. Molluscan Stud. 2000, 66, 573–575. [Google Scholar] [CrossRef]
- Sá-Pinto, A.; Branco, M.; Harris, D.J.; Alexandrino, P. Phylogeny and phylogeography of the genus Patella based on mitochondrial DNA sequence data. J. Exp. Mar. Biol. Ecol. 2005, 325, 95–110. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.J.; Gibson, T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choisechoice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit, a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ezzine, I.K.; Pfarrer, B.; Dimassi, N.; Said, K.; Neubert, E. At home at least: The taxonomic position of some North African Xerocrassa species (Pulmonata, Geomitridae). Zookeys 2017, 712, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chueca, L.J.; Gomez-Moliner, B.J.; Madeira, M.J.; Pfenninger, M. Molecular phylogeny of Candidula (Geomitridae) land snails inferred from mitochondrial and nuclear markers reveals the polyphyly of the genus. Mol. Phylogenet. Evol. 2018, 118, 357–368. [Google Scholar] [CrossRef]
- Caro, A.; Neiber, M.T.; Gomez-Moliner, B.J.; Madeira, M.J. Molecular phylogeny and biogeography of the land snail subfamily Leptaxinae (Gastropoda: Hygromiidae). Mol. Phylogenet. Evol. 2019, 139, 106570. [Google Scholar] [CrossRef]
- Sauer, J.; Hausdorf, B. A comparison of DNA-based methods for delimiting species in a Cretan land snail radiation reveals shortcomings of exclusively molecular taxonomy. Cladistics 2012, 28, 300–316. [Google Scholar] [CrossRef]
- Neiber, M.T.; Razkin, O.; Hausdorf, B. Molecular phylogeny and biogeography of the land snail family Hygromiidae (Gastropoda: Helicoidea). Mol. Phylogenet. Evol. 2017, 111, 169–184. [Google Scholar] [CrossRef]
- Boeckers, A.; Greve, C.; Hutterer, R.; Misof, B.; Haase, M. Testing heterogeneous base composition as potential cause for conflicting phylogenetic signal between mitochondrial and nuclear DNA in the land snail genus Theba Risso 1826 (Gastropoda, Stylommatophora, Helicoidea). Org. Divers. Evol. 2016, 16, 835–846. [Google Scholar] [CrossRef]
- Hatzoglou, E.; Rodakis, G.C.; Lecanidou, R. Complete sequence and gene organization of the mitochondrial genome of the land snail Albinaria caerulea. Genetics 1995, 140, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, G.; Bodon, M.; Favilli, L.; Giusti, F. Gastropoda Pulmonata. In Checklist Delle Specie Della Fauna Italiana; Minelli, A., Ruffo, S., La Posta, S., Eds.; Calderini: Bologna, Italy, 1995; Volume 16, pp. 1–60. [Google Scholar]
- Guo, X.; Su, H.; Shi, Q.; Fu, S.; Wang, J.; Zhang, X.; Hu, Z.; Han, F. De Novo Centromere Formation and Centromeric Sequence Expansion in Wheat and its Wide Hybrids. PLoS Genet. 2016, 12, e1005997. [Google Scholar] [CrossRef] [PubMed]
- King, M. Species Evolution: The Role of Chromosome Change; Cambridge University Press: Cambridge, UK, 1993; pp. xxi–336. [Google Scholar]
- Ayala, D.; Fontaine, M.C.; Cohuet, A.; Fontenille, D.; Vitalis, R.; Simard, F. Chromosomal inversions, natural selection and adaptation in the malaria vector Anopheles funestus. Mol. Biol. Evol. 2011, 28, 745–758. [Google Scholar] [CrossRef]
- Huang, K.; Rieseberg, L.H. Frequency, Origins, and Evolutionary Role of Chromosomal Inversions in Plants. Front. Plant Sci. 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, S.; Cozzolino, S.; Migliaccio, M.; Musacchio, A.; Innocenti, M.; Vendramin, G.G. Sicily represents the Italian reservoir of chloroplast DNA diversity of Quercus ilex L. (Fagaceae). Ann. For. Sci. 2005, 62, 79–84. [Google Scholar] [CrossRef]
- Fiorentino, V.; Salomone, N.; Manganelli, G.; Giusti, F. Phylogeography and morphological variability in land snails: The Sicilian Marmorana (Pulmonata, Helicidae). Biol. J. Linn. Soc. 2008, 94, 809–823. [Google Scholar] [CrossRef]
- Stöck, M.; Grifoni, G.; Armor, N.; Scheidt, U.; Sicilia, A.; Novarini, N. On the origin of the recent herpetofauna of Sicily: Comparative phylogeography using homologous mitochondrial and nuclear genes. Zool. Anz. 2016, 261, 70–81. [Google Scholar] [CrossRef]
- Lo Valvo, M.; Russo, R.; Mancuso, F.P.; Palla, F. mtDNA diversity in a rabbit population from Sicily (Italy). Turk. J. Zool. 2017, 41, 7. [Google Scholar] [CrossRef]
- Scalercio, S.; Cini, A.; Menchetti, M.; Vodă, R.; Bonelli, S.; Bordoni, A.; Casacci, L.P.; Dincă, V.; Balletto, E.; Vila, R.; et al. How long is 3 km for a butterfly? Ecological constraints and functional traits explain high mitochondrial genetic diversity between Sicily and the Italian Peninsula. J. Anim. Ecol. 2020, 89, 2013–2026. [Google Scholar] [CrossRef]
- La Greca, M. La situazione paleoclimatica nel Quaternario. Biogeographia 1998, 19, 7–29. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Sanmartìn, I. Dispersal vs. vicariance in the Mediterranean: Historical biogeography of the Palearctic Pachydeminae (Coleoptera, Scarabaeoidea). J. Biogeogr. 2003, 30, 1883–1897. [Google Scholar] [CrossRef]
- Duggen, S.; Hoernle, K.; van den Bogaard, P.; Rüpke, L.; Morgan, J.P. Deep roots of the Messinian Salinity Crisis. Nature 2003, 422, 602–606. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.-Y.; Boeuf, G. The Mediterranean Region. Biological Diversity in Space and Time, 2nd ed.; Oxford University Press: New York, NJ, USA, 2010; p. 376. [Google Scholar]
- Sacchi, C.F. Contributo alla conoscenza faunistica della Campania. Ricerche malacologiche nella regione sorrentina. I. Note sistematiche di alcune elicelline. Annuar. Ist. Mus. Zool. Univ. Napoli 1954, 6, 1–14. [Google Scholar]
- Sacchi, C.F. Il contributo dei molluschi terrestri alle ipotesi del “Ponte Siciliano”. Elementi tirrenici ed orientali nella malacofauna del Magreb. Arch. Zool. Ital. 1955, 40, 49–181. [Google Scholar]
- Ezzine, I.K.; Dimassi, N.; Pfarrer, B.; Said, K.; Neubert, E. New records of the endemic Sicilian land snail species Marmorana (Murella) muralis (Muller, O.F. 1774) from the North of Tunisia (Eupulmonata, Gastropoda). Zookeys 2018, 775, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.T. Cytotaxonomic studies of the family Helicidae (Gastropoda, Pulmonata). Genét. Ibér. 1981, 33, 211–224. [Google Scholar]
- Patterson, C.M.; Burch, J.B. Chromosomes of Pulmonated Molluscs. In Pulmonates; Systematics, Evolution and Ecology; Fretter, V., Peake, J., Eds.; Academic Press: New York, NY, USA, 1978; Volume 2A, pp. 171–217. [Google Scholar]
- Fukagawa, T.; Earnshaw, W.C. Neocentromeres. Curr. Biol. 2014, 24, R946–R947. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, E.; Piras, F.M.; Sola, L.; Santagostino, M.; Abdelgadir, W.A.; Raimondi, E.; Lescai, F.; Nergadze, S.G.; Giulotto, E. Robertsonian Fusion and Centromere Repositioning Contributed to the Formation of Satellite-free Centromeres During the Evolution of Zebras. Mol. Biol. Evol. 2022, 39, msac162. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).