Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Amphiprion ocellaris and Its Role in Antibacterial Immunity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Cells, Bacterial Strains, and Ethical Statement
2.2. Cloning of the AoLEAP2 Sequence
2.3. RNA Isolation and Reverse-Transcription Quantitative PCR (RT-qPCR)
2.4. Protein Modeling and Synthesis of AoLEAP2 Peptides
2.5. The Expression of Immune-Related Genes in AoLEAP2-Stimulated Clownfish
2.6. Antibacterial Activity of AoLEAP2 In Vitro
2.7. Hydrolytic Effect on Bacterial gDNA
2.8. Cell Counting Kit-8 (CCK-8) Assay
2.9. Fish Survival Assay
2.10. Statistical Analysis
3. Results
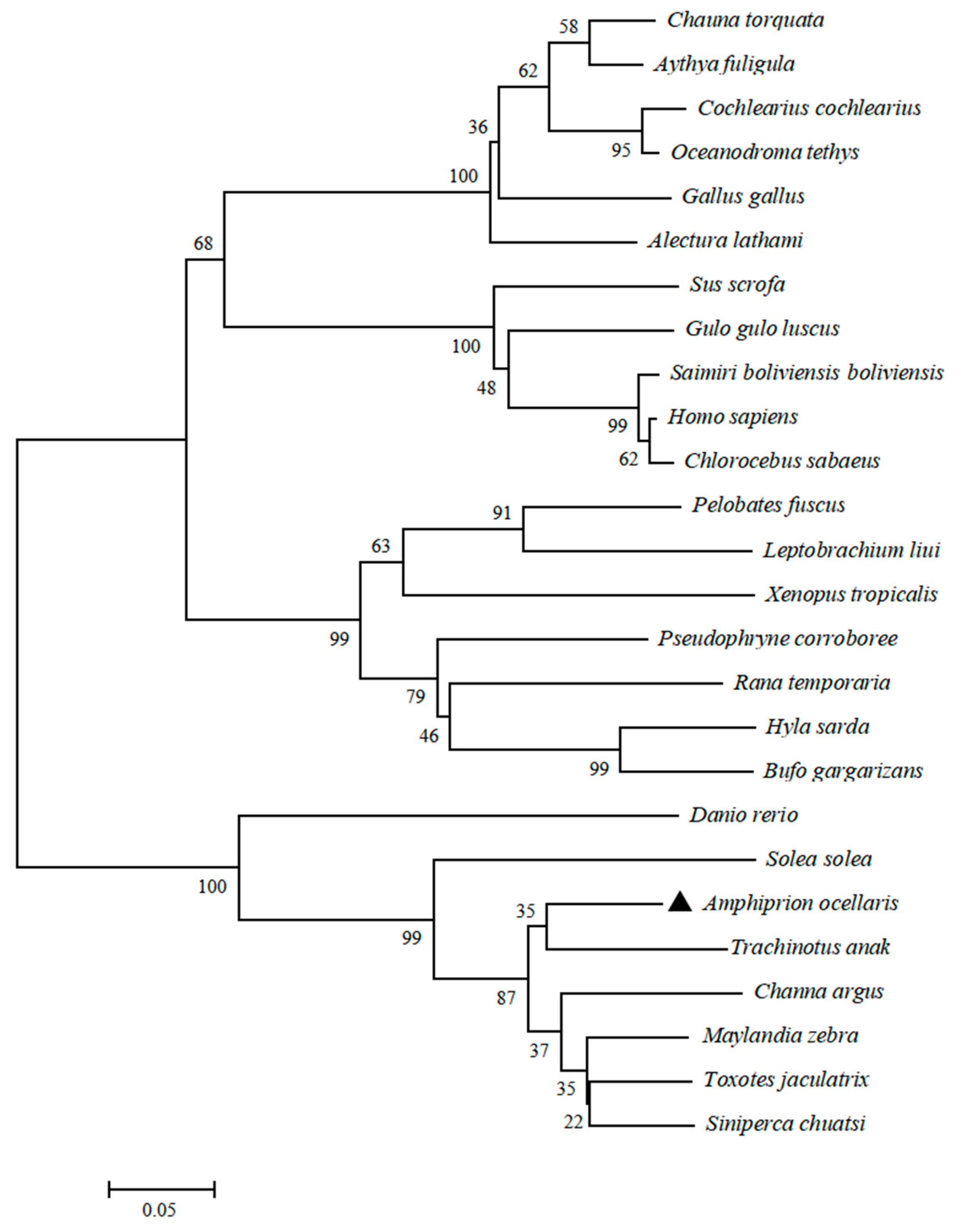
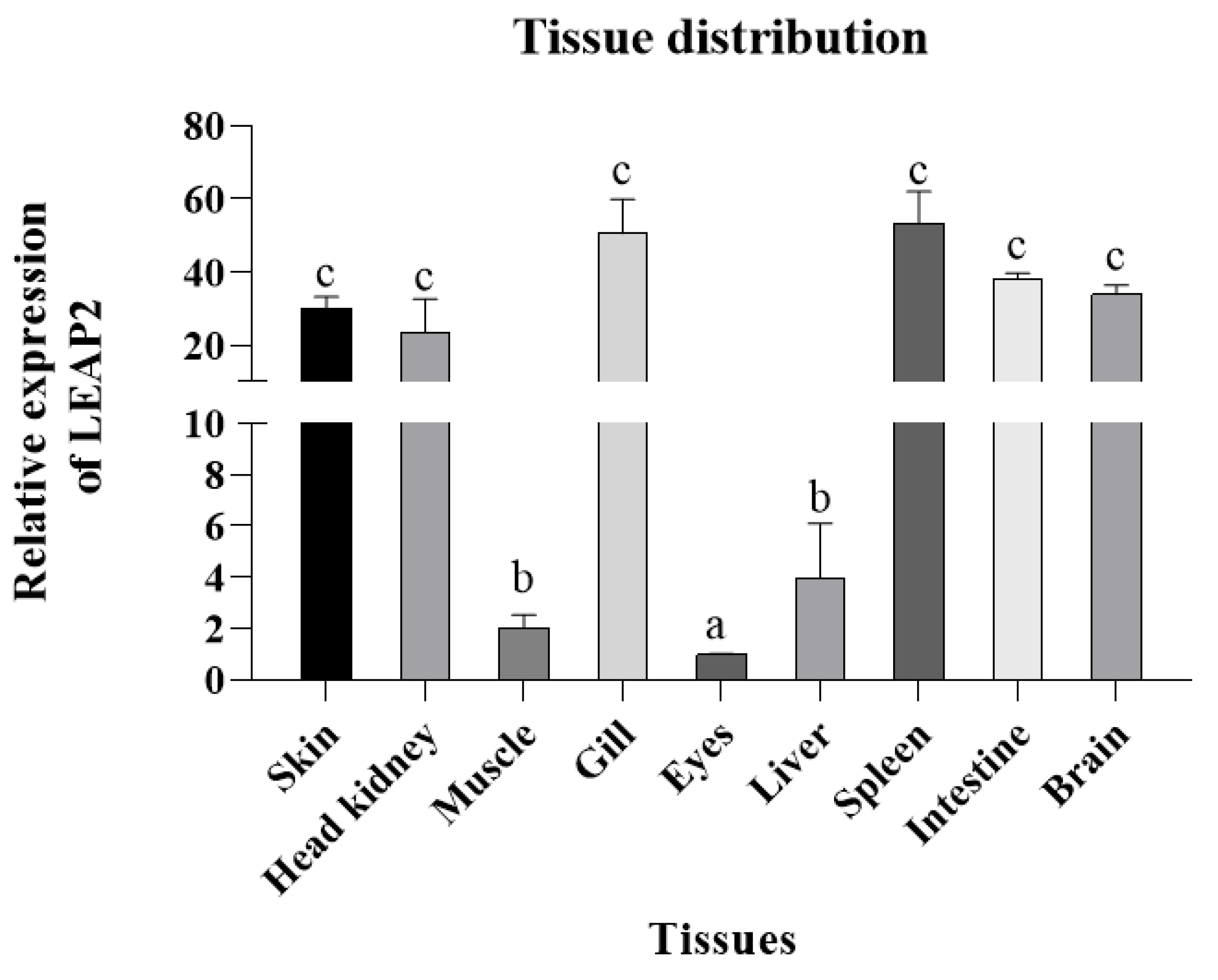
3.1. LEAP2 Sequence Characteristics in Clownfish
3.2. Expression of AoLEAP2 in Response to Bacterial Infection
3.3. Chemically Synthesized AoLEAP2 Shows Antibacterial Activity In Vitro
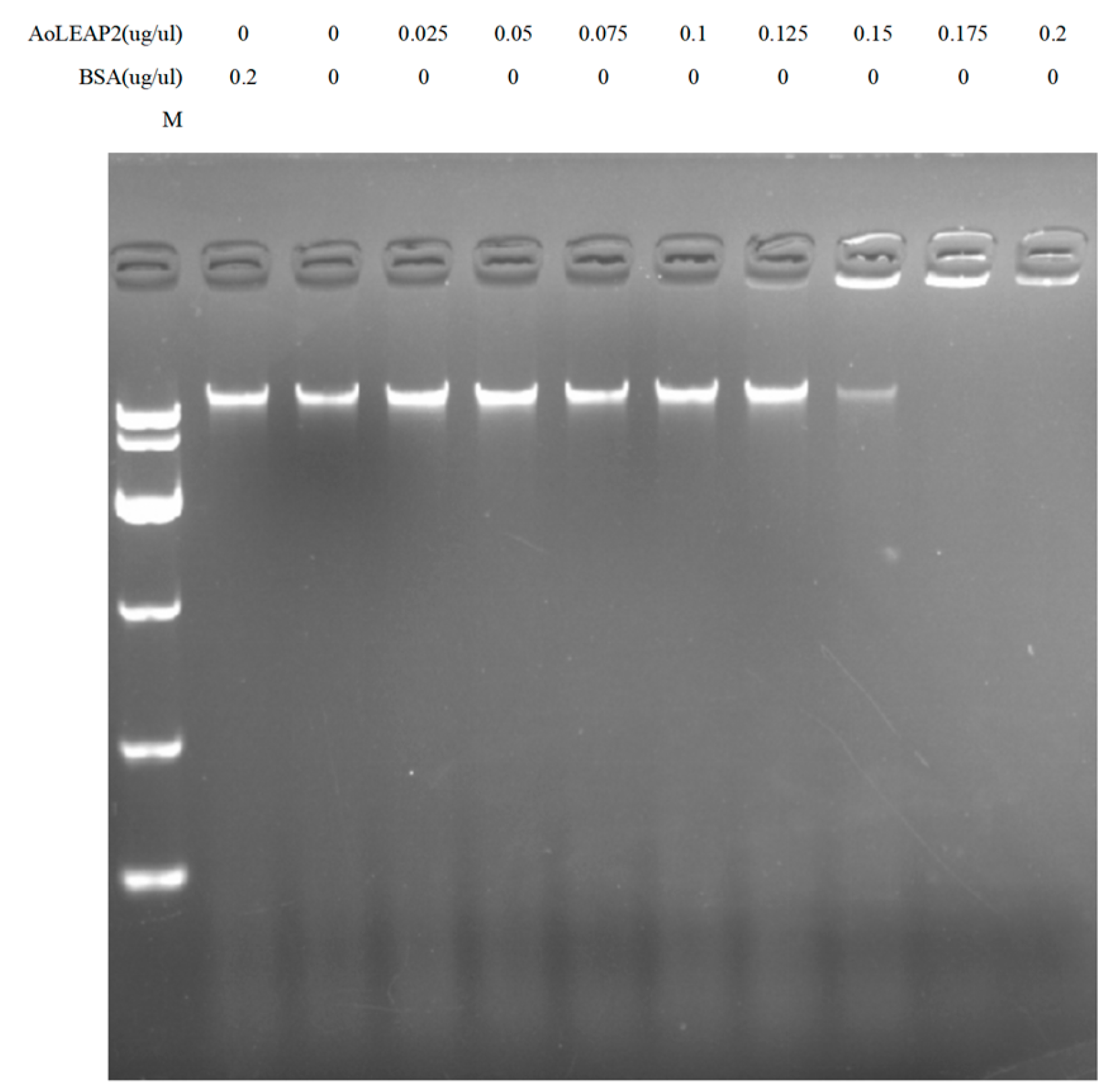
3.4. Mature AoLEAP2 Binds to Bacterial Genomic DNA
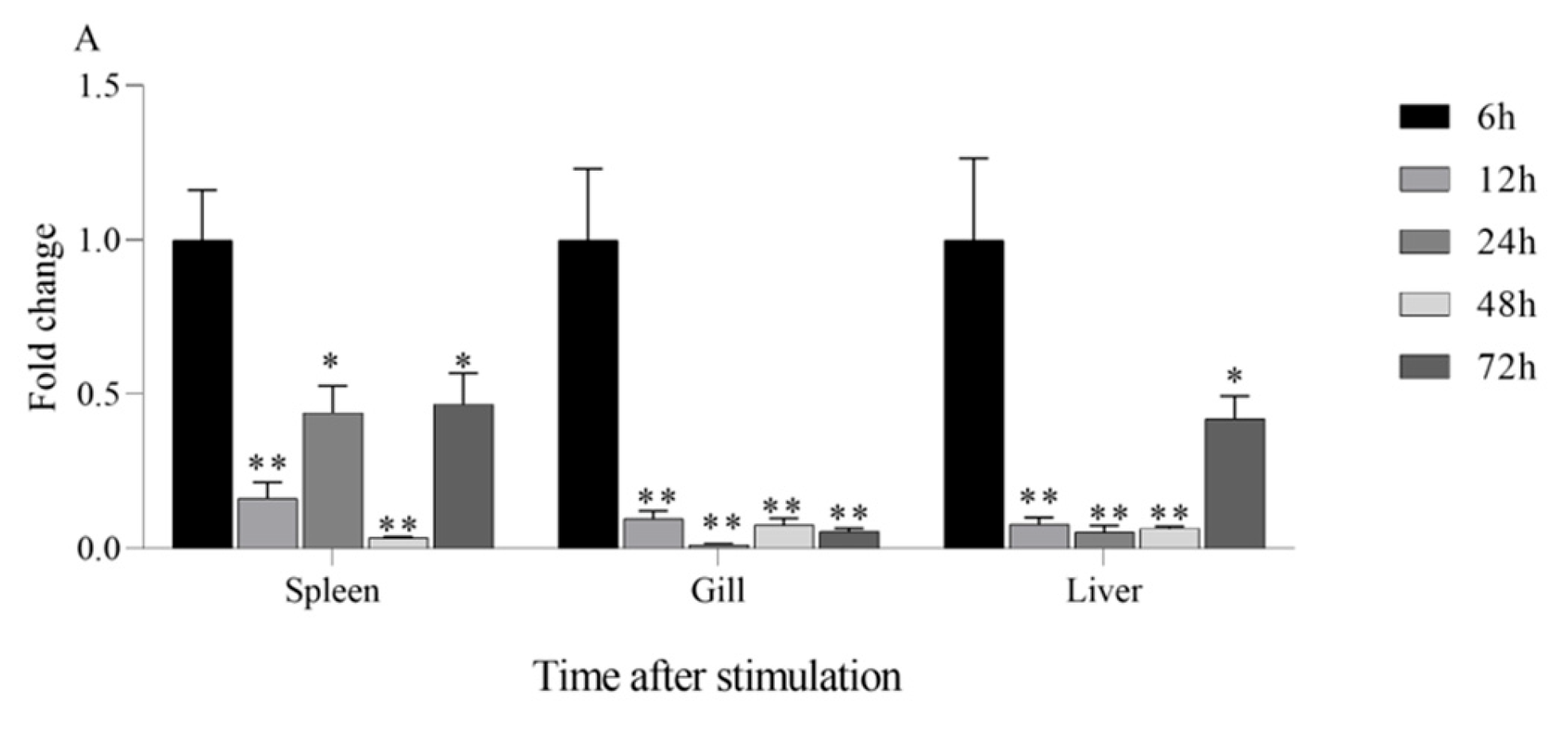
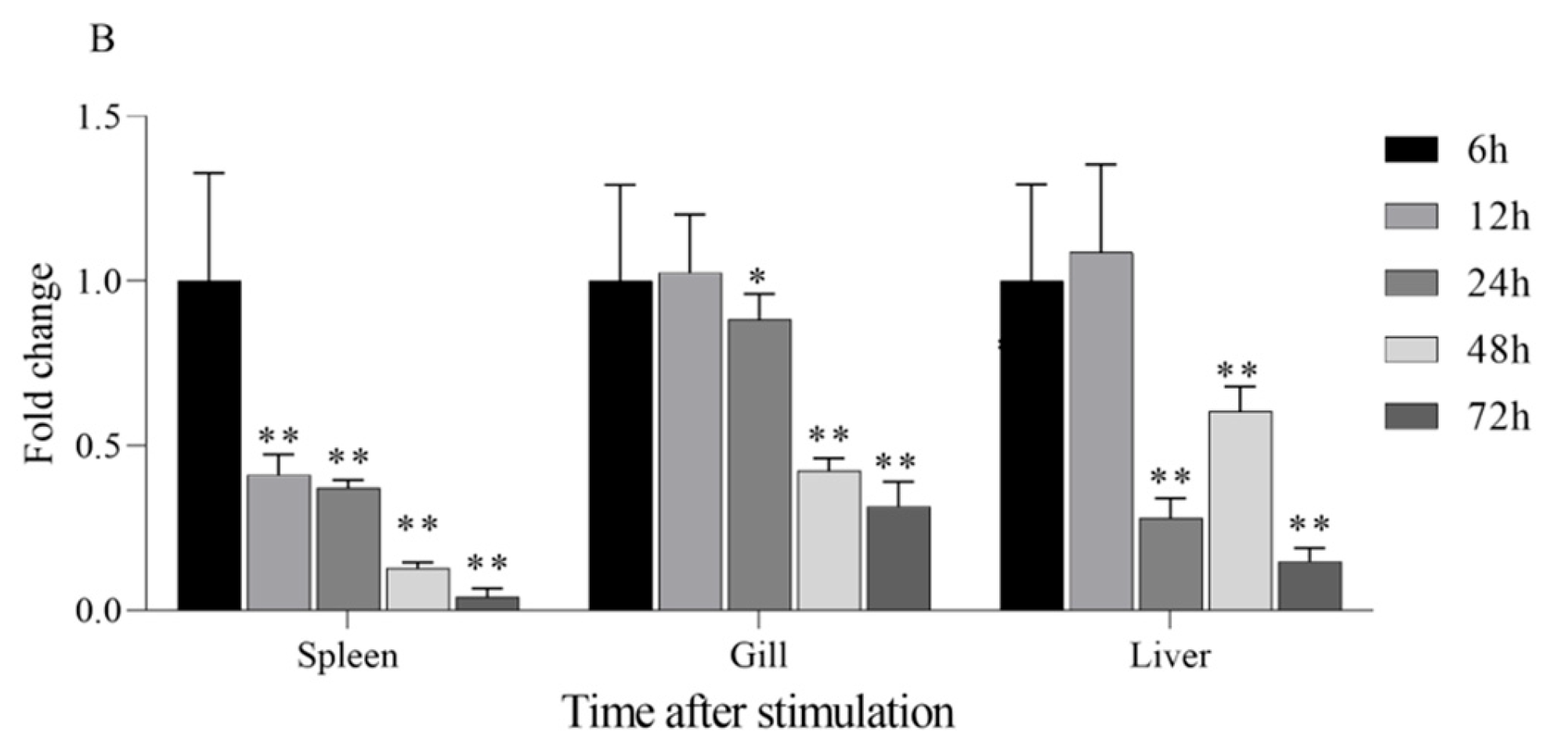
3.5. Change in IL1β and TNFα Expression of AoLEAP2 in Fish Tissue After V. harveyi Infection
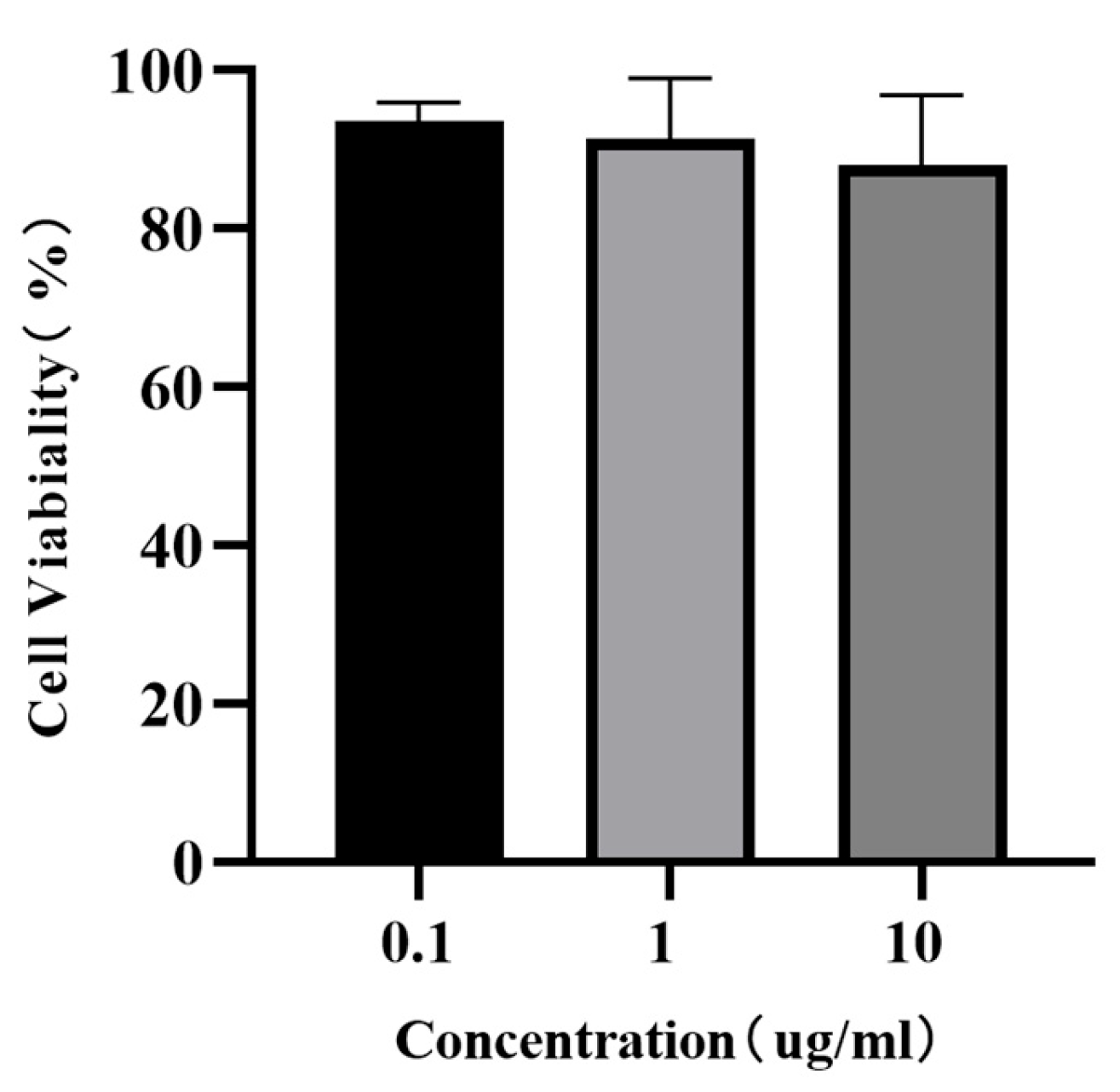
3.6. Effect of AoLEAP2 on FHM Cells
3.7. Effect of AoLEAP2 on the Survival of V. harveyi-Infected Clownfish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial Peptide Biological Activity, Delivery Systems and Clinical Translation Status and Challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef]
- Odunitan, T.T.; Apanisile, B.T.; Afolabi, J.A.; Adeniwura, P.O.; Akinboade, M.W.; Ibrahim, N.O.; Alare, K.P.; Saibu, O.A.; Adeosun, O.A.; Opeyemi, H.S.; et al. Beyond Conventional Drug Design: Exploring the Broad-Spectrum Efficacy of Antimicrobial Peptides. Chem. Biodivers. 2025, 22, e202401349. [Google Scholar] [CrossRef]
- Gani, Z.; Kumar, A.; Raje, M.; Raje, C.I. Antimicrobial Peptides: An Alternative Strategy to Combat Antimicrobial Resistance. Drug Discov. Today 2025, 30, 104305. [Google Scholar] [CrossRef] [PubMed]
- Owliaee, I.; Khaledian, M.; Shojaeian, A.; Madanchi, H.; Yarani, R.; Boroujeni, A.K.; Shoushtari, M. Antimicrobial Peptides Against Arboviruses: Mechanisms, Challenges, and Future Directions. Probiotics Antimicrob. Proteins 2025, 17, 2058–2085. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Moscoso, C.A.; Merlano, J.A.R.; Olivera Gálvez, A.; Volcan Almeida, D. Antimicrobial Peptides (AMPs) from Microalgae as an Alternative to Conventional Antibiotics in Aquaculture. Prep. Biochem. Biotechnol. 2025, 55, 26–35. [Google Scholar] [CrossRef]
- Peteláková, M.; Neprašová, B.; Šmotková, Z.; Myšková, A.; Holá, L.; Petelák, A.; Áčová, A.; Cantel, S.; Fehrentz, J.-A.; Sýkora, D.; et al. Simultaneous Treatment with Palm-LEAP2(1-14) and Feeding High-Fat Diet Attenuates Liver Lipid Metabolism but Not Obesity: Sign of Selective Resistance to Palm-LEAP2(1-14). Mol. Cell Endocrinol. 2025, 597, 112442. [Google Scholar] [CrossRef]
- Johansen, V.B.I.; Gradel, A.K.J.; Holm, S.K.; Cuenco, J.; Merrild, C.; Petersen, N.; Demozay, D.; Mani, B.K.; Suppli, M.P.; Grøndahl, M.F.G.; et al. Regulation of LEAP2 by Insulin and Glucagon in Mice and Humans. Cell Rep. Med. 2025, 6, 101996. [Google Scholar] [CrossRef]
- Ball, S.L.; Siou, G.P.; Wilson, J.A.; Howard, A.; Hirst, B.H.; Hall, J. Expression and Immunolocalisation of Antimicrobial Peptides within Human Palatine Tonsils. J. Laryngol. Otol. 2007, 121, 973–978. [Google Scholar] [CrossRef]
- Liang, T.; Ji, W.; Zhang, G.-R.; Wei, K.-J.; Feng, K.; Wang, W.-M.; Zou, G.-W. Molecular Cloning and Expression Analysis of Liver-Expressed Antimicrobial Peptide 1 (LEAP-1) and LEAP2 Genes in the Blunt Snout Bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2013, 35, 553–563. [Google Scholar] [CrossRef]
- Li, H.-X.; Lu, X.-J.; Li, C.-H.; Chen, J. Molecular Characterization and Functional Analysis of Two Distinct Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Genes in Large Yellow Croaker (Larimichthys crocea). Fish Shellfish Immunol. 2014, 38, 330–339. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.-L.; Yue, G.-H.; Fu, J.-J.; Zhou, Z.-F. Molecular Cloning and Expression Analysis of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Gene in Grass Carp. Vet. Immunol. Immunopathol. 2010, 133, 133–143. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Y.; Wang, R.; Xu, T. Characterization, Evolution and Functional Analysis of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Gene in Miiuy Croaker. Fish Shellfish Immunol. 2014, 41, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial Peptides: Premises and Promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Hocquellet, A.; le Senechal, C.; Garbay, B. Importance of the Disulfide Bridges in the Antibacterial Activity of Human Hepcidin. Peptides 2012, 36, 303–307. [Google Scholar] [CrossRef]
- Hocquellet, A.; Odaert, B.; Cabanne, C.; Noubhani, A.; Dieryck, W.; Joucla, G.; Le Senechal, C.; Milenkov, M.; Chaignepain, S.; Schmitter, J.-M.; et al. Structure–Activity Relationship of Human Liver-Expressed Antimicrobial Peptide 2. Peptides 2010, 31, 58–66. [Google Scholar] [CrossRef]
- Thiébaud, P.; Garbay, B.; Auguste, P.; Sénéchal, C.L.; Maciejewska, Z.; Fédou, S.; Gauthereau, X.; Costaglioli, P.; Thézé, N. Overexpression of Leap2 Impairs Xenopus Embryonic Development and Modulates FGF and Activin Signals. Peptides 2016, 83, 21–28. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Zou, J.; Chang, C.-I.; Secombes, C.J. Discovery and Characterization of Two Types of Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Genes in Rainbow Trout. Vet. Immunol. Immunopathol. 2004, 101, 259–269. [Google Scholar] [CrossRef]
- Bo, J.; Yang, Y.; Zheng, R.; Fang, C.; Jiang, Y.; Liu, J.; Chen, M.; Hong, F.; Bailey, C.; Segner, H.; et al. Antimicrobial Activity and Mechanisms of Multiple Antimicrobial Peptides Isolated from Rockfish Sebastiscus marmoratus. Fish Shellfish Immunol. 2019, 93, 1007–1017. [Google Scholar] [CrossRef]
- Krause, A.; Sillard, R.; Kleemeier, B.; Klüver, E.; Maronde, E.; Conejo-García, J.R.; Forssmann, W.G.; Schulz-Knappe, P.; Nehls, M.C.; Wattler, F.; et al. Isolation and Biochemical Characterization of LEAP2, a Novel Blood Peptide Expressed in the Liver. Protein Sci. 2003, 12, 143–152. [Google Scholar] [CrossRef]
- Li, H.-Z.; Shou, L.-L.; Shao, X.-X.; Liu, Y.-L.; Xu, Z.-G.; Guo, Z.-Y. Identifying Key Residues and Key Interactions for the Binding of LEAP2 to Receptor GHSR1a. Biochem. J. 2020, 477, 3199–3217. [Google Scholar] [CrossRef]
- M’Kadmi, C.; Cabral, A.; Barrile, F.; Giribaldi, J.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; Dutertre, S.; Péraldi-Roux, S.; et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J. Med. Chem. 2019, 62, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Lu, X.-J.; Li, C.-H.; Chen, J. Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) in a Teleost Fish, Plecoglossus altivelis: Antimicrobial Activity and Molecular Mechanism. Mol. Immunol. 2015, 65, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Q.; Lu, X.-J.; Chen, J. The Protection Effect of LEAP2 on the Mudskipper (Boleophthalmus pectinirostris) against Edwardsiella Tarda Infection Is Associated with Its Immunomodulatory Activity on Monocytes/Macrophages. Fish Shellfish Immunol. 2016, 59, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Chen, J.; Nie, L.; Chen, J. MOSPD2 Is a Receptor Mediating the LEAP2 Effect on Monocytes/Macrophages in a Teleost, Boleophthalmus pectinirostris. Zool. Res. 2020, 41, 644–655. [Google Scholar] [CrossRef]
- Roux, N.; Salis, P.; Lee, S.-H.; Besseau, L.; Laudet, V. Anemonefish, a Model for Eco-Evo-Devo. Evodevo 2020, 11, 20. [Google Scholar] [CrossRef]
- Liyanage, D.S.; Omeka, W.K.M.; Yang, H.; Lim, C.; Kwon, H.; Choi, C.Y.; Lee, J. Expression Profiling, Immune Functions, and Molecular Characteristics of the Tetraspanin Molecule CD63 from Amphiprion clarkii. Dev. Comp. Immunol. 2021, 123, 104168. [Google Scholar] [CrossRef]
- Chen, J.; Yu, D.; Li, Y.; Xia, H.; Xia, L.; Lei, Y.; Dong, Z.; Ye, J.; Lu, Y. Study on the Expression of Nk-Lysin from Nile Tilapia (Oreochromis niloticus) in Pichia pastoris and Its Biological Function. Aquaculture 2022, 557, 738321. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Sun, L. NKLP27: A Teleost NK-Lysin Peptide That Modulates Immune Response, Induces Degradation of Bacterial DNA, and Inhibits Bacterial and Viral Infection. PLoS ONE 2014, 9, e106543. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.-Y.; Chen, J.-Y.; Seah, R.W.X.; Zhang, L.; Ma, L.; Ding, G.-H. Host Defence Peptide LEAP2 Contributes to Antimicrobial Activity in a Mustache Toad (Leptobrachium liui). BMC Vet. Res. 2023, 19, 47. [Google Scholar] [CrossRef]
- Li, C.-H.; Lu, X.-J.; Li, M.-Y.; Chen, J. Cathelicidin Modulates the Function of Monocytes/Macrophages via the P2X7 Receptor in a Teleost, Plecoglossus altivelis. Fish Shellfish Immunol. 2015, 47, 878–885. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), Interferons, Transforming Growth Factor β, and TNF-α: Receptors, Functions, and Roles in Diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]



| Primer Name | Sequence (5′-3′) | Tm (°C) | Products (bp) | Used |
|---|---|---|---|---|
| AoLEAP2-OF | ATGGAAAGAATTTCAATCCTG | 56 | 324 | Amplification of ORF of AoLEAP2 |
| AoLEAP2-OR | TCCATGCATAGGATGTACAG | |||
| RT-AoLEAP2-F | GGGAACAGACCGTCCACATAC | 55 | 135 | Real-time PCR |
| RT-AoLEAP2-R | GCAGAGTCCTGTTAAGCATTCG | |||
| RT-AoIL1β-F | CAGTGACAACCGCAAAGT | 53 | 140 | |
| RT-AoIL1β-R | GAGATTAGTGTCCCTGATGC | |||
| RT-AoTNFα-F | GCCCAACAGGAACG | 54 | 120 | |
| RT-AoTNFα-R | TTCACGCAGATTACGAT | |||
| RT-β-actin-F | GGGCCAAAAGGACAGCTAC | 57 | 156 | |
| RT-β-actin-R | CAGGGTCAGGATACCCCTCT |
| Bacterial Strains | Minimal Inhibitory Concentration (MIC; μg/mL) | ||
|---|---|---|---|
| AoLEAP2 | Kana+ | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC6538 | >250 | + |
| Streptococcus iniae ATCC29178 | 125 | + | |
| Gram-negative bacteria | Edwardsiella tarda ATCC15947 | 7.81 | + |
| Escherichia coli ATCC8739 | 7.81 | + | |
| Aeromonas hydrophila ATCC8739 | 31.25 | + | |
| Shigella sonnei CMCC(B)51529 | 7.81 | + | |
| Pseudomonas aeruginosa ATCC27853 | 31.25 | + | |
| Aeromonas caviae BNCC139095 | 7.81 | + | |
| Proteus vulgaris CMCC(B)49027 | >250 | + | |
| Salmonella typhimurium ATCC14028 | >250 | + | |
| Proteus mirabilis CMCC(B)49005 | 7.81 | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Li, T.; Wang, K.; Zhang, M.; Mo, J.; Chen, J.; Xia, H.; Lu, Y.; Cai, J. Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Amphiprion ocellaris and Its Role in Antibacterial Immunity. Animals 2025, 15, 2590. https://doi.org/10.3390/ani15172590
Yu D, Li T, Wang K, Zhang M, Mo J, Chen J, Xia H, Lu Y, Cai J. Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Amphiprion ocellaris and Its Role in Antibacterial Immunity. Animals. 2025; 15(17):2590. https://doi.org/10.3390/ani15172590
Chicago/Turabian StyleYu, Dapeng, Tao Li, Kang Wang, Meiling Zhang, Jingyi Mo, Jianlin Chen, Hongli Xia, Yishan Lu, and Jia Cai. 2025. "Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Amphiprion ocellaris and Its Role in Antibacterial Immunity" Animals 15, no. 17: 2590. https://doi.org/10.3390/ani15172590
APA StyleYu, D., Li, T., Wang, K., Zhang, M., Mo, J., Chen, J., Xia, H., Lu, Y., & Cai, J. (2025). Molecular Characterization of the Liver-Expressed Antimicrobial Peptide 2 (LEAP2) from Amphiprion ocellaris and Its Role in Antibacterial Immunity. Animals, 15(17), 2590. https://doi.org/10.3390/ani15172590





