Comprehensive Evaluation of the Immune Response of Angus Cattle to Live Attenuated and Inactivated Goatpox Virus Vaccines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Grouping Scheme
2.2. Vaccines
2.3. Antibody Level Sample Collection and Index Determination
2.4. Blood Index Sample Collection and Index Determination
2.5. Gut Microbiota Sample Collection and Index Determination
2.6. Data Statistical Analysis
3. Results
3.1. Results of Antibody Level Determination
3.2. Blood Routine Test Results
3.3. Serum Biochemical Determination Results
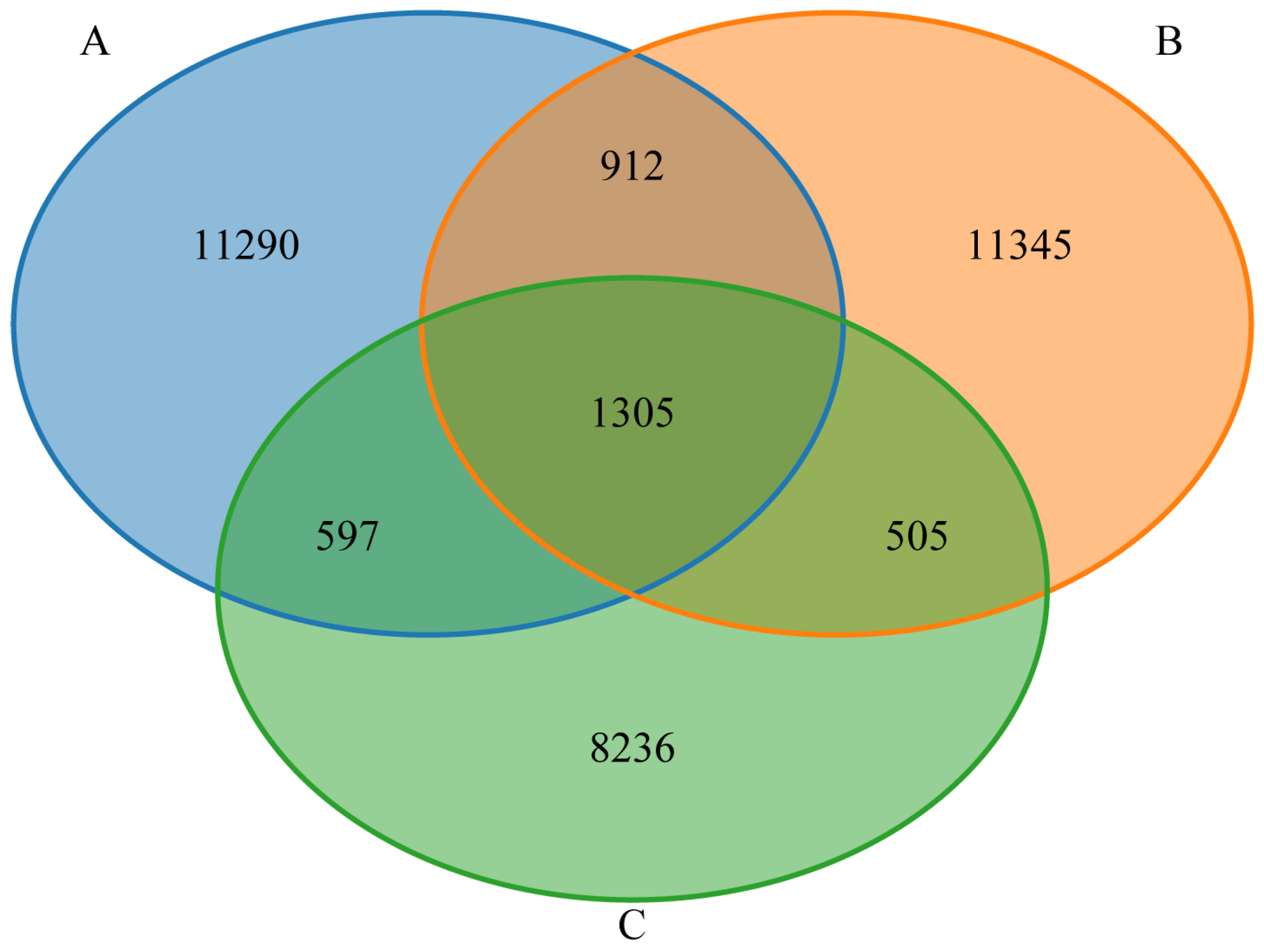
3.4. Gut Microbiota Venn Diagram Analysis
3.5. Alpha Diversity Analysis of the Gut Microbiota
3.6. Species Richness at the Phylum Level
3.7. Correlation Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Vazquez, J.A.; van der Werf, J.; Clark, S.A. Genetic and phenotypic associations of feed efficiency with growth and carcass traits in Australian Angus cattle. J. Anim. Sci. 2018, 96, 4521–4531. [Google Scholar] [CrossRef]
- Baneh, H.; Elatkin, N.; Gentzbittel, L. Genome-wide association studies and genetic architecture of carcass traits in Angus beef cattle using imputed whole-genome sequences data. Genet. Sel. Evol. 2025, 57, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Moghaddar, N.; Clark, S.; van der Werf, J.H.J.; Heras-Saldana, S.d.L.; Chaubey, G. Genome-wide scan for signatures of selection in Hanwoo and Angus cattle using whole-genome sequence data. PLoS ONE 2025, 20, e0324034. [Google Scholar] [CrossRef]
- Pandey, N.; Hopker, A.; Prajapati, G.; Rahangdale, N.; Gore, K.; Sargison, N. Observations on presumptive lumpy skin disease in native cattle and Asian water buffaloes around the tiger reserves of the central Indian highlands. N. Z. Vet. J. 2022, 70, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Adamu, K.; Abayneh, T.; Getachew, B.; Mohammed, H.; Deresse, G.; Zekarias, M.; Chala, W.; Gelaye, E. Lumpy skin disease virus isolation, experimental infection, and evaluation of disease development in a calf. Sci. Rep. 2024, 14, 20460. [Google Scholar] [CrossRef]
- Agianniotaki, E.I.; Chaintoutis, S.C.; Haegeman, A.; De Clercq, K.; Chondrokouki, E.; Dovas, C.I. A TaqMan probe-based multiplex real-time PCR method for the specific detection of wild type lumpy skin disease virus with beta-actin as internal amplification control. Mol. Cell Probe 2021, 60, 101778. [Google Scholar] [CrossRef]
- Akther, M.; Akter, S.H.; Sarker, S.; Aleri, J.W.; Annandale, H.; Abraham, S.; Uddin, J.M. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses 2023, 15, 1861. [Google Scholar] [CrossRef]
- Kumar, N.; Barua, S.; Kumar, R.; Khandelwal, N.; Kumar, A.; Verma, A.; Singh, L.; Godara, B.; Chander, Y.; Kumar, G.; et al. Evaluation of the safety, immunogenicity and efficacy of a new live-attenuated lumpy skin disease vaccine in India. Virulence 2023, 14, 2190647. [Google Scholar] [CrossRef]
- Moudgil, G.; Chadha, J.; Khullar, L.; Chhibber, S.; Harjai, K. Lumpy skin disease: Insights into current status and geographical expansion of a transboundary viral disease. Microb. Pathog. 2024, 186, 106485. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH); The World Forum of Accountants (WOFA). Manual of Diagnostic Tests and Vaccines for Terrestrial; Chapter 3.4.12; WOAH/OIE: Paris, France, 2023; pp. 1–16. [Google Scholar]
- Fawzi, E.M.; Morsi, A.M.; Abd-Elfatah, E.B. Molecular diagnosis of three outbreaks during three successive years (2018, 2019, and 2020) of Lumpy skin disease virus in cattle in Sharkia Governorate, Egypt. Open Vet. J. 2022, 12, 451–462. [Google Scholar] [CrossRef]
- Haegeman, A.; De Leeuw, I.; Philips, W.; De Regge, N. Development and Validation of a New DIVA Real-Time PCR Allowing to Differentiate Wild-Type Lumpy Skin Disease Virus Strains, Including the Asian Recombinant Strains, from Neethling-Based Vaccine Strains. Viruses 2023, 15, 870. [Google Scholar] [CrossRef]
- Liang, Z.; Yao, K.; Wang, S.; Yin, J.; Ma, X.; Yin, X.; Wang, X.; Sun, Y. Understanding the research advances on lumpy skin disease: A comprehensive literature review of experimental evidence. Front. Microbiol. 2022, 13, 1065894. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, J.; Boumart, Z.; Daouam, S.; El Arkam, A.; Bamouh, Z.; Jazouli, M.; Tadlaoui, K.O.; Fihri, O.F.; Gavrilov, B.; El Harrak, M. Development and Evaluation of an Inactivated Lumpy Skin Disease Vaccine for Cattle. Vet. Microbiol. 2020, 245, 108689. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Babin, Y.; Pestova, Y.; Kononova, S.; Wallace, D.B.; Van Schalkwyk, A.; Byadovskaya, O.; Diev, V.; Lozovoy, D.; Melcher, U.; et al. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS ONE 2018, 13, e0207480. [Google Scholar] [CrossRef] [PubMed]
- Bedeković, T.; Šimić, I.; Krešić, N.; Lojkić, I. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound. Emerg. Dis. 2018, 65, 491–496. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Tuppurainen, E.S. Serological and clinical evaluation of the Yugoslavian RM65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transbound. Emerg. Dis. 2018, 65, 1657–1663. [Google Scholar] [CrossRef]
- Kumar, N.; Chander, Y.; Kumar, R.; Khandelwal, N.; Riyesh, T.; Chaudhary, K.; Shanmugasundaram, K.; Kumar, S.; Kumar, A.; Gupta, M.K.; et al. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS ONE 2021, 16, e0241022. [Google Scholar] [CrossRef]
- Tuppurainen, E.; Dietze, K.; Wolff, J.; Bergmann, H.; Beltran-Alcrudo, D.; Fahrion, A.; Lamien, C.E.; Busch, F.; Sauter-Louis, C.; Conraths, F.J.; et al. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines 2021, 9, 1136. [Google Scholar] [CrossRef]
- Morgenstern, M.; Klement, E. The Effect of Vaccination with Live Attenuated Neethling Lumpy Skin Disease Vaccine on Milk Production and Mortality-An Analysis of 77 Dairy Farms in Israel. Vaccines 2020, 8, 324. [Google Scholar] [CrossRef]
- Hamdi, J.; Bamouh, Z.; Jazouli, M.; Boumart, Z.; Tadlaoui, K.O.; Fihri, O.F.; EL Harrak, M. Experimental evaluation of the cross-protection between Sheeppox and bovine Lumpy skin vaccines. Sci. Rep. 2020, 10, 8888. [Google Scholar] [CrossRef]
- Uzar, S.; Sarac, F.; Gulyaz, V.; Enul, H.; Yılmaz, H.; Turan, N. Comparison and efficacy of two different sheep pox vaccines prepared from the Bakirkoy strain against lumpy skin disease in cattle. Clin. Exp. Vaccine Res. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Cinicola, B.; Conti, M.G.; Terrin, G.; Sgrulletti, M.; Elfeky, R.; Carsetti, R.; Salinas, A.F.; Mortari, E.P.; Brindisi, G.; De Curtis, M.; et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021, 9, 638871. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, Y.; Cui, L.; Zhang, Y.; Chen, T.; Xue, W.; Wang, H.; Liu, H.; Zhang, Y.; Chen, L.; et al. Immunogenicity and cellular response of a herpes zoster virus gEgI fusion protein adjuvanted with CpG-emulsion in mice. J. Nanobiotechnol. 2025, 23, 395. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.N.; Spinard, E.; Azzinaro, P.A.; Rodriguez-Calzada, M.; Gutkoska, J.; Kloc, A.; Rieder, E.A.; Taillon, B.E.; Mueller, S.; Santos, T.D.L.; et al. Deoptimization of FMDV P1 Region Results in Robust Serotype-Independent Viral Attenuation. Viruses 2023, 15, 1332. [Google Scholar] [CrossRef]
- Wu, N.; Liu, Y.; Miao, C.; Yu, Z.; Ma, G.; Wu, J. Enhancing the Deformability of the Adjuvant: Achieving Lymph Node Targeted Delivery to Elicit a Long-Lasting Immune Protection. Adv. Healthc. Mater. 2025, 14, e2401520. [Google Scholar] [CrossRef]
- Zamora, C.; Diaz-Torne, C.; Ortiz, M.A.; Moya, P.; Park, H.S.; Pitarch, C.; Cantó, E.; Osuna-Gomez, R.; Mulet, M.; Garcia-Arguinzonis, M.; et al. Platelet-Derived Soluble CD40L and Its Impact on Immune Modulation and Anti-IL6R Antibody Treatment Outcome in Rheumatoid Arthritis. Cells 2025, 14, 625. [Google Scholar] [CrossRef]
- Wang, S.; Kun, T.; Tian, Z.; Huang, M.; Fan, Y.; Li, B.; Zheng, H.; Xu, R.; Wang, M.; Zhao, J.; et al. The MFGE8/integrin beta3 axis mitigates experimental neutrophilic asthma by suppressing NLRP3-Caspase-1 pathway-mediated NETosis. Resp. Res. 2025, 26, 229. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, T.; Bejjani, S.; Kim, M.S.; Lee, J.H.; Shin, Y.; Woo, S.-J.; Cheon, B.M.; Kim, D.; Lee, S.; et al. Systems serology-based comparison of humoral immune responses induced by liposome or aluminum hydroxide adjuvanted SARS-CoV-2 spike protein. Sci. Rep 2025, 15, 18734. [Google Scholar] [CrossRef]
- Hakobyan, V.; Sargsyan, K.; Kharatyan, S.; Elbakyan, H.; Sargsyan, V.; Markosyan, T.; Vardanyan, T.; Badalyan, M.; Achenbach, J.E. The Serological Response in Cattle following Administration of a Heterologous Sheep Pox Virus Strain Vaccine for Protection from Lumpy Skin Disease; Current Situation in Armenia. Vet. Sci. 2023, 10, 102. [Google Scholar] [CrossRef]
- Chi, L.; Tu, P.; Ru, H.; Lu, K. Studies of xenobiotic-induced gut microbiota dysbiosis: From correlation to mechanisms. Gut Microbes 2021, 13, 1921912. [Google Scholar] [CrossRef]
- Tomkinson, S.; Triscott, C.; Schenk, E.; Foey, A. The Potential of Probiotics as Ingestible Adjuvants and Immune Modulators for Antiviral Immunity and Management of SARS-CoV-2 Infection and COVID-19. Pathogens 2023, 12, 928. [Google Scholar] [CrossRef]
- Patel, S.P.; Bhoraniya, S.J.; Kalola, S.D.; Rukadikar, A.; Ravi, R.; Farooqui, S.; Rukadikar, C. Gut Microbiota and its Impact on Chronic Diseases: A Comprehensive Review. J. Pharm. Bioallied Sci. 2025, 17, S1080–S1082. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashidi, H.E. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi J. Biol. Sci. 2022, 29, 1628–1643. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Haile, A.F.; Woodfint, R.M.; Kim, E.; Joldrichsen, M.R.; Berhe, N.; Gebreyes, W.A.; Boyaka, P.N. Broad-Spectrum and Gram-Negative-Targeting Antibiotics Differentially Regulate Antibody Isotype Responses to Injected Vaccines. Vaccines 2021, 9, 1240. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhou, M.; Luo, Z.; Lv, L.; Pei, J.; Wang, C.; Chai, B.; Sui, B.; Huang, F.; et al. Composition of the murine gut microbiome impacts humoral immunity induced by rabies vaccines. Clin. Transl. Med. 2020, 10, e161. [Google Scholar] [CrossRef]
- Yu, B.; Pei, C.; Peng, W.; Zheng, Y.; Fu, Y.; Wang, X.; Wang, W.; Wang, Z.; Chen, Y.; Wang, Q.; et al. Microbiota-derived butyrate alleviates asthma via inhibiting Tfh13-mediated IgE production. Signal Transduct. Tar. 2025, 10, 181. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Tar. 2023, 8, 283. [Google Scholar] [CrossRef]
- Wai, K.K.; Liang, Y.; Zhou, L.; Cai, L.; Liang, C.; Liu, L.; Lin, X.; Wu, H.; Lin, J. The protective effects of Acanthus ilicifolius alkaloid A and its derivatives on pro- and anti-inflammatory cytokines in rats with hepatic fibrosis. Biotechnol. Appl. Bioc. 2015, 62, 537–546. [Google Scholar] [CrossRef]



| Groups | Numbers | Inoculation Dose 1 | Second Immune | Inoculation Time |
|---|---|---|---|---|
| A | 10 | 2 mL | none | a.m. |
| B | 10 | 2 mL | 14 d | a.m. |
| C | 10 | 2 mL | none | a.m. |
| Items 1 | Groups | SEM 2 | p-Value | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Day 14 | |||||
| RBC (1012/L) | 6.84 b | 7.27 ab | 7.63 a | 0.12 | 0.005 |
| PLT (109/L) | 216.31 b | 250.97 a | 156.63 c | 8.70 | <0.001 |
| WBC (109/L) | 7.15 a | 6.49 b | 6.23 b | 0.13 | 0.004 |
| NEUT (%) | 76.53 a | 70.82 b | 68.51 c | 0.75 | <0.001 |
| LYM (%) | 16.11 a | 14.90 a | 12.23 b | 0.44 | <0.001 |
| Day 28 | |||||
| RBC (1012/L) | 7.49 | 7.53 | 7.58 | 0.15 | 0.973 |
| PLT (109/L) | 202.00 b | 270.90 a | 160.94 c | 9.65 | <0.001 |
| WBC (109/L) | 6.69 ab | 7.10 a | 6.13 b | 0.16 | 0.049 |
| NEUT (%) | 75.80 b | 88.09 a | 69.24 c | 1.78 | <0.001 |
| LYM (%) | 18.60 a | 19.20 a | 11.54 b | 0.76 | <0.001 |
| Day 42 | |||||
| RBC (1012/L) | 7.54 | 7.74 | 7.66 | 0.25 | 0.951 |
| PLT (109/L) | 161.50 b | 184.73 a | 162.13 b | 3.94 | 0.017 |
| WBC (109/L) | 6.83 | 6.91 | 6.17 | 0.16 | 0.107 |
| NEUT (%) | 70.73 | 71.64 | 69.91 | 0.64 | 0.555 |
| LYM (%) | 12.50 ab | 13.53 a | 11.28 b | 0.32 | 0.009 |
| Day 56 | |||||
| RBC (1012/L) | 7.46 | 7.54 | 7.77 | 0.18 | 0.781 |
| PLT (109/L) | 164.08 | 168.65 | 163.48 | 4.52 | 0.888 |
| WBC (109/L) | 6.42 | 6.44 | 6.35 | 0.18 | 0.976 |
| NEUT (%) | 69.41 | 70.81 | 69.34 | 0.60 | 0.541 |
| LYM (%) | 12.20 | 12.70 | 11.51 | 0.42 | 0.529 |
| Items 1 | Groups | SEM 2 | p-Value | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Day 14 | |||||
| AST (U/L) | 105.28 a | 79.26 b | 60.03 c | 3.58 | <0.001 |
| TP (g/L) | 69.80 a | 59.36 b | 57.06 b | 1.16 | <0.001 |
| BUN (mmol/L) | 5.92 a | 5.34 b | 2.55 c | 0.29 | <0.001 |
| UA (umol/L) | 98.49 a | 78.05 b | 49.67 c | 4.06 | <0.001 |
| GGT (U/L) | 54.26 a | 32.63 b | 23.07 c | 2.46 | <0.001 |
| CREA (umol/L) | 122.46 | 118.70 | 109.78 | 2.34 | 0.071 |
| Day 28 | |||||
| AST (U/L) | 72.77 a | 68.04 a | 60.69 b | 1.50 | 0.002 |
| TP (g/L) | 80.04 a | 74.49 b | 57.31 c | 1.83 | <0.001 |
| BUN (mmol/L) | 3.94 | 4.33 | 2.56 | 0.33 | 0.061 |
| UA (umol/L) | 72.89 | 68.80 | 50.14 | 4.70 | 0.106 |
| GGT (U/L) | 32.94 ab | 41.15 a | 23.06 b | 2.50 | 0.008 |
| CREA (umol/L) | 116.26 | 114.36 | 110.22 | 1.62 | 0.309 |
| Day 42 | |||||
| AST (U/L) | 63.62 | 65.76 | 60.55 | 1.05 | 0.121 |
| TP (g/L) | 68.75 ab | 77.75 a | 57.20 b | 2.86 | 0.008 |
| BUN (mmol/L) | 3.21 | 3.69 | 2.55 | 0.24 | 0.160 |
| UA (umol/L) | 56.80 | 60.32 | 49.41 | 2.18 | 0.111 |
| GGT (U/L) | 30.48 | 31.34 | 23.16 | 1.80 | 0.124 |
| CREA (umol/L) | 110.79 | 112.68 | 110.88 | 1.06 | 0.726 |
| Day 56 | |||||
| AST (U/L) | 62.18 | 63.32 | 60.77 | 0.87 | 0.504 |
| TP (g/L) | 60.91 | 65.34 | 57.07 | 2.32 | 0.358 |
| BUN (mmol/L) | 2.54 | 3.01 | 2.44 | 0.17 | 0.374 |
| UA (umol/L) | 51.61 | 56.54 | 50.11 | 1.46 | 0.174 |
| GGT (U/L) | 24.77 | 26.79 | 23.84 | 1.15 | 0.580 |
| CREA (umol/L) | 111.25 | 113.08 | 110.06 | 1.34 | 0.667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Chen, C.; Yang, L.; Abulaiti, A.; Hua, J. Comprehensive Evaluation of the Immune Response of Angus Cattle to Live Attenuated and Inactivated Goatpox Virus Vaccines. Animals 2025, 15, 2592. https://doi.org/10.3390/ani15172592
Tian S, Chen C, Yang L, Abulaiti A, Hua J. Comprehensive Evaluation of the Immune Response of Angus Cattle to Live Attenuated and Inactivated Goatpox Virus Vaccines. Animals. 2025; 15(17):2592. https://doi.org/10.3390/ani15172592
Chicago/Turabian StyleTian, Shijun, Chao Chen, Lei Yang, Adili Abulaiti, and Jinling Hua. 2025. "Comprehensive Evaluation of the Immune Response of Angus Cattle to Live Attenuated and Inactivated Goatpox Virus Vaccines" Animals 15, no. 17: 2592. https://doi.org/10.3390/ani15172592
APA StyleTian, S., Chen, C., Yang, L., Abulaiti, A., & Hua, J. (2025). Comprehensive Evaluation of the Immune Response of Angus Cattle to Live Attenuated and Inactivated Goatpox Virus Vaccines. Animals, 15(17), 2592. https://doi.org/10.3390/ani15172592





