Hippotherapy in the Treatment of CMD and Bruxism in Dentistry
Simple Summary
Abstract
1. Introduction
1.1. Current State in the Treatment of CMD
1.2. Current State of CMD Hippotherapy
1.3. Present Therapeutical Concept for Bruxism/CMD
1.4. Associated Clinical Measures Within the Multi-Disciplinary Therapy of Bruxism/CMD
1.5. Goal of the Study
1.6. Cortisol Measurement
1.7. HRV Measurement
1.8. Stress Measurements in a Horse
1.9. Baevsky Stress Index (SI)
2. Methods
2.1. HRV and HF Measurement
2.2. Animal Protection Issues
3. Results and Findings
3.1. Salivary Cortisol Measurement
3.1.1. Findings with Female Riders in the Intervention Group (Germany)
3.1.2. Findings with Female Riders in the Control Group (Switzerland)
3.2. HRV Measurement Findings in Intervention Group Germany
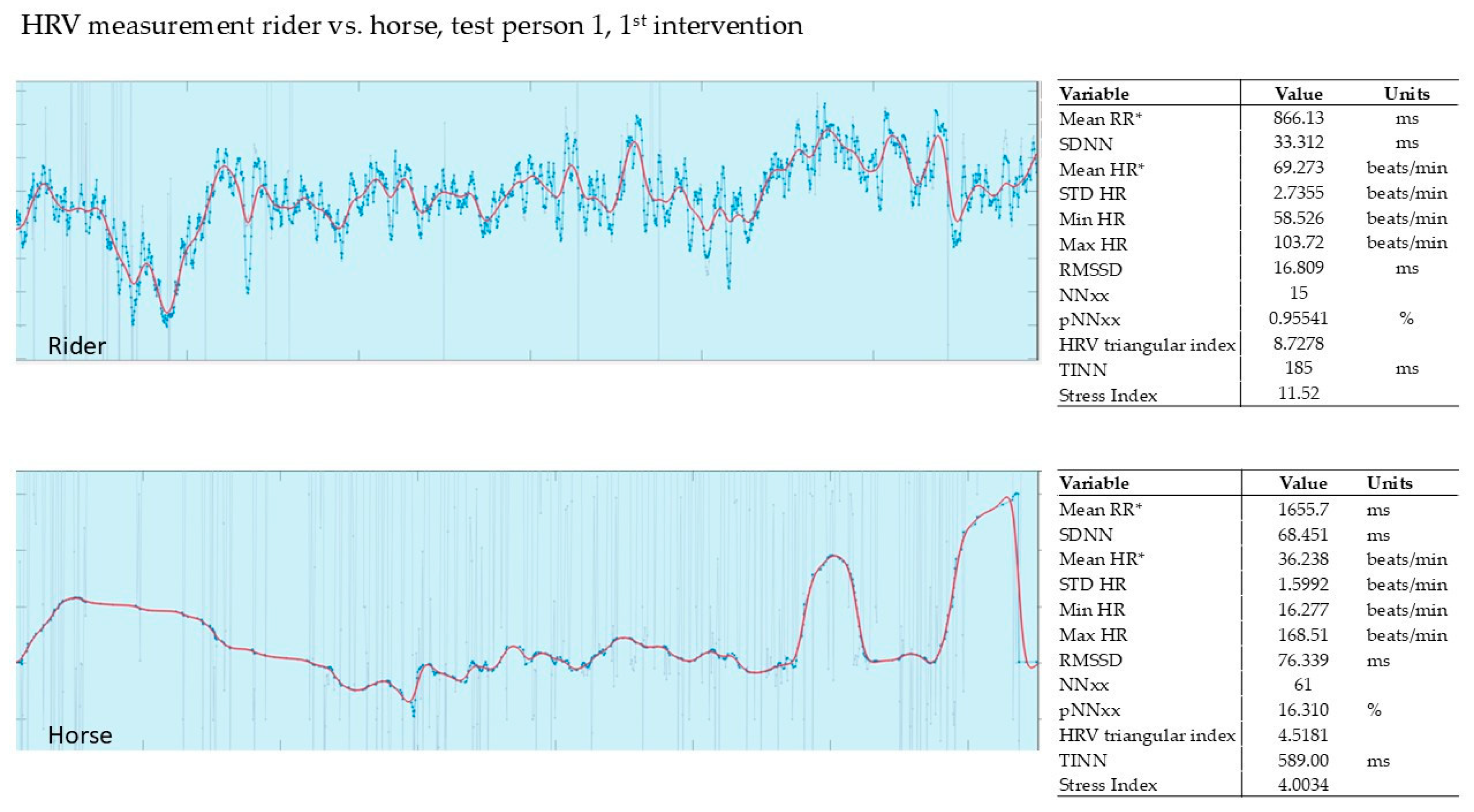
3.2.1. Evaluation of the 1st Intervention—Germany
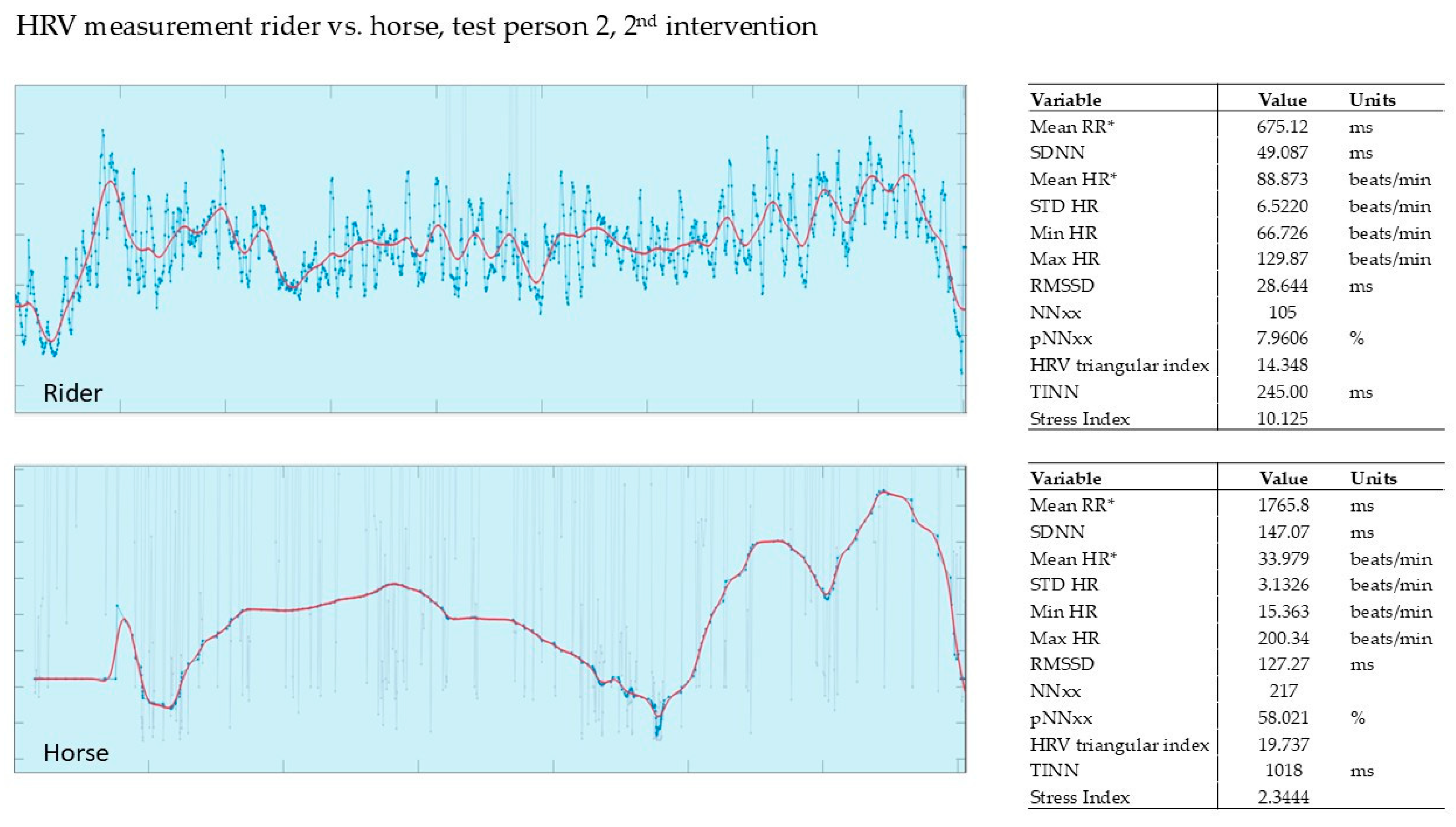
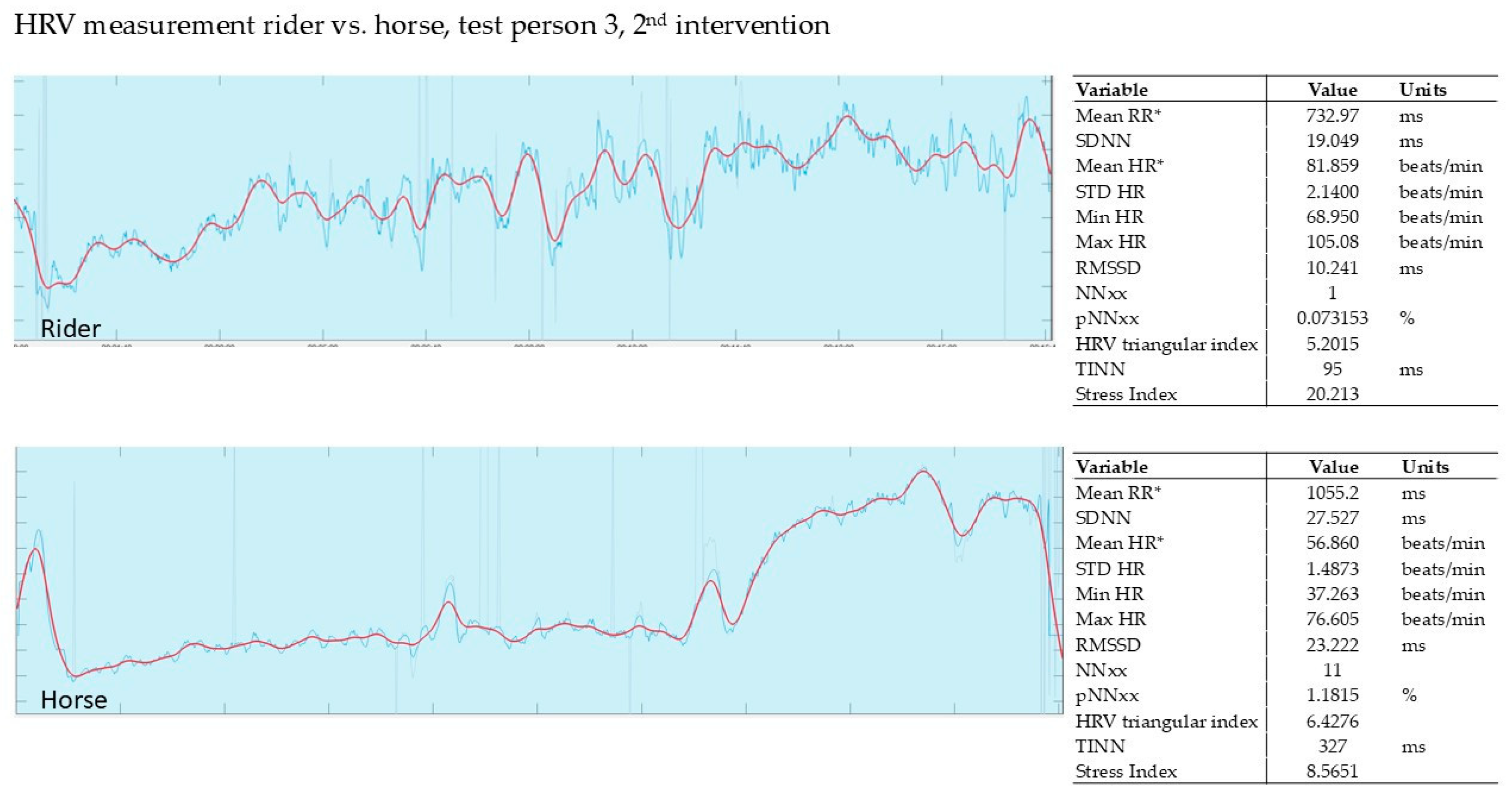
3.2.2. Evaluation of the 2nd Intervention—Germany—After Three Months
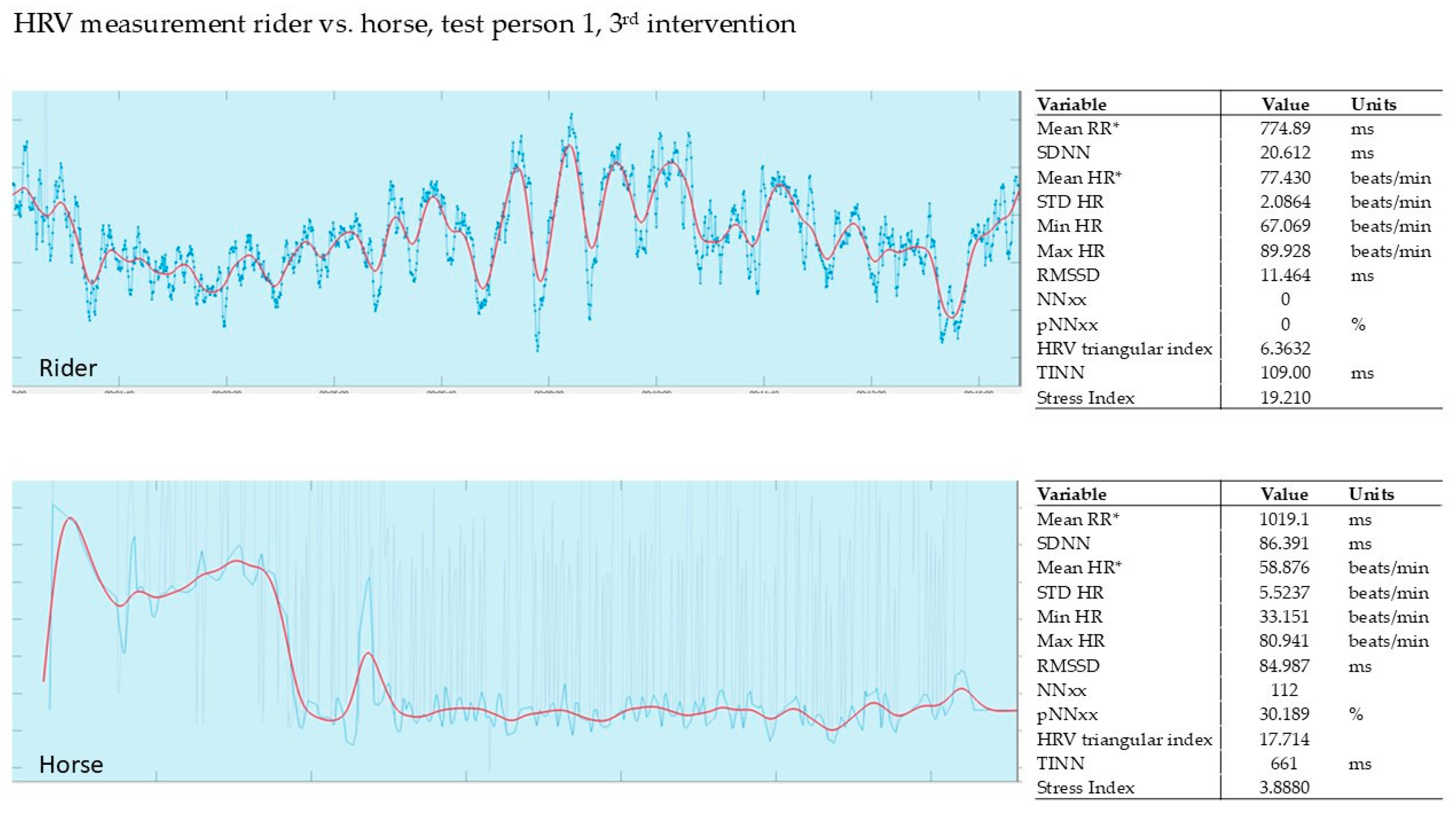
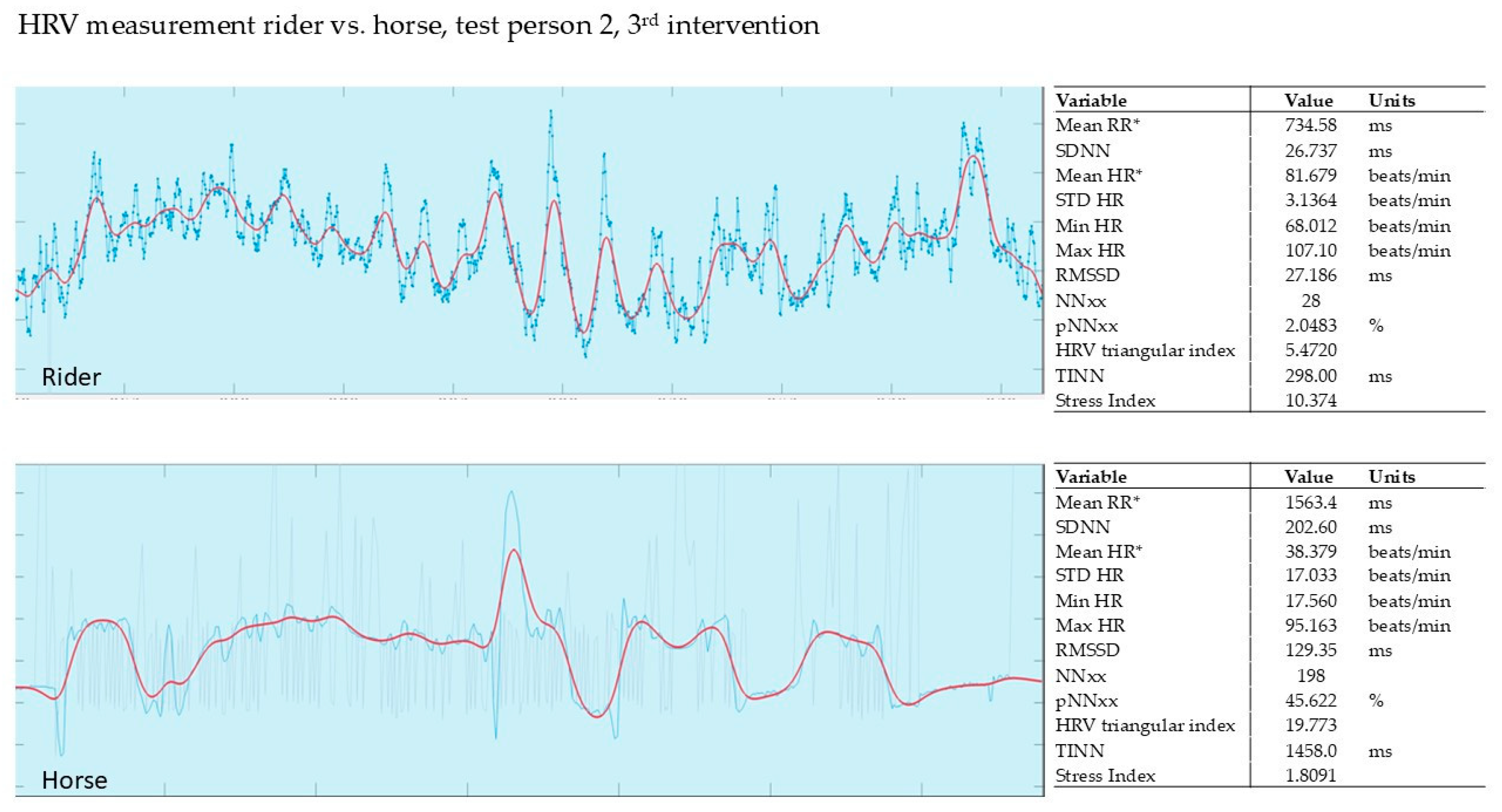
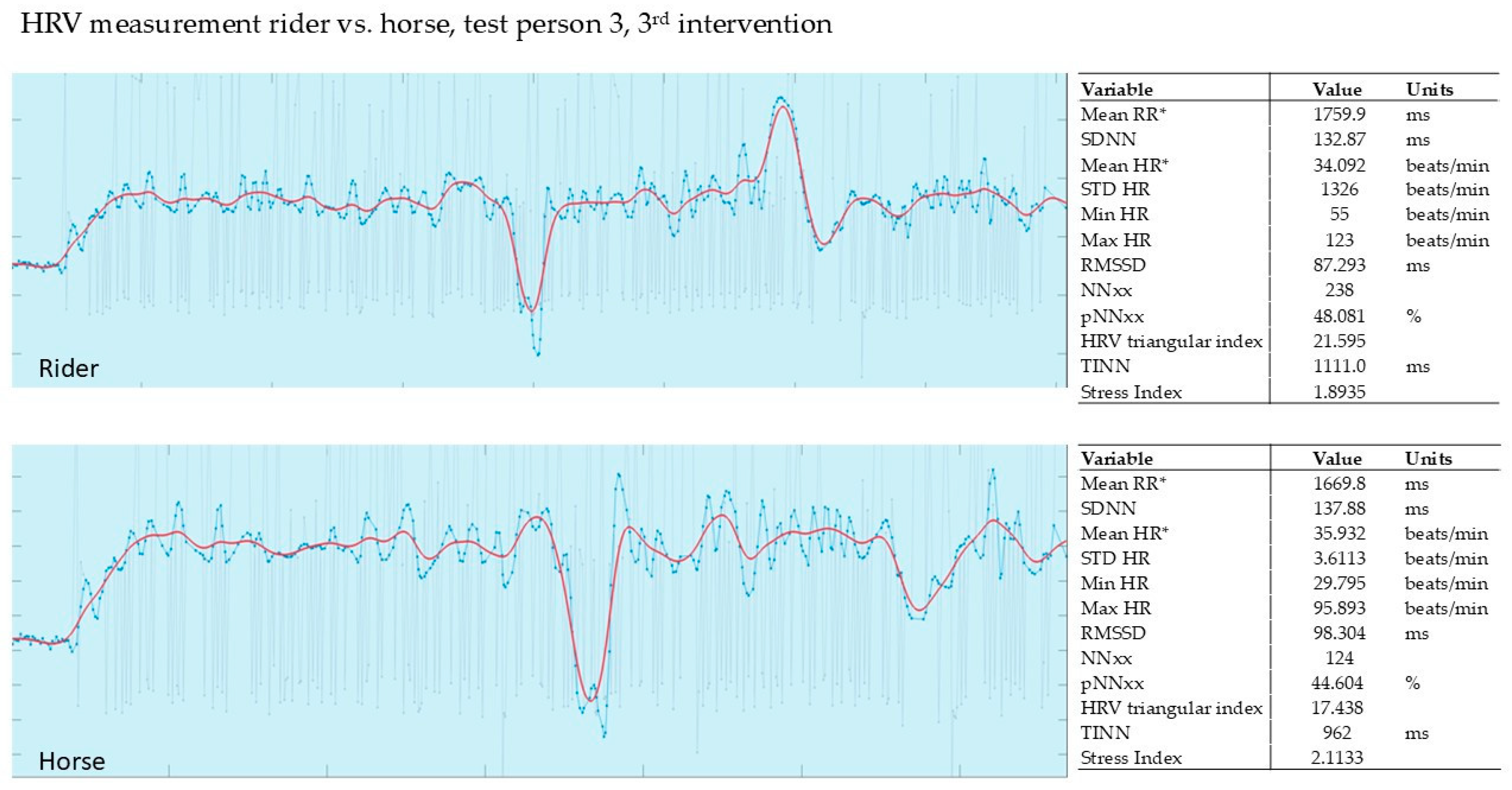
3.2.3. Evaluation of the 3rd Intervention—Germany—After Six Months
4. Discussion
4.1. Stress Measurement
4.2. Aspects of Animal Welfare
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugger, A.; Lange, M.; Schindler, H.J.; Türp, J.C. Begriffsbestimmungen: Funktionsstörung, Dysfunktion, Craniomandibuläre Dysfunktion (CMD), Myoarthropathie des Kausystems (MAP), Deutsche Gesellschaft für Funktionsdiagnostik Und-Therapie (DGFT), Archived: 01/2016. Available online: https://www.dgfdt.de/documents/266840/266917/Begriffsbestimmungen+NEU/3cc28f96-978a-447d-a154-e08e0b5cd9bd (accessed on 1 July 2025).
- AWMF Leitlinie S3 Diagnosik und Behandlung des Bruxismus. 2019. Available online: https://register.awmf.org/de/leitlinien/detail/083-027 (accessed on 1 July 2025).
- De Leeuw, R.; Klasser, G.D.; Reyes, M.R. Orofacial Pain, Guidelines for Assessment, Diagnosis, and Management. In American Academy of Orofacial Pain, 7th ed.; Quintessence Publishing Co. Limited: New Malden, UK; Surrey, UK, 2009; ISBN 978-0-86715-413-9. [Google Scholar]
- Deutsches Kuratorium für Therapeutisches Reiten, E.V. Available online: https://dkthr.de/ (accessed on 1 July 2025).
- Wahlund, K.; Nilsson, I.M.; Larsson, B. Treating temporomandibular disorders in adolescents: A randomized, controlled, sequential comparison of relaxation training and occlusal appliance therapy. J. Oral Facial Pain Headache 2015, 29, 41–50. [Google Scholar] [CrossRef]
- Jacobson, E. Progressive Relaxation: A Physiological and Clinical Investigation of Muscular States and Their Significance in Psychology and Medical Practice, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1938. [Google Scholar]
- Torales, J.; O’Higgins, M.; Barrios, I.; Gonzalez, I.; Almirón, M. An Overview of Jacobson’s Progressive Muscle Relaxation in Managing Anxiety. Rev. Argent. Clin. Psicol. 2020, XXIX, 17–23. [Google Scholar] [CrossRef]
- Stresshormon Verringert Knochenstabilität Bei Kindern. 2014. Available online: https://www.aerzteblatt.de/news/stresshormon-verringert-knochenstabilitaet-bei-kindern-18c0b7c4-2d9e-4816-a972-e6a01bd87287 (accessed on 1 July 2025).
- Shi, L.; Sánchez-Guijo, A.; Hartmann, M.F.; Schönau, E.; Esche, J.; Wudy, S.A.; Remer, T. Higher glucocorticoid secretion in the physiological range is associated with lower bone strength at the proximal radius in healthy children: Importance of protein intake adjustment. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Dauerstress Behindert Die Knochenheilung Nach Brüchen. 2019. Available online: https://www.aerzteblatt.de/news/dauerstress-behindert-die-knochenheilung-nach-bruechen-3d73772b-ad70-4175-8ce9-64d7951ad9d6 (accessed on 1 July 2025).
- Haffner-Luntzer, M.; Foertsch, S.; Fischer, V.; Prystaz, K.; Tschaffon, M.; Mödinger, Y.; Bahney, C.S.; Marcucio, R.S.; Miclau, T.; Ignatius, A.; et al. Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via β-AR signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 8615–8622. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Schneider, E.M. Editorial: Depression: An Insight and Need for Personalized Psychological Stress Monitoring and Management. J. Basic Appl. Sci. 2014, 10, 177–182. [Google Scholar] [CrossRef]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous measurement of salivary cortisol and alpha-amylase: Application and recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Pilger, A.; Haslacher, H.; Meyer, B.M.; Lackner, A.; Nassan-Agha, S.; Nistler, S.; Stanglmaier, C.; Endler, G.; Mikulits, A.; Priemer, I.; et al. Midday and nadir salivary cortisol appear superior to cortisol awakening response in burnout assessment and monitoring. Sci. Rep. 2018, 8, 9151. [Google Scholar] [CrossRef]
- Wollschläger, T. Normwerte der Herzfrequenzvariabilität: Was Bedeuten Sie für Ihre Gesundheit? (archived on 24 February 2022). Available online: https://www.meduni.com/herzfrequenzvariabilitaet-normwerte-bedeutung-fuer-gesundheit/ (accessed on 1 July 2025).
- Hughson, R.; Yamamoto, Y.; McCullough, R.; Sutton, J.; Reeves, J. Sympathetic and parasympathetic indicators of heart rate control at altitude studied by spectral analysis. J. Appl. Physiol. 1994, 77, 2537–2542. [Google Scholar] [CrossRef]
- Chiu, H.W.; Kao, T. A mathematical model for autonomic control of heart rate variation. IEEE Eng. Med. Biol. Mag. 2001, 20, 69–76. [Google Scholar] [CrossRef]
- Hottenrott, K.; Hoos, O.; Esperer, H.D. Herzfrequenzvariabilität: Gesundheitsförderung, Trainingssteuerung, Biofeedback. Satellitensymposium: Training und Therapie in Künstlicher Höhe; Schriftenreihe der Deutschen Vereinigung für Sportwissenschaft Band; Feldhaus Verlag: Hamburg, Germany, 2011; Volume 214. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, S.; Teferra, M.N.; Baumert, M.; Bidargaddi, N. Heart Rate Variability for Evaluating Psychological Stress Changes in Healthy Adults: A Scoping Review. Neuropsychobiology 2023, 82, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Herzfrequenzvariabilität: Was Sie Bedeutet und Wie Sie Ihre Werte Verbessern. Available online: https://www.barmer.de/gesundheit-verstehen/sport/bewegung-und-fitness/herzfrequenzvariabilitaet-1296634#:~:text=Herzfrequenzvariabilität%3A%20Normwerte.-Die%20Herzfrequenzvariabilität%20kann&text=Als%20Faustregel%20gilt%3A%20„Bei%20einer.normal”%2C%20erk (accessed on 1 July 2025).
- Miller, K.F. Kennen Sie Die Normalwerte Ihres Pferdes—Wie Man Die Wichtigsten Vitalwerte Erfasst, Misst und Verfolgt (archived on 11 July 2023). Available online: https://haygain.de/blogs/news-events/kennen-sie-die-normalwerte-ihres-pferdes-wie-man-die-wichtigsten-vitalwerte-erfasst-misst-und-verfolgt (accessed on 1 July 2025).
- Uhlendorf, F. Vergleichende Untersuchung Zu elektrokardiographischen Techniken und der Analyse der Herzfrequenzvariabilität Mit Dem Langzeit-EKG Bei Warmblutpferden. Dissertation Hannover 2009 URN (urn:nbn:de:gbv:95-97168). Available online: https://elib.tiho-hannover.de/receive/etd_mods_00001360 (accessed on 1 July 2025).
- Löllgen, H. Serie: Neue Methoden Der Kardialen Funktionsdiagnostik—Herzfrequenzvariabilität. Deutsches Ärzteblatt, 1999, Ausgabe: 31-32/1999. Available online: https://www.aerzteblatt.de/archiv/serie-neue-methoden-in-der-kardialen-funktionsdiagnostik-herzfrequenzvariabilitaet-2be7facc-833c-44e8-83b1-767542c59208 (accessed on 1 July 2025).
- Naber, A.; Kreuzer, L.; Zink, R.; Millesi, E.; Palme, R.; Hediger, K.; Glenk, L.M. Heart rate, heart rate variability and salivary cortisol as indicators of arousal and synchrony in clients with intellectual disability, horses and therapist during equine-assisted interventions. Pet Behav. Sci. 2019, 7, 17–23. [Google Scholar] [CrossRef]
- Hödl, S. Transportstress Bei Pferden, Einfluss von wiederholten Transporten Auf Der Strasse Auf Die Belastung Von Transportunerfahrenen Pferden. Masterarbeit. 2010. Available online: https://unipub.uni-graz.at/obvugrhs/download/pdf/213031 (accessed on 1 July 2025).
- Baevsky, R.M.; Chernikova, A.G. Heart Rate Variability Analysis: Physiological Foundations and Main Methods. Cardiometry 2017, 10, 66–76. [Google Scholar] [CrossRef]
- Methodical Recommendations Use KARDiVAR System for Determination of the Stress Level and Estimation of the Body Adaptability. Standards of Measurements and Physiological Interpretation. 2009. Available online: https://api.semanticscholar.org/CorpusID:29215863 (accessed on 1 July 2025).
- Wundrak, S.; Paul, J.; Ulrici, J.; Hell, E.; Geibel, M.A.; Bernhardt, P.; Rottbauer, W.; Rasche, V. A self-gating method for time-resolved imaging of nonuniform motion. Magn. Reson. Med. 2016, 76, 919–925. [Google Scholar] [CrossRef]
- Kaiser, L.; Heleski, C.R.; Siegford, J.; Smith, K.A. Stress-related behaviors among horses used in a therapeutic riding program. J. Am. Vet. Med. Assoc. 2006, 228, 39–45. [Google Scholar] [CrossRef]
- Brelsford, V.L.; Dimolareva, M.; Gee, N.R.; Meints, K. Best Practice Standards in Animal-Assisted Interventions: How the LEAD Risk Assessment Tool Can Help. Animals 2020, 10, 974. [Google Scholar] [CrossRef]
- McKinney, C.; Mueller, M.K.; Frank, N. Effects of Therapeutic Riding on Measures of Stress in Horses. J. Equine Vet. Sci. 2015, 35, 922–928. [Google Scholar] [CrossRef]
- Welsch, U.; Deller, T. Lehrbuch Histologie, 3rd ed.; Elsevier Verlag: Amsterdam, The Netherlands, 2010; ISBN 9783437593819. [Google Scholar]
- Tarvainen, M.P.; Lipponen, J.; Niskanen, J.-P.; Ranta-Aho, P.O. Kubios HRV Software USER’S GUIDE. November 2021. Available online: https://www.kubios.com/downloads/Kubios_HRV_Users_Guide.pdf (accessed on 31 August 2025).
- Gussgard, A.M.; Weese, J.S.; Hensten, A.; Jokstad, A. Dog-assisted therapy in the dental clinic: Part A-Hazards and assessment of potential risks to the health and safety of humans. Clin. Exp. Dent. Res. 2019, 5, 692–700. [Google Scholar] [CrossRef]
- Gussgard, A.M.; Carlstedt, K.; Meirik, M. Intraoral clinical examinations of pediatric patients with anticipatory anxiety and situational fear facilitated by therapy dog assistance: A pilot RCT. Clin. Exp. Dent. Res. 2023, 9, 122–133. [Google Scholar] [CrossRef]
- Geibel, M.A. Orale Medizin Band 1, Gender Dentistry Grundlagen und Konsequenzen für Den Zahnmedizinischen Alltag; Lehmanns Media: Berlin, Germany, 2021; pp. 203–214. [Google Scholar]
- Johnson, R.A.; Johnson, P.J.; Megarani, D.V.; Patel, S.D.; Yaglom, H.D.; Osterlind, S.; Grindler, K.; Vogelweid, C.M.; Parker, T.M.; Pascua, C.K.; et al. Horses Working in Therapeutic Riding Programs: Cortisol, Adrenocorticotropic Hormone, Glucose, and Behavior Stress Indicators. J. Equine Vet. Sci. 2017, 57, 77–85. [Google Scholar] [CrossRef]
- Malinowski, K.; Yee, C.; Tevlin, J.M.; Birks, E.K.; Durando, M.M.; Pournajafi-Nazarloo, H.; Cavaiola, A.A.; McKeever, K.H. The Effects of Equine Assisted Therapy on Plasma Cortisol and Oxytocin Concentrations and Heart Rate Variability in Horses and Measures of Symptoms of Post-Traumatic Stress Disorder in Veterans. J. Equine Vet. Sci. 2018, 64, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Carrillo, A.; Iniesta, P.; Ferrer, P. Pilot Study of the Influence of Equine Assisted Therapy on Physiological and Behavioral Parameters Related to Welfare of Horses and Patients. Animals 2021, 11, 3527. [Google Scholar] [CrossRef]
- Seery, R.; Wells, D. An Exploratory Study into the Backgrounds and Perspectives of Equine-Assisted Service Practitioners. Animals 2024, 14, 347. [Google Scholar] [CrossRef]
- Fine, A.H.; Beck, A.M.; Zenithson, N. The State of Animal-Assisted Interventions: Addressing the Contemporary Issues That Will Shape the Future. Int. J. Environ. Res. Public Health 2019, 16, 3997. [Google Scholar] [CrossRef]
- International Association of Human-Animal Interaction (IAHAIO). International Guidelines on Care, Training and Welfare Requirements for Equines in Equine-Assisted Services. Version 1, 2021 (17.12.24). Available online: https://iahaio.org (accessed on 1 July 2025).
- Serpell, J.A.; Coppinger, R.; Fine, A.H.; Peralta, J.M. Welfare considerations in therapy and assistance animals. In Handbook on Animal-Assisted Therapy; Fine, A.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- TVT, Tierärztliche Vereinigung Für Tierschutz. Nutzung Von Tieren Im Sozialen Einsatz, 131.9 Pferde. June 2012. Available online: https://www.tierschutz-tvt.de (accessed on 1 July 2025).

| Age Group | HRV Standard Values |
|---|---|
| 20 to 25 years | HRV 55 to 105 |
| 30 to 40 years | HRV 38 to 80 |
| 50 to 55 years | HRV 30 to 55 |
| 60 to 65 years | HRV 25 to 45 |
| 1st Intervention T1 | Δ | 2nd Intervention T2 | Δ | 3rd Intervention T3 | Δ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Participant No. | 1–1 | 1–2 | 2–1 | 2–2 | 3–1 | 3–3 | |||
| 1 | 8.28 | 2.44 | −71% | 5.47 | 3.26 | −40% | 10.40 | 5.48 | −47% |
| 2 | 8.91 | 0.19 | −98% | 18.60 | 2.63 | −86% | 9.91 | 4.41 | −55% |
| 3 | 13.40 | 5.63 | −58% | 10.10 | 1.35 | −87% | 2.66 | 1.98 | −26% |
| 4 | 7.62 | 2.81 | −63% | 3.63 | 2.00 | −45% | 9.72 | 3.79 | −61% |
| 5 | 7.45 | 0.54 | −93% | 9.32 | 1.83 | −80% | * | * | * |
| 6 | 10.80 | 3.54 | −67% | 3.06 | 2.73 | −11% | 13.20 | 4.43 | −66% |
| 7 | 5.19 | 2.53 | −51% | 8.75 | 3.00 | −66% | 9.90 | 3.50 | −65% |
| SD | 17.6% | 28.5% | 15.1% * | ||||||
| mean | −72% | −59% | −53% * | ||||||
| Control Group Jumpers Switzerland | |||||
|---|---|---|---|---|---|
| Tournament | |||||
| Participant No. | 1st Sample | 2nd Sample | T1 | T2 | Δ |
| 1 | 6:10 | 18:40 | 4.80 | 3.47 | −28% |
| 2 | 5:15 | 13:00 | 5.86 | 0.14 | −98% |
| 3 | 5:50 | 12:50 | 15.80 | 7.41 | −53% |
| 4 | 5:35 | 13:00 | 12.60 | 12.20 | −3% |
| 5 | 7:00 | 17:40 | 11.30 | 9.36 | −17% |
| 6 | 6:40 | 17:40 | 11.40 | 1.47 | −87% |
| 9 | 7:00 | 14:00 | 5.48 | 2.33 | −57% |
| 10 | 4:21 | 14:57 | 2.97 | 1.89 | −36% |
| 11 | 6:30 | 15:30 | 15.40 | 9.70 | −37% |
| SD | 31.1% | ||||
| mean | −46% | ||||
| Control Group Jumpers Switzerland | |||||
|---|---|---|---|---|---|
| Jumping Instruction | |||||
| Participant No. | 1st Sample | 2nd Sample | T1 | T2 | Δ |
| 1 | 13:00 | 14:00 | 6.86 | 4.39 | −36% |
| 2 | 7:30 | 18:30 | 6.83 | 6.04 | −12% |
| 3 | 6:00 | 18:30 | 9.87 | 8.02 | −19% |
| 4 | 8:00 | 18:30 | 14.80 | 14.70 | −1% |
| 5 | 7:00 | 19:00 | 2.98 | 2.65 | −11% |
| SD | 13.0% | ||||
| mean | −16% | ||||
| Control Group Jumpers Switzerland | |||||
|---|---|---|---|---|---|
| Leisure Riding | |||||
| Participant No. | 1st Sample | 2nd Sample | T1 | T2 | Δ |
| 1 | 7:00 | 11:30 | 15.50 | 12.80 | −17% |
| 2 | 7:00 | 13:00 | 9.94 | 8.69 | −13% |
| 3 | 9:00 | 17:00 | 6.40 | 4.43 | −31% |
| 4 | 7:30 | 17:00 | 13.20 | 5.29 | −60% |
| SD | 21.3% | ||||
| mean | −30% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geibel, M.-A.; Kildal, D.; Geibel, A.M.; Ott, S. Hippotherapy in the Treatment of CMD and Bruxism in Dentistry. Animals 2025, 15, 2587. https://doi.org/10.3390/ani15172587
Geibel M-A, Kildal D, Geibel AM, Ott S. Hippotherapy in the Treatment of CMD and Bruxism in Dentistry. Animals. 2025; 15(17):2587. https://doi.org/10.3390/ani15172587
Chicago/Turabian StyleGeibel, Margrit-Ann, Daniela Kildal, Amina Maria Geibel, and Sibylle Ott. 2025. "Hippotherapy in the Treatment of CMD and Bruxism in Dentistry" Animals 15, no. 17: 2587. https://doi.org/10.3390/ani15172587
APA StyleGeibel, M.-A., Kildal, D., Geibel, A. M., & Ott, S. (2025). Hippotherapy in the Treatment of CMD and Bruxism in Dentistry. Animals, 15(17), 2587. https://doi.org/10.3390/ani15172587






