Hemoparasites in Wild Birds: A Systematic Review of Their Ecology and Clinical Implications
Simple Summary
Abstract
1. Introduction
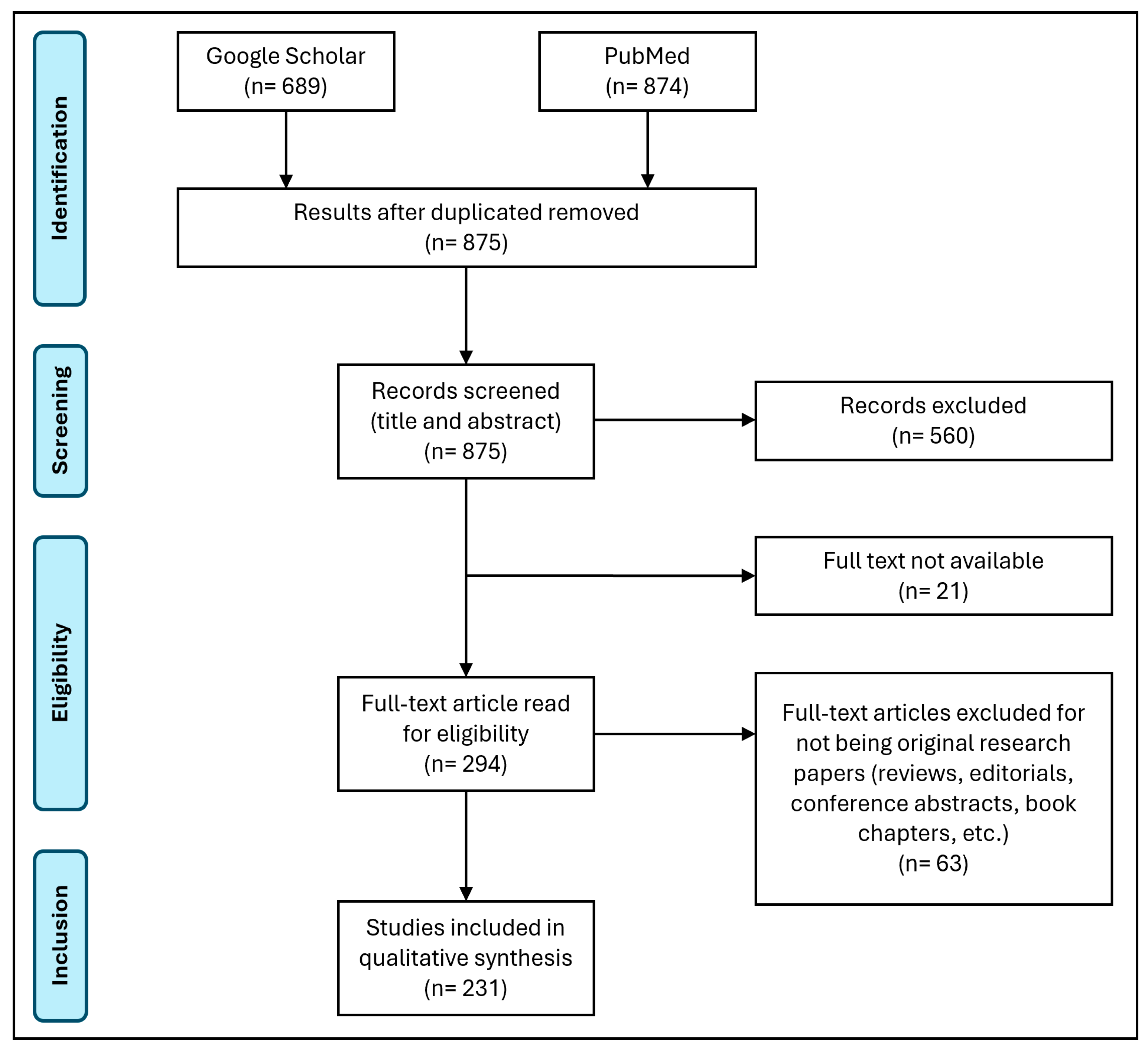
2. Materials and Methods
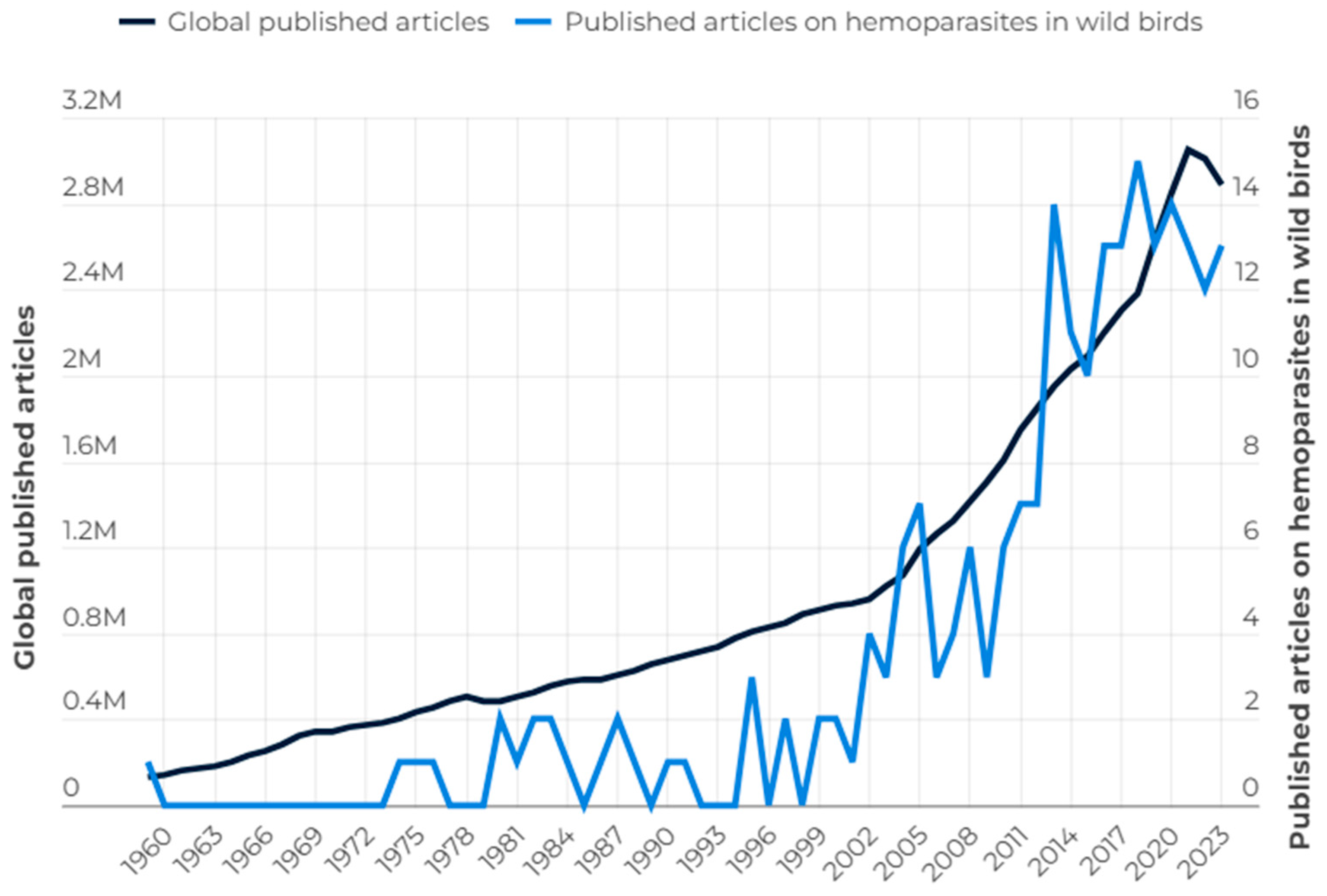
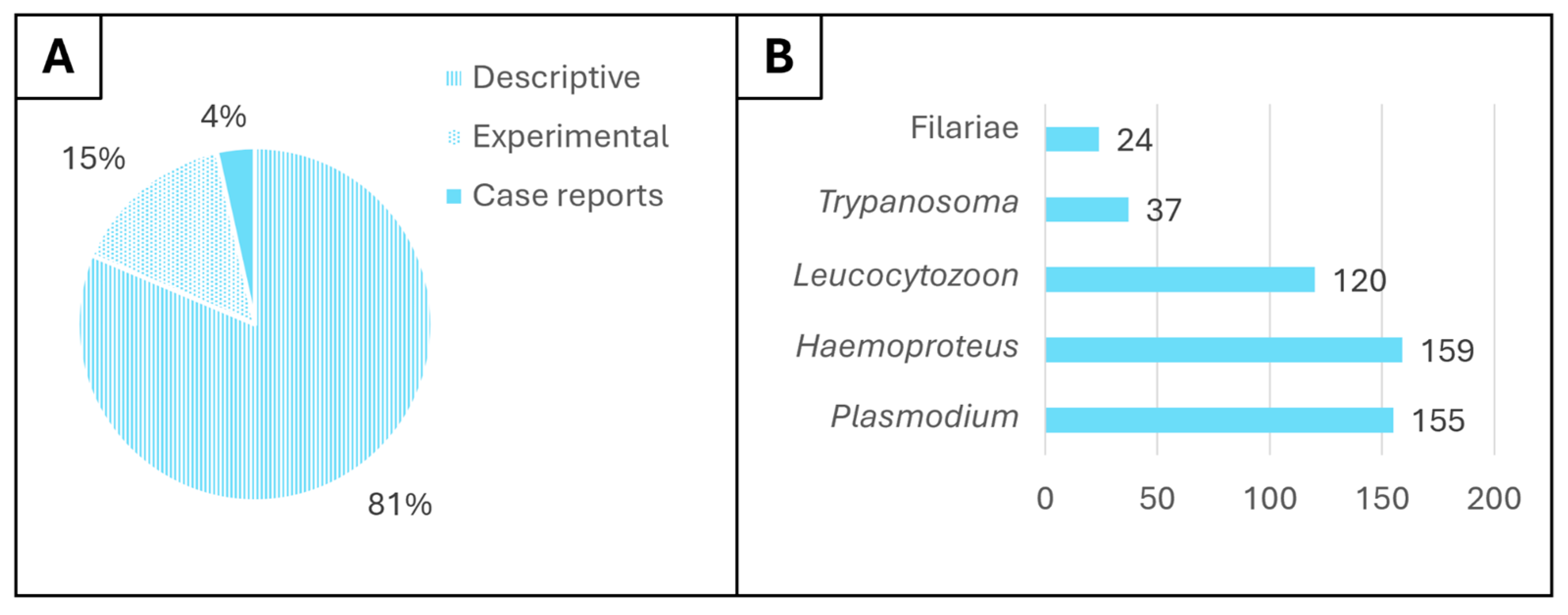
3. Bibliometric Analysis
4. Clinical Signs Related to Hemoparasites in Wild Birds
4.1. Effects on Body Condition, Mass, and Growth
4.2. Effects on Reproductive Success and Survival
4.3. Blood Parameters and Immune Response Alterations
4.4. Effects on Bird Activity, Body Temperature, and Behavior
4.5. Effects on Feather Quality or Growth
4.6. Interactions with Ectoparasites
5. Lesions and Mortality
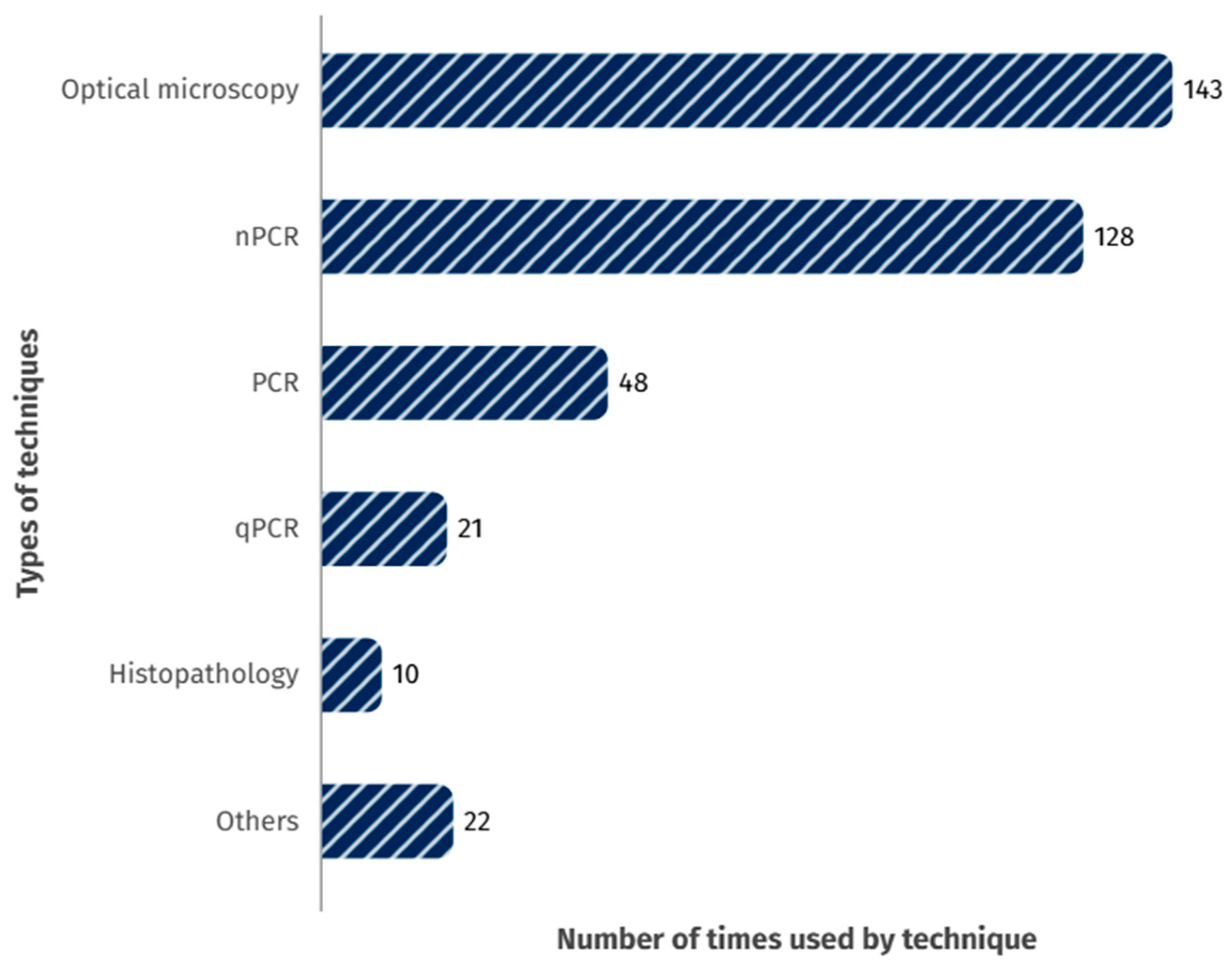
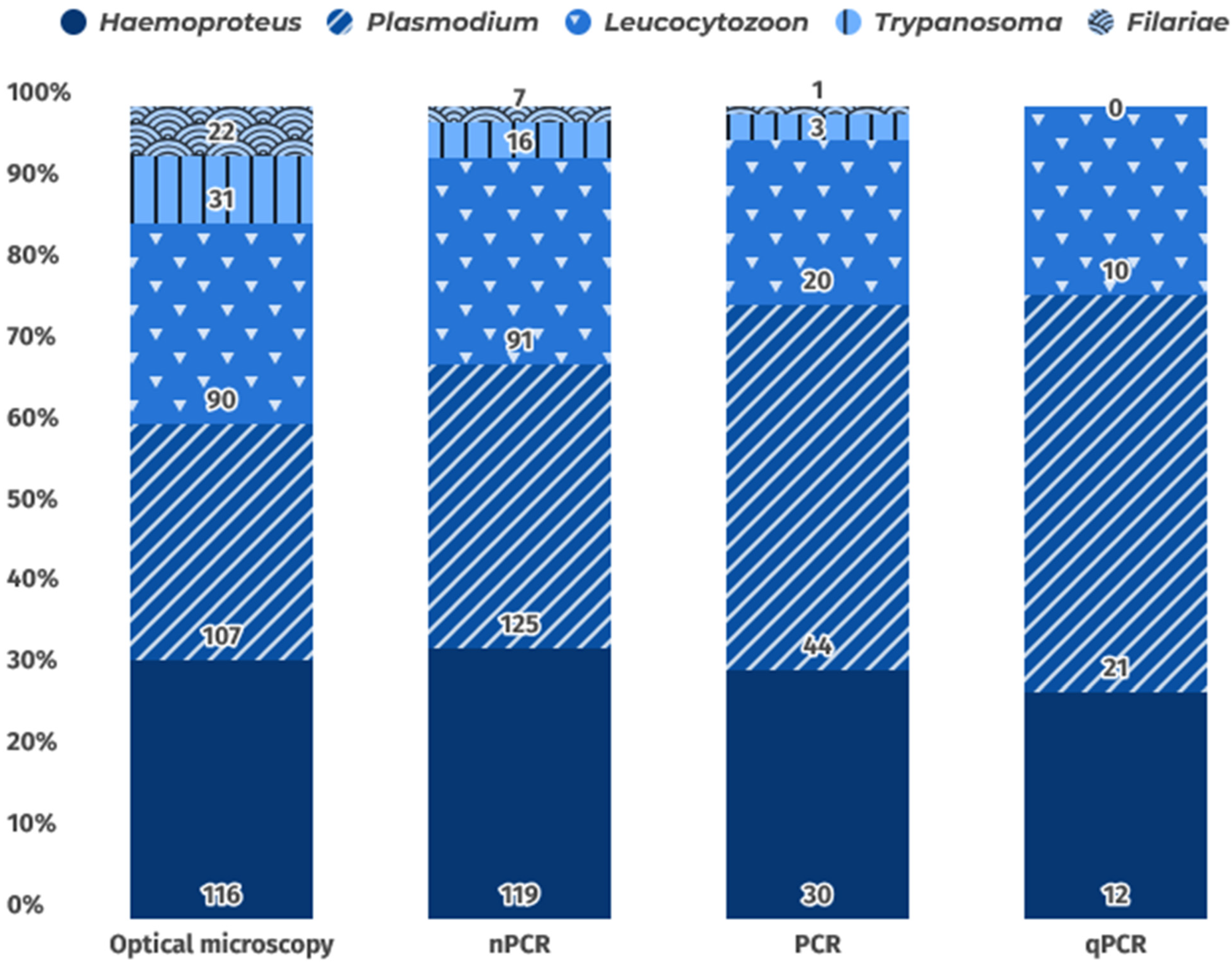
6. Diagnostic Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, T.C.; Hernández Saint-Aubin, L.M.; Madrigal Sesma, M.J. Parasitología General, 2nd ed.; Traducción de: General Parasitology; Editorial Limusa: Mexico City, Mexico, 1978. [Google Scholar]
- Clayton, D.; Moore, J. Host-Parasite Evolution: General Principles and Avian Models; Oxford University Press (OUP): Oxford, UK, 1997. [Google Scholar]
- Poulin, R. Evolutionary Ecology of Parasites: From Individuals to Communities; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Omeragic, J.; Seric-Haracic, S.; Kapo, N. Zoonotic Parasites and Vector-Borne Parasitoses. In Zoonosis of Public Health Interest; IntechOpen: London, UK, 2022; Available online: https://ideas.repec.org//h/ito/pchaps/263127.html (accessed on 16 October 2024).
- de La Rocque, S.; Balenghien, T.; Halos, L.; Dietze, K.; Claes, F.; Ferrari, G.; Guberti, V.; Slingenbergh, J. A review of trends in the distribution of vector-borne diseases: Is international trade contributing to their spread? Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2011, 30, 119–130. [Google Scholar] [CrossRef]
- Ryser-Degiorgis, M.-P. Wildlife health investigations: Needs, challenges and recommendations. BMC Vet. Res. 2013, 9, 223. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Dunn, A.M.; Smith, M.J.; Telfer, S. Wildlife diseases: From individuals to ecosystems. J. Anim. Ecol. 2011, 80, 19–38. [Google Scholar] [CrossRef]
- Swangneat, K.; Srikacha, N.; Soulinthone, N.; Paudel, S.; Srisanyong, W.; Stott, C.J.; Mahawan, T.; Pornpanom, P. Molecular Prevalence of Avian Haemosporidian Parasites in Southeast Asia: Systematic Review and Meta-Analysis. Animals 2025, 15, 636. [Google Scholar] [CrossRef]
- Yan, W.-L.; Sun, H.-T.; Zhao, Y.-C.; Hou, X.-W.; Zhang, M.; Zhao, Q.; Elsheikha, H.M.; Ni, H.-B. Global prevalence of Plasmodium infection in wild birds: A systematic review and meta-analysis. Res. Vet. Sci. 2024, 168, 105136. [Google Scholar] [CrossRef] [PubMed]
- Villalva-Pasillas, D.; Medina, J.P.; Soriano-Vargas, E.; Martínez-Hernández, D.A.; García-Conejo, M.; Galindo-Sánchez, K.P.; Sánchez-Jasso, J.M.; Talavera-Rojas, M.; Salgado-Miranda, C. Haemoparasites in endemic and non-endemic passerine birds from central Mexico highlands. Int. J. Parasitol. Parasites Wildl. 2020, 11, 88–92. [Google Scholar] [CrossRef]
- Greiner, E.C.; Bennett, G.F.; White, E.M.; Coombs, R.F. Distribution of the avian hematozoa of North America. Can. J. Zool. 1975, 53, 1762–1787. [Google Scholar] [CrossRef]
- Adl, S.M.; Leander, B.S.; Simpson, A.G.B.; Archibald, J.M.; Anderson, O.R.; Bass, D.; Bowser, S.S.; Brugerolle, G.; Farmer, M.A.; Karpov, S.; et al. Diversity, nomenclature, and taxonomy of protists. Syst. Biol. 2007, 56, 684–689. [Google Scholar] [CrossRef]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.J.; Sabatier, P. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg. Infect. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, C.; Chen, J.; Sun, Y.; Tang, T.; Zhang, Y.; Yang, Y.; Wang, G.; Xu, Q.; Zhang, X.; et al. The global distribution and diversity of wild-bird-associated pathogens: An integrated data analysis and modeling study. Med 2025, 6, 100553. [Google Scholar] [CrossRef]
- Jindal, M.; Stone, H.; Lim, S.; MacIntyre, C.R. A Geospatial Perspective Toward the Role of Wild Bird Migrations and Global Poultry Trade in the Spread of Highly Pathogenic Avian Influenza H5N1. GeoHealth 2025, 9, e2024GH001296. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Zirintunda, G.; Ssempijja, F.; Buyinza, B.; Alzahrani, K.J.; Matama, K.; Nakimbugwe, H.N.; Alkazmi, L.; Onanyang, D.; Bogere, P.; et al. Epidemiology of Trypanosomiasis in Wildlife—Implications for Humans at the Wildlife Interface in Africa. Front. Vet. Sci. 2021, 8, 621699. [Google Scholar] [CrossRef]
- Leal Filho, W.; Nagy, G.J.; Gbaguidi, G.J.; Paz, S.; Dinis, M.A.P.; Luetz, J.M.; Sharifi, A. The role of climatic changes in the emergence and re-emergence of infectious diseases: Bibliometric analysis and literature-supported studies on zoonoses. One Health Outlook 2025, 7, 12. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; de la Hera, I.; Fernández-González, S.; Pérez-Tris, J. Global warming will reshuffle the areas of high prevalence and richness of three genera of avian blood parasites. Glob. Change Biol. 2014, 20, 2406–2416. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Muñoz, E.; Ferrer, D.; Molina, R.; Adlard, R.D. Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet. Rec. 1999, 144, 632–636. [Google Scholar] [CrossRef]
- Merino, S.; Moreno, J.; José Sanz, J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. London. Ser. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Liutkevicius, G.; Iezhova, T.A. Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera, Ceratopogonidae). J. Parasitol. 2002, 88, 864–868. [Google Scholar] [CrossRef]
- Votýpka, J.; Oborník, M.; Volf, P.; Svobodová, M.; Lukes, J. Trypanosoma avium of raptors (Falconiformes): Phylogeny and identification of vectors. Parasitology 2002, 125 Pt 3, 253–263. [Google Scholar] [CrossRef]
- Pérez-Tris, J.; Bensch, S. Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology 2005, 131, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, H.; Waldenström, J.; Hansson, B.; Hasselquist, D.; von Schantz, T.; Bensch, S. Associations between malaria and MHC genes in a migratory songbird. Proc. R. Soc. B Biol. Sci. 2005, 272, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.L.; Knowles, S.C.L.; Day, K.P.; Sheldon, B.C. No evidence for avian malaria infection during the nestling phase in a passerine bird. J. Parasitol. 2006, 92, 1302–1304. [Google Scholar] [CrossRef]
- Martínez de la Puente, J.; Merino, S.; Tomás, G.; Moreno Klemming, J.; Morales, J.; Lobato, E.; García-Fraile, S. Can the host immune system promote multiple invasions of erythrocytes in vivo? Differential effects of medication and host sex in a wild malaria-like model. Parasitology 2007, 134, 651–655. [Google Scholar] [CrossRef]
- Ortego, J.; Cordero, P.J.; Aparicio, J.M.; Calabuig, G. No relationship between individual genetic diversity and prevalence of avian malaria in a migratory kestrel. Mol. Ecol. 2007, 16, 4858–4866. [Google Scholar] [CrossRef]
- Marzal, A.; Bensch, S.; Reviriego, M.; Balbontin, J.; De Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef]
- Stjernman, M.; Råberg, L.; Nilsson, J.-Å. Maximum Host Survival at Intermediate Parasite Infection Intensities. PLoS ONE 2008, 3, e2463. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Palinauskas, V.; Sheldon, B.C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.C.L.; Wood, M.J.; Sheldon, B.C. Context-dependent effects of parental effort on malaria infection in a wild bird population, and their role in reproductive trade-offs. Oecologia 2010, 164, 87–97. [Google Scholar] [CrossRef]
- La Puente, J.M.; Merino, S.; Tomás, G.; Moreno, J.; Morales, J.; Lobato, E.; García-Fraile, S.; Belda, E.J. The blood parasite Haemoproteus reduces survival in a wild bird: A medication experiment. Biol. Lett. 2010, 6, 663–665. [Google Scholar] [CrossRef]
- Wojczulanis-Jakubas, K.; Svoboda, A.; Kruszewicz, A.; Johnsen, A. No Evidence of Blood Parasites in Little Auks (Alle alle) Breeding on Svalbard. J. Wildl. Dis. 2010, 46, 574–578. [Google Scholar] [CrossRef]
- Karell, P.; Ahola, K.; Karstinen, T.; Kolunen, H.; Siitari, H.; Brommer, J.E. Blood parasites mediate morph-specific maintenance costs in a colour polymorphic wild bird. J. Evol. Biol. 2011, 24, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.C.L.; Wood, M.J.; Alves, R.; Wilkin, T.A.; Bensch, S.; Sheldon, B.C. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol. Ecol. 2011, 20, 1062–1076. [Google Scholar] [CrossRef]
- Lachish, S.; Knowles, S.C.L.; Alves, R.; Wood, M.J.; Sheldon, B.C. Fitness effects of endemic malaria infections in a wild bird population: The importance of ecological structure. J. Anim. Ecol. 2011, 80, 1196–1206. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Martínez, J.; Rivero-de Aguilar, J.; Herrero, J.; Merino, S. On the specificity of avian blood parasites: Revealing specific and generalist relationships between haemosporidians and biting midges. Mol. Ecol. 2011, 20, 3275–3287. [Google Scholar] [CrossRef]
- Yohannes, E.; Palinauskas, V.; Valkiūnas, G.; Lee, R.W.; Bolshakov, C.V.; Bensch, S. Does avian malaria infection affect feather stable isotope signatures? Oecologia 2011, 167, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Glaizot, O.; Fumagalli, L.; Iritano, K.; Lalubin, F.; Rooyen, J.V.; Christe, P. High Prevalence and Lineage Diversity of Avian Malaria in Wild Populations of Great Tits (Parus major) and Mosquitoes (Culex pipiens). PLoS ONE 2012, 7, e34964. [Google Scholar] [CrossRef]
- Garroway, C.J.; Radersma, R.; Sepil, I.; Santure, A.W.; De Cauwer, I.; Slate, J.; Sheldon, B.C. Fine-scale genetic structure in a wild bird population: The role of limited dipersal and environmentally based selection as casual factors. Evolution 2013, 67, 3488–3500. [Google Scholar] [CrossRef]
- Isaksson, C.; Sepil, I.; Baramidze, V.; Sheldon, B.C. Explaining variance of avian malaria infection in the wild: The importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Marzal, A.; Reviriego, M.; Hermosell, I.G.; Balbontín, J.; Bensch, S.; Relinque, C.; Rodríguez, L.; Garcia-Longoria, L.; de Lope, F. Malaria infection and feather growth rate predict reproductive success in house martins. Oecologia 2013, 171, 853–861. [Google Scholar] [CrossRef]
- Mendes, L.; Pardal, S.; Morais, J.; Antunes, S.; Ramos, J.A.; Perez-Tris, J.; Piersma, T. Hidden haemosporidian infections in Ruffs (Philomachus pugnax) staging in Northwest Europe en route from Africa to Arctic Europe. Parasitol. Res. 2013, 112, 2037–2043. [Google Scholar] [CrossRef]
- Palinauskas, V.; Iezhova, T.A.; Križanauskienė, A.; Markovets, M.Y.; Bensch, S.; Valkiūnas, G. Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): A pathogenic avian parasite. Parasitol. Int. 2013, 62, 358–363. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; de la Puente, J.; Onrubia, A.; Pérez-Tris, J. Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae). Int. J. Parasitol. 2013, 43, 381–387. [Google Scholar] [CrossRef]
- Sepil, I.; Lachish, S.; Hinks, A.E.; Sheldon, B.C. Mhc supertypes confer both qualitative and quantitative resistance to avian malaria infections in a wild bird population. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130134. [Google Scholar] [CrossRef]
- González, A.D.; Matta, N.E.; Ellis, V.A.; Miller, E.T.; Ricklefs, R.E.; Gutiérrez, H.R. Mixed Species Flock, Nest Height, and Elevation Partially Explain Avian Haemoparasite Prevalence in Colombia. PLoS ONE 2014, 9, e100695. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Wood, M.J.; Alves, R.; Sheldon, B.C. Dispersal in a patchy landscape reveals contrasting determinants of infection in a wild avian malaria system. J. Anim. Ecol. 2014, 83, 429–439. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Žiegytė, R.; Palinauskas, V.; Iezhova, T.A.; Dimitrov, D.; Ilgūnas, M.; Bernotienė, R.; Markovets, M.Y.; Valkiūnas, G. Biting midges (Culicoides, Diptera) transmit Haemoproteus parasites of owls: Evidence from sporogony and molecular phylogeny. Parasites Vectors 2015, 8, 303. [Google Scholar] [CrossRef]
- Chakarov, N.; Linke, B.; Boerner, M.; Goesmann, A.; Krüger, O.; Hoffman, J.I. Apparent vector-mediated parent-to-offspring transmission in an avian malaria-like parasite. Mol. Ecol. 2015, 24, 1355–1363. [Google Scholar] [CrossRef]
- Dinhopl, N.; Nedorost, N.; Mostegl, M.M.; Weissenbacher-Lang, C.; Weissenböck, H. In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol. Res. 2015, 114, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Delhaye, J.; Christe, P. Testing Local Adaptation in a Natural Great Tit-Malaria System: An Experimental Approach. PLoS ONE 2015, 10, e0141391. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A.; Palinauskas, V.; Ilgūnas, M.; Bernotienė, R. The evidence for rapid gametocyte viability changes in the course of parasitemia in Haemoproteus parasites. Parasitol. Res. 2015, 114, 2903–2909. [Google Scholar] [CrossRef]
- Bernotienė, R.; Palinauskas, V.; Iezhova, T.; Murauskaitė, D.; Valkiūnas, G. Avian haemosporidian parasites (Haemosporida): A comparative analysis of different polymerase chain reaction assays in detection of mixed infections. Exp. Parasitol. 2016, 163, 31–37. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef]
- Delhaye, J.; Jenkins, T.; Christe, P. Plasmodium infection and oxidative status in breeding great tits, Parus major. Malar. J. 2016, 15, 531. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A.; Dinhopl, N.; Nedorost, N.; Weissenbacher-Lang, C.; Weissenböck, H.; Valkiūnas, G. Mortality and pathology in birds due to Plasmodium (Giovannolaia) homocircumflexum infection, with emphasis on the exoerythrocytic development of avian malaria parasites. Malar. J. 2016, 15, 256. [Google Scholar] [CrossRef]
- Mukhin, A.; Palinauskas, V.; Platonova, E.; Kobylkov, D.; Vakoliuk, I.; Valkiūnas, G. The Strategy to Survive Primary Malaria Infection: An Experimental Study on Behavioural Changes in Parasitized Birds. PLoS ONE 2016, 11, e0159216. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Iezhova, T.A. Description of Haemoproteus ciconiae sp. Nov. (Haemoproteidae, Haemosporida) from the white stork Ciconia ciconia, with remarks on insensitivity of established polymerase chain reaction assays to detect this infection. Parasitol. Res. 2016, 115, 2609–2616. [Google Scholar] [CrossRef]
- Žiegytė, R.; Bernotienė, R.; Palinauskas, V.; Valkiūnas, G. Haemoproteus tartakovskyi (Haemoproteidae): Complete sporogony in Culicoides nubeculosus (Ceratopogonidae), with implications for avian haemoproteid experimental research. Exp. Parasitol. 2016, 160, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, F.; Rao, M.; Huang, X.; Bensch, S. Estimating prevalence of avian haemosporidians in natural populations: A comparative study on screening protocols. Parasites Vectors 2017, 10, 127. [Google Scholar] [CrossRef]
- Padilla, D.P.; Illera, J.C.; Gonzalez-Quevedo, C.; Villalba, M.; Richardson, D.S. Factors affecting the distribution of haemosporidian parasites within an oceanic island. Int. J. Parasitol. 2017, 47, 225–235. [Google Scholar] [CrossRef]
- Videvall, E.; Cornwallis, C.K.; Ahrén, D.; Palinauskas, V.; Valkiūnas, G.; Hellgren, O. The transcriptome of the avian malaria parasite Plasmodium ashfordi displays host-specific gene expression. Mol. Ecol. 2017, 26, 2939–2958. [Google Scholar] [CrossRef]
- Arriero, E.; Pérez-Tris, J.; Ramírez, A.; Remacha, C. Trade-off between tolerance and resistance to infections: An experimental approach with malaria parasites in a passerine bird. Oecologia 2018, 188, 1001–1010. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Bukauskaitė, D.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G. A new blood parasite of leaf warblers: Molecular characterization, phylogenetic relationships, description and identification of vectors. Parasites Vectors 2018, 11, 538. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological determinants of avian malaria infections: An integrative analysis at landscape, mosquito and vertebrate community levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Masello, J.F.; Martínez, J.; Calderón, L.; Wink, M.; Quillfeldt, P.; Sanz, V.; Theuerkauf, J.; Ortiz-Catedral, L.; Berkunsky, I.; Brunton, D.; et al. Can the intake of antiparasitic secondary metabolites explain the low prevalence of hemoparasites among wild Psittaciformes? Parasites Vectors 2018, 11, 357. [Google Scholar] [CrossRef]
- Pigeault, R.; Cozzarolo, C.-S.; Choquet, R.; Strehler, M.; Jenkins, T.; Delhaye, J.; Bovet, L.; Wassef, J.; Glaizot, O.; Christe, P. Haemosporidian infection and co-infection affect host survival and reproduction in wild populations of great tits. Int. J. Parasitol. 2018, 48, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Dadam, D.; Robinson, R.A.; Clements, A.; Peach, W.J.; Bennett, M.; Rowcliffe, J.M.; Cunningham, A.A. Avian malaria-mediated population decline of a widespread iconic bird species. R. Soc. Open Sci. 2019, 6, 182197. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; García-Longoria, L.; Soriguer, R.; Figuerola, J.; Marzal, A. From Africa to Europe: Evidence of transmission of a tropical Plasmodium lineage in Spanish populations of house sparrows. Parasites Vectors 2019, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.; Fragner, K.; Platonova, E.; Weissenböck, H.; Valkiūnas, G. Patterns of Plasmodium homocircumflexum virulence in experimentally infected passerine birds. Malar. J. 2019, 18, 174. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Palinauskas, V.; Platonova, E.; Iezhova, T.; Valkiūnas, G. The experimental study on susceptibility of common European songbirds to Plasmodium elongatum (lineage pGRW6), a widespread avian malaria parasite. Malar. J. 2019, 18, 290. [Google Scholar] [CrossRef]
- Jiménez-Peñuela, J.; Ferraguti, M.; Martínez-de la Puente, J.; Soriguer, R.; Figuerola, J. Urbanization and blood parasite infections affect the body condition of wild birds. Sci. Total Environ. 2019, 651, 3015–3022. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Wecker, C.; Marek, C.; Wassmuth, M.; Bentele, A.; Willems, H.; Reiner, G.; Quillfeldt, P. Blood parasites in Passeriformes in central Germany: Prevalence and lineage diversity of Haemosporida (Haemoproteus, Plasmodium and Leucocytozoon) in six common songbirds. PeerJ 2019, 6, e6259. [Google Scholar] [CrossRef] [PubMed]
- Bichet, C.; Brischoux, F.; Ribout, C.; Parenteau, C.; Meillère, A.; Angelier, F. Physiological and morphological correlates of blood parasite infection in urban and non-urban house sparrow populations. PLoS ONE 2020, 15, e0237170. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Binkienė, R.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G. The buffy coat method: A tool for detection of blood parasites without staining procedures. Parasites Vectors 2020, 13, 104. [Google Scholar] [CrossRef]
- Díez-Fernández, A.; Martínez-de la Puente, J.; Gangoso, L.; López, P.; Soriguer, R.; Martín, J.; Figuerola, J. Mosquitoes are attracted by the odour of Plasmodium-infected birds. Int. J. Parasitol. 2020, 50, 569–575. [Google Scholar] [CrossRef]
- Duc, M.; Ilgūnas, M.; Valkiūnas, G. Patterns of Haemoproteus majoris (Haemosporida, Haemoproteidae) megalomeront development. Acta Trop. 2020, 212, 105706. [Google Scholar] [CrossRef] [PubMed]
- Emmenegger, T.; Alves, J.A.; Rocha, A.D.; Costa, J.S.; Schmid, R.; Schulze, M.; Hahn, S. Population- and age-specific patterns of haemosporidian assemblages and infection levels in European bee-eaters (Merops apiaster). Int. J. Parasitol. 2020, 50, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Himmel, T.; Harl, J.; Pfanner, S.; Nedorost, N.; Nowotny, N.; Weissenböck, H. Haemosporidioses in wild Eurasian blackbirds (Turdus merula) and song thrushes (T. philomelos): An in situ hybridization study with emphasis on exo-erythrocytic parasite burden. Malar. J. 2020, 19, 69. [Google Scholar] [CrossRef]
- Huang, X.; Jönsson, J.; Bensch, S. Persistence of avian haemosporidians in the wild: A case study to illustrate seasonal infection patterns in relation to host life stages. Int. J. Parasitol. 2020, 50, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Himmel, T.; Harl, J.; Matt, J.; Weissenböck, H. A citizen science-based survey of avian mortality focusing on haemosporidian infections in wild passerine birds. Malar. J. 2021, 20, 417. [Google Scholar] [CrossRef] [PubMed]
- Platonova, E.; Aželytė, J.; Iezhova, T.; Ilgūnas, M.; Mukhin, A.; Palinauskas, V. Experimental study of newly described avian malaria parasite Plasmodium (Novyella) collidatum n. sp., genetic lineage pFANTAIL01 obtained from South Asian migrant bird. Malar. J. 2021, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Schumm, Y.R.; Bakaloudis, D.; Barboutis, C.; Cecere, J.G.; Eraud, C.; Fischer, D.; Hering, J.; Hillerich, K.; Lormée, H.; Mader, V.; et al. Prevalence and genetic diversity of avian haemosporidian parasites in wild bird species of the order Columbiformes. Parasitol. Res. 2021, 120, 1405–1420. [Google Scholar] [CrossRef]
- Wiegmann, A.; Springer, A.; Rinaud, T.; Ottensmann, M.; Legler, M.; Krüger, O.; Fehr, M.; Chakarov, N.; Strube, C. The prevalence of Leucocytozoon spp. In nestlings of three wild raptor species including implications on haematological and blood chemistry values. Int. J. Parasitol. Parasites Wildl. 2021, 16, 236–243. [Google Scholar] [CrossRef]
- Aželytė, J.; Platonova, E.; Bensch, S.; Hellgren, O.; Palinauskas, V. A comparative analysis of the dynamics of Plasmodium relictum (GRW4) development in the blood during single and co-infections. Acta Trop. 2022, 226, 106247. [Google Scholar] [CrossRef]
- Ellis, V.A.; Kalbskopf, V.; Ciloglu, A.; Duc, M.; Huang, X.; Inci, A.; Bensch, S.; Hellgren, O.; Palinauskas, V. Genomic sequence capture of Plasmodium relictum in experimentally infected birds. Parasites Vectors 2022, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Ilgūnas, M.; Himmel, T.; Harl, J.; Dagys, M.; Valkiūnas, G.; Weissenböck, H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Anim. Open Access J. MDPI 2022, 12, 2212. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Duc, M.; Himmel, T.; Eigirdas, V.; Weissenböck, H.; Valkiūnas, G. Exo-erythrocytic development of Leucocytozoon parasites (Haemosporida, Leucocytozoidae) in song thrushes Turdus philomelos. Int. J. Parasitol. Parasites Wildl. 2023, 22, 60–68. [Google Scholar] [CrossRef]
- de Francisco, O.N.; Sacristán, I.; Ewbank, A.C.; Velarde, R.; Afonso, I.; Garcia-Ferré, D.; Martín-Maldonado, B.; Esperón, F.; Iglesias, I.; de la Torre, A.; et al. First detection of herpesvirus and hemosporidians in the endangered Pyrenean Capercaillie (Tetrao urogallus aquitanicus). Sci. Rep. 2023, 13, 21936. [Google Scholar] [CrossRef]
- Gomes, J.; Leitão, M.; Louro, M.C.; Brandão, R.; Mateus, T.L. Avian Malaria in wild birds from a wildlife rehabilitation center in Central Portugal. Vet. Parasitol. Reg. Stud. Rep. 2023, 43, 100904. [Google Scholar] [CrossRef]
- González-Olvera, M.; Hernandez-Colina, A.; Chantrey, J.; Allen, S.; Lopez, J.; Baylis, M. A non-invasive feather-based methodology for the detection of blood parasites (Haemosporida). Sci. Rep. 2023, 13, 16712. [Google Scholar] [CrossRef]
- Jiménez-Peñuela, J.; Ferraguti, M.; Martínez-De La Puente, J.; Soriguer, R.C.; Figuerola, J. Oxidative status in relation to blood parasite infections in house sparrows living along an urbanization gradient. Environ. Pollut. 2023, 316, 120712. [Google Scholar] [CrossRef] [PubMed]
- Lynton-Jenkins, J.G.; Chaine, A.S.; Russell, A.F.; Bonneaud, C. Parasite detection and quantification in avian blood is dependent on storage medium and duration. Ecol. Evol. 2023, 13, e9819. [Google Scholar] [CrossRef]
- Martín-Maldonado, B.; Mencía-Gutiérrez, A.; Andreu-Vázquez, C.; Fernández, R.; Pastor-Tiburón, N.; Alvarado, A.; Carrero, A.; Fernández-Novo, A.; Esperón, F.; González, F. A Four-Year Survey of Hemoparasites from Nocturnal Raptors (Strigiformes) Confirms a Relation between Leucocytozoon and Low Hematocrit and Body Condition Scores of Parasitized Birds. Vet. Sci. 2023, 10, 54. [Google Scholar] [CrossRef]
- Himmel, T.; Harl, J.; Matt, J.; Nedorost, N.; Lunardi, M.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G.; Weissenböck, H. Co-infecting Haemoproteus species (Haemosporida, Apicomplexa) show different host tissue tropism during exo-erythrocytic development in Fringilla coelebs (Fringillidae). Int. J. Parasitol. 2024, 54, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, E.C.; Martin, C.A.; Armstrong, C.; González-Quevedo, C.; Illera, J.C.; Suh, A.; Spurgin, L.G.; Richardson, D.S. Genotype–environment associations reveal genes potentially linked to avian malaria infection in populations of an endemic island bird. Mol. Ecol. 2024, 33, e17329. [Google Scholar] [CrossRef]
- Zehtindjiev, P.; Ilieva, M.; Westerdahl, H.; Hansson, B.; Valkiūnas, G.; Bensch, S. Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird, the great reed warbler Acrocephalus arundinaceus. Exp. Parasitol. 2008, 119, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Shurulinkov, P.; Spasov, L.; Stoyanov, G.; Chakarov, N. Blood parasite infections in a wild population of ravens (Corvus corax) in Bulgaria. Malar. J. 2018, 17, 33. [Google Scholar] [CrossRef]
- Hahn, S.; Bauer, S.; Dimitrov, D.; Emmenegger, T.; Ivanova, K.; Zehtindjiev, P.; Buttemer, W.A. Low intensity blood parasite infections do not reduce the aerobic performance of migratory birds. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172307. [Google Scholar] [CrossRef]
- Iezhova, T.A.; Valkiūnas, G.; Bairlein, F. Vertebrate Host Specificity of Two Avian Malaria Parasites of the Subgenus Novyella Plasmodium nucleophilum and Plasmodium vaughani. J. Parasitol. 2005, 91, 472–474. [Google Scholar] [CrossRef]
- Jovani, R.; Sol, D. How predictable is the abundance of double gametocyte infections? Parasitol. Res. 2005, 97, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.C. Microfilariae and Other Blood Parasites in Mexican Wild Doves and Pigeons. J. Parasitol. 1959, 45, 69–75. [Google Scholar] [CrossRef]
- Forrester, D.J.; Hon, L.T.; Williams, L.E.; Austin, D.H. Blood protozoa of wild turkeys in Florida. J. Protozool. 1974, 21, 494–497. [Google Scholar] [CrossRef]
- Noblet, R.; Moore, H.S. Prevalence and distribution of Leucocytozoon smithi and Haemoproteus meleagridis in wild turkeys in South Carolina. J. Wildl. Dis. 1975, 11, 516–518. [Google Scholar] [CrossRef]
- Clark, G.W. Hematozoa of mallard ducks (Anas platyrhynchos) of the Pacific Flyway, Washington. J. Wildl. Dis. 1980, 16, 529–531. [Google Scholar] [CrossRef]
- Forrester, D.J.; Humphrey, P.P.; Telford, S.R.; Williams, L.E. Effects of blood-induced infections of Plasmodium hermani on domestic and wild turkey poults. J. Wildl. Dis. 1980, 16, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Greiner, E.C.; Forrester, D.J. Haemoproteus meleagridis Levine 1961: Redescription and developmental morphology of the gametocytes in turkeys. J. Parasitol. 1980, 66, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.F.; Turner, B.; Holton, G. Blood parasites of trumpeter swans, Olor buccinator (Richardson), from Alberta. J. Wildl. Dis. 1981, 17, 213–215. [Google Scholar] [CrossRef]
- Bennett, G.F.; Nieman, D.J.; Turner, B.; Kuyt, E.; Whiteway, M.; Greiner, E.C. Blood parasites of prairie anatids and their implication in waterfowl management in Alberta and Saskatchewan. J. Wildl. Dis. 1982, 18, 287–296. [Google Scholar] [CrossRef]
- Christensen, B.M.; Barnes, H.J.; Rowley, W.A. Vertebrate host specificity and experimental vectors of Plasmodium (Novyella) kempi sp. N. From the eastern wild turkey in Iowa. J. Wildl. Dis. 1983, 19, 204–213. [Google Scholar] [CrossRef]
- Castle, M.D.; Christensen, B.M. Blood and gastrointestinal parasites of eastern wild turkeys from Kentucky and Tennessee. J. Wildl. Dis. 1984, 20, 190–196. [Google Scholar] [CrossRef]
- Barnard, W.H.; Bair, R.D. Prevalence of avian hematozoa in central Vermont. J. Wildl. Dis. 1986, 22, 365–374. [Google Scholar] [CrossRef]
- Allan, R.A.; Mahrt, J.L. Populations of Leucocytozoon gametocytes in blue grouse (Dendragapus obscurus) from Hardwicke Island, British Columbia. J. Protozool. 1987, 34, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.T.; Forrester, D.J. Myopathy associated with megaloschizonts of Haemoproteus meleagridis in a wild turkey from Florida. J. Wildl. Dis. 1987, 23, 495–498. [Google Scholar] [CrossRef]
- Fix, A.S.; Waterhouse, C.; Greiner, E.C.; Stoskopf, M.K. Plasmodium relictum as a cause of avian malaria in wild-caught magellanic penguins (Spheniscus magellanicus). J. Wildl. Dis. 1988, 24, 610–619. [Google Scholar] [CrossRef]
- Hopkins, B.A.; Skeeles, J.K.; Houghten, G.E.; Slagle, D.; Gardner, K. A survey of infectious diseases in wild turkeys (Meleagridis gallopavo silvestris) from Arkansas. J. Wildl. Dis. 1990, 26, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Fedynich, A.M.; Rhodes, O.E., Jr. Hemosporid (Apicomplexa, Hematozoea, Hemosporida) Community Structure and Pattern in Wintering Wild Turkeys. J. Wildl. Dis. 1995, 31, 404–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michot, T.C.; Garvin, M.C.; Weidner, E.H. Survey for blood parasites in redheads (Aythya americana) wintering at the Chandeleur Islands, Louisiana. J. Wildl. Dis. 1995, 31, 90–92. [Google Scholar] [CrossRef][Green Version]
- Durden, L.A.; McLean, R.G.; Oliver, J.H.; Ubico, S.R.; James, A.M. Ticks, Lyme disease spirochetes, trypanosomes, and antibody to encephalitis viruses in wild birds from coastal Georgia and South Carolina. J. Parasitol. 1997, 83, 1178–1182. [Google Scholar] [CrossRef]
- Morishita, T.Y.; Aye, P.P.; Ley, E.C.; Harr, B.S. Survey of pathogens and blood parasites in free-living passerines. Avian Dis. 1999, 43, 549–552. [Google Scholar] [CrossRef]
- DeJong, R.J.; Muzzall, P.M. Hematozoa of Waterfowl from Michigan. J. Wildl. Dis. 2000, 36, 767–773. [Google Scholar] [CrossRef][Green Version]
- Ricklefs, R.E.; Fallon, S.M. Diversification and host switching in avian malaria parasites. Proc. Biol. Sci. 2002, 269, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.C.; Basbaum, J.P.; Ducore, R.M.; Bell, K.E. Patterns of Haemoproteus beckeri parasitism in the gray catbird (Dumatella carolinensis) during the breeding season. J. Wildl. Dis. 2003, 39, 582–587. [Google Scholar] [CrossRef]
- Garvin, M.C.; Greiner, E.C. Epizootiology of Haemoproteus danilewskyi (Haemosporina: Haemoproteidae) in blue jays (Cyanocitta cristata) in southcentral Florida. J. Wildl. Dis. 2003, 39, 1–9. [Google Scholar] [CrossRef][Green Version]
- Dusek, R.J.; Spalding, M.G.; Forrester, D.J.; Greiner, E.C. Haemoproteus balearicae and other blood parasites of free-ranging Florida sandhill crane chicks. J. Wildl. Dis. 2004, 40, 682–687. [Google Scholar] [CrossRef]
- Szymanski, M.M.; Lovette, I.J. High lineage diversity and host sharing of malarial parasites in a local avian assemblage. J. Parasitol. 2005, 91, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Heard, D.J.; Mulcahy, D.M.; Iverson, S.A.; Rizzolo, D.J.; Greiner, E.C.; Hall, J.; Ip, H.; Esler, D. A Blood Survey of Elements, Viral Antibodies, and Hemoparasites in Wintering Harlequin Ducks (Histrionicus histrionicus) and Barrow’s Goldeneyes (Bucephala islandica). J. Wildl. Dis. 2008, 44, 486–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leppert, L.L.; Dufty, A.M., Jr.; Stock, S.; Oleyar, M.D.; Kaltenecker, G.S. Survey of Blood Parasites in Two Forest Owls, Northern Saw-whet Owls and Flammulated Owls, of Western North America. J. Wildl. Dis. 2008, 44, 475–479. [Google Scholar] [CrossRef]
- Shutler, D.; Lowe, A.G.; Robinson, S.R. Relationships between circulating leucocytes and Leucocytozoon simondi in mallard, Anas platyrhynchos, ducklings. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 46–49. [Google Scholar] [CrossRef]
- Grillo, E.L.; Fithian, R.C.; Cross, H.; Wallace, C.; Viverette, C.; Reilly, R.; Mayer, D.C.G. Presence of Plasmodium and Haemoproteus in Breeding Prothonotary Warblers (Protonotaria citrea: Parulidae): Temporal and Spatial Trends in Infection Prevalence. J. Parasitol. 2012, 98, 93–102. [Google Scholar] [CrossRef]
- Astudillo, V.G.; Hernández, S.M.; Kistler, W.M.; Boone, S.L.; Lipp, E.K.; Shrestha, S.; Yabsley, M.J. Spatial, temporal, molecular, and intraspecific differences of haemoparasite infection and relevant selected physiological parameters of wild birds in Georgia, USA. Int. J. Parasitol. Parasites Wildl. 2013, 2, 178–189. [Google Scholar] [CrossRef][Green Version]
- Carlson, J.S.; Martínez-Gómez, J.E.; Valkiūnas, G.; Loiseau, C.; Bell, D.A.; Sehgal, R.N.M. Diversity and Phylogenetic Relationships of Hemosporidian Parasites in Birds of Socorro Island, México, and Their Role in the Re-Introduction of the Socorro Dove (Zenaida graysoni). J. Parasitol. 2013, 99, 270–276. [Google Scholar] [CrossRef]
- Davis, A.K.; Hood, W.R.; Hill, G.E. Prevalence of Blood Parasites in Eastern Versus Western House Finches: Are Eastern Birds Resistant to Infection? EcoHealth 2013, 10, 290–297. [Google Scholar] [CrossRef]
- Kistler, W.M.; Hernandez, S.M.; Gibbs, S.E.J.; Ballard, J.R.; Arnold, S.L.; Johnson, T.; Yabsley, M.J. Evaluation of a Restriction Fragment Length Enzyme Assay for Differentiation of Haemoproteus and Plasmodium Across a Standard Region of the Mitochondrial Genome. J. Parasitol. 2013, 99, 1133–1136. [Google Scholar] [CrossRef]
- Charles-Smith, L.E.; Rutledge, M.E.; Meek, C.J.; Baine, K.; Massey, E.; Ellsaesser, L.N.; DePerno, C.S.; Moorman, C.E.; Degernes, L.A. Hematologic Parameters and Hemoparasites of Nonmigratory Canada Geese (Branta canadensis) From Greensboro, North Carolina, USA. J. Avian Med. Surg. 2014, 28, 16–23. [Google Scholar] [CrossRef]
- Ellis, V.A.; Kunkel, M.R.; Ricklefs, R.E. The ecology of host immune responses to chronic avian haemosporidian infection. Oecologia 2014, 176, 729–737. [Google Scholar] [CrossRef]
- Ferguson, L.M.; Norton, T.M.; Cray, C.; Oliva, M.; Jodice, P.G.R. Health assessments of brown pelican (Pelecanus occidentalis) nestlings from colonies in South Carolina and Georgia, U.S.A. J. Zoo Wildl. Med. 2014, 45, 802–812. [Google Scholar] [CrossRef]
- Ramey, A.M.; Schmutz, J.A.; Reed, J.A.; Fujita, G.; Scotton, B.D.; Casler, B.; Fleskes, J.P.; Konishi, K.; Uchida, K.; Yabsley, M.J. Evidence for intercontinental parasite exchange through molecular detection and characterization of haematozoa in northern pintails (Anas acuta) sampled throughout the North Pacific Basin. Int. J. Parasitol. Parasites Wildl. 2014, 4, 11–21. [Google Scholar] [CrossRef]
- Thurber, M.I.; Gamble, K.C.; Krebs, B.; Goldberg, T.L. Molecular detection of Plasmodium in free-ranging birds and captive flamingos (Phoenicopterus Chilensis) in Chicago. J. Zoo Wildl. Med. 2014, 45, 749–754. [Google Scholar] [CrossRef]
- Smith, M.M.; Schmutz, J.; Apelgren, C.; Ramey, A.M. A real-time, quantitative PCR protocol for assessing the relative parasitemia of Leucocytozoon in waterfowl. J. Microbiol. Methods 2015, 111, 72–77. [Google Scholar] [CrossRef]
- Zylberberg, M.; Derryberry, E.P.; Breuner, C.W.; Macdougall-Shackleton, E.A.; Cornelius, J.M.; Hahn, T.P. Haemoproteus infected birds have increased lifetime reproductive success. Parasitology 2015, 142, 1033–1043. [Google Scholar] [CrossRef]
- Meixell, B.W.; Arnold, T.W.; Lindberg, M.S.; Smith, M.M.; Runstadler, J.A.; Ramey, A.M. Detection, prevalence, and transmission of avian hematozoa in waterfowl at the Arctic/sub-Arctic interface: Co-infections, viral interactions, and sources of variation. Parasites Vectors 2016, 9, 390. [Google Scholar] [CrossRef]
- Reinoso-Pérez, M.T.; Canales-Delgadillo, J.C.; Chapa-Vargas, L.; Riego-Ruiz, L. Haemosporidian parasite prevalence, parasitemia, and diversity in three resident bird species at a shrubland dominated landscape of the Mexican highland plateau. Parasites Vectors 2016, 9, 307. [Google Scholar] [CrossRef]
- Bertram, M.R.; Hamer, S.A.; Hartup, B.K.; Snowden, K.F.; Medeiros, M.C.; Outlaw, D.C.; Hamer, G.L. A novel Haemosporida clade at the rank of genus in North American cranes (Aves: Gruiformes). Mol. Phylogenetics Evol. 2017, 109, 73–79. [Google Scholar] [CrossRef]
- Bertram, M.R.; Hamer, G.L.; Hartup, B.K.; Snowden, K.F.; Medeiros, M.C.; Hamer, S.A. Haemosporida prevalence and diversity are similar in endangered wild whooping cranes (Grus americana) and sympatric sandhill cranes (Grus canadensis). Parasitology 2017, 144, 629–640. [Google Scholar] [CrossRef]
- Slowinski, S.P.; Fudickar, A.M.; Hughes, A.M.; Mettler, R.D.; Gorbatenko, O.V.; Spellman, G.M.; Ketterson, E.D.; Atwell, J.W. Sedentary songbirds maintain higher prevalence of haemosporidian parasite infections than migratory conspecifics during seasonal sympatry. PLoS ONE 2018, 13, e0201563. [Google Scholar] [CrossRef]
- Townsend, A.K.; Wheeler, S.S.; Freund, D.; Sehgal, R.N.M.; Boyce, W.M. Links between blood parasites, blood chemistry, and the survival of nestling American crows. Ecol. Evol. 2018, 8, 8779–8790. [Google Scholar] [CrossRef]
- Barrow, L.N.; Allen, J.M.; Huang, X.; Bensch, S.; Witt, C.C. Genomic sequence capture of haemosporidian parasites: Methods and prospects for enhanced study of host–parasite evolution. Mol. Ecol. Resour. 2019, 19, 400–410. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Parish, C.N.; Hauck, T.J.; Aguilar, R.F.; Escalante, A.A. The endangered California Condor (Gymnogyps californianus) population is exposed to local haemosporidian parasites. Sci. Rep. 2020, 10, 17947. [Google Scholar] [CrossRef]
- Reinoso-Pérez, M.T.; Dhondt, K.V.; Sydenstricker, A.V.; Heylen, D.; Dhondt, A.A. Complex interactions between bacteria and haemosporidia in coinfected hosts: An experiment. Ecol. Evol. 2020, 10, 5801–5814. [Google Scholar] [CrossRef]
- Adams, D.R.; Golnar, A.J.; Hamer, S.A.; Slotman, M.A.; Hamer, G.L. Culex quinquefasciatus (Diptera: Culicidae) survivorship following the ingestion of bird blood infected with Haemoproteus sp. parasites. Parasitol. Res. 2021, 120, 2343–2350. [Google Scholar] [CrossRef]
- Theodosopoulos, A.N.; Grabenstein, K.C.; Bensch, S.; Taylor, S.A. A highly invasive malaria parasite has expanded its range to non-migratory birds in North America. Biol. Lett. 2021, 17, 20210271. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; Oria-Martínez, B.; Rendón-Franco, E.; Villalobos, G.; Muñoz-García, C.I. Trypanosoma cruzi, beyond the dogma of non-infection in birds. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2022, 99, 105239. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Ferreira, F.C.; Logan, C.J.; McCune, K.B.; MacPherson, M.P.; Miranda, S.A.; Santiago-Alarcon, D.; Escalante, A.A. Great-tailed Grackles (Quiscalus mexicanus) as a tolerant host of avian malaria parasites. PLoS ONE 2022, 17, e0268161. [Google Scholar] [CrossRef]
- Talbott, K.M.; Ketterson, E.D. Physiological impacts of chronic and experimental Plasmodium infection on breeding-condition male songbirds. Sci. Rep. 2023, 13, 13091. [Google Scholar] [CrossRef]
- Kelly, T.R.; Cannon, A.L.; Stansberry, K.R.; Kimball, M.G.; Lattin, C.R. Changes in hypothalamic–pituitary–adrenal axis function, immunity, and glucose during acute Plasmodium relictum infection in house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 2024, 345, 114388. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Saili, K.S.; Utzurrum, R.B.; Jarvi, S.I. Experimental Evidence for Evolved Tolerance to Avian Malaria in a Wild Population of Low Elevation Hawai‘i ‘Amakihi (Hemignathus virens). EcoHealth 2013, 10, 366–375. [Google Scholar] [CrossRef]
- Seidl, C.M.; Ferreira, F.C.; Parise, K.L.; Paxton, K.L.; Paxton, E.H.; Atkinson, C.T.; Fleischer, R.C.; Foster, J.T.; Marm Kilpatrick, A. Linking avian malaria parasitemia estimates from quantitative PCR and microscopy reveals new infection patterns in Hawai’i. Int. J. Parasitol. 2024, 54, 123–130. [Google Scholar] [CrossRef]
- Murata, K. Prevalence of blood parasites in Japanese wild birds. J. Vet. Med. Sci. 2002, 64, 785–790. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamaguchi, T.; Kitahara, M.; Hirai, K.; Murata, K. Leucocytozoon lovati Infections in Wild Rock Ptarmigan (Lagopus mutus) in Japan. J. Wildl. Dis. 2004, 40, 804–807. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Gering, E.; Rappole, J.H.; Rahmani, A.R.; Jhala, Y.V.; Dove, C.J.; Milensky, C.; Olson, S.L.; Peirce, M.A.; Fleischer, R.C. Prevalence and diversity of avian hematozoan parasites in Asia: A regional survey. J. Wildl. Dis. 2007, 43, 382–398. [Google Scholar] [CrossRef]
- Sato, Y.; Hagihara, M.; Yamaguchi, T.; Yukawa, M.; Murata, K. Phylogenetic Comparison of Leucocytozoon spp. from Wild Birds of Japan. J. Vet. Med. Sci. 2007, 69, 55–59. [Google Scholar] [CrossRef][Green Version]
- Murata, K.; Nii, R.; Yui, S.; Sasaki, E.; Ishikawa, S.; Sato, Y.; Matsui, S.; Horie, S.; Akatani, K.; Takagi, M.; et al. Avian Haemosporidian Parasites Infection in Wild Birds Inhabiting Minami-Daito Island of the Northwest Pacific, Japan. J. Vet. Med. Sci. 2008, 70, 501–503. [Google Scholar] [CrossRef][Green Version]
- Imura, T.; Suzuki, Y.; Ejiri, H.; Sato, Y.; Ishida, K.; Sumiyama, D.; Murata, K.; Yukawa, M. Prevalence of avian haematozoa in wild birds in a high-altitude forest in Japan. Vet. Parasitol. 2012, 183, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Salakij, J.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Salakij, C. Plasmodium circumflexum in a Shikra (Accipiter badius): Phylogeny and ultra-structure of the haematozoa. Jpn. J. Vet. Res. 2012, 60, 105–109. [Google Scholar] [PubMed]
- Tanigawa, M.; Sato, Y.; Ejiri, H.; Imura, T.; Chiba, R.; Yamamoto, H.; Kawaguchi, M.; Tsuda, Y.; Murata, K.; Yukawa, M. Molecular identification of avian haemosporidia in wild birds and mosquitoes on Tsushima Island, Japan. J. Vet. Med. Sci. 2013, 75, 319–326. [Google Scholar] [CrossRef]
- Elahi, R.; Islam, A.; Hossain, M.S.; Mohiuddin, K.; Mikolon, A.; Paul, S.K.; Hosseini, P.R.; Daszak, P.; Alam, M.S. Prevalence and Diversity of Avian Haematozoan Parasites in Wetlands of Bangladesh. J. Parasitol. Res. 2014, 2014, 493754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Zhang, Q.; Su, D.; Zou, F. Prevalence Patterns of Avian Plasmodium and Haemoproteus Parasites and the Influence of Host Relative Abundance in Southern China. PLoS ONE 2014, 9, e99501. [Google Scholar] [CrossRef]
- Borkataki, S.; Katoch, R.; Goswami, P.; Godara, R.; Khajuria, J.K.; Yadav, A.; Kour, R.; Mir, I. Incidence of Haemoproteus columbae in pigeons of Jammu district. J. Parasit. Dis. 2015, 39, 426–428. [Google Scholar] [CrossRef][Green Version]
- Nourani, L.; Aliabadian, M.; Dinparast-Djadid, N.; Mirshamsi, O. New Host Records for Haemoproteus spp. (Apicomplexa: Haemosporidiasina) in Passeriformes from North-West of Iran. J. Arthropod-Borne Dis. 2017, 11, 236–241. [Google Scholar][Green Version]
- Ishtiaq, F.; Barve, S. Do avian blood parasites influence hypoxia physiology in a high elevation environment? BMC Ecol. 2018, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Nourani, L.; Aliabadian, M.; Mirshamsi, O.; Djadid, N.D. Correction: Molecular detection and genetic diversity of avian haemosporidian parasites in Iran. PLoS ONE 2018, 14, e0212453. [Google Scholar] [CrossRef]
- Rhim, H.; Bae, J.; Kim, H.; Han, J.-I. Prevalence and phylogenetic analysis of avian haemosporidia in wild birds in the republic of Korea. J. Wildl. Dis. 2018, 54, 772–781. [Google Scholar] [CrossRef]
- Gupta, P.; Vishnudas, C.K.; Ramakrishnan, U.; Robin, V.V.; Dharmarajan, G. Geographical and host species barriers differentially affect generalist and specialist parasite community structure in a tropical sky-island archipelago. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190439. [Google Scholar] [CrossRef]
- Yuda, P. Detection of avian malaria in wild birds at Trisik Beach of Yogyakarta, Java (Indonesia). Ann. Parasitol. 2019, 65, 171–175. [Google Scholar] [CrossRef]
- Gupta, P.; Vishnudas, C.K.; Robin, V.V.; Dharmarajan, G. Host phylogeny matters: Examining sources of variation in infection risk by blood parasites across a tropical montane bird community in India. Parasites Vectors 2020, 13, 536. [Google Scholar] [CrossRef]
- Nourani, L.; Djadid, N.D.; Rabiee, K.; Mezerji, M.S.; Shakiba, M.; Bakhshi, H.; Shokrollahi, B.; Farahani, R.K. Detection of haemosporidian parasites in wild and domestic birds in northern and central provinces of Iran: Introduction of new lineages and hosts. Int. J. Parasitol. Parasites Wildl. 2020, 13, 203–212. [Google Scholar] [CrossRef]
- Dharmarajan, G.; Gupta, P.; Vishnudas, C.K.; Robin, V.V. Anthropogenic disturbance favours generalist over specialist parasites in bird communities: Implications for risk of disease emergence. Ecol. Lett. 2021, 24, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Inumaru, M.; Odaya, Y.; Sato, Y.; Marzal, A. First records of prevalence and diversity of avian haemosporidia in snipe species (genus Gallinago) of Japan. Int. J. Parasitol. Parasites Wildl. 2021, 16, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lertwatcharasarakul, P.; Salakij, C.; Prasopsom, P.; Kasorndorkbua, C.; Jakthong, P.; Santavakul, M.; Suwanasaeng, P.; Ploypan, R. Molecular and Morphological Analyses of Leucocytozoon Parasites (Haemosporida: Leucocytozoidae) in Raptors from Thailand. Acta Parasitol. 2021, 66, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Nourani, L.; Baghkheirati, A.A.; Zargar, M.; Karimi, V.; Djadid, N.D. Haemoproteosis and avian malaria in Columbidae and Corvidae from Iran. Vet. Med. Sci. 2021, 7, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Shokrani, H.; Norouzian, H.; Dezfoulian, O. Exo-erythrocytic stages of Haemoproteus sp. in common buzzard (Buteo buteo): A histopathological and molecular study. Int. J. Parasitol. Parasites Wildl. 2021, 16, 64–69. [Google Scholar] [CrossRef]
- Honjo, Y.; Fukumoto, S.; Sakamoto, H.; Hikosaka, K. New PCR primers targeting the cytochrome b gene reveal diversity of Leucocytozoon lineages in an individual host. Parasitol. Res. 2022, 121, 3313–3320. [Google Scholar] [CrossRef]
- Inumaru, M.; Nishiumi, I.; Kawakami, K.; Sato, Y. A widespread survey of avian haemosporidia in deceased wild birds of Japan: The hidden value of personally collected samples. J. Vet. Med. Sci. 2022, 84, 1253–1260. [Google Scholar] [CrossRef]
- Han, Y.; Hellgren, O.; Wu, Q.; Liu, J.; Jin, T.; Bensch, S.; Ding, P. Seasonal variations of intensity of avian malaria infection in the Thousand Island Lake System, China. Parasites Vectors 2023, 16, 218. [Google Scholar] [CrossRef]
- Kim, M.; Bae, J.; Oh, B.; Rhim, H.; Yang, M.-S.; Yang, S.; Kim, B.; Han, J.-I. Surveillance of wild animals carrying infectious agents based on high-throughput screening platform in the Republic of Korea. BMC Vet. Res. 2023, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Adriano, E.A.; Cordeiro, N.S. Prevalence and intensity of Haemoproteus columbae in three species of wild doves from Brazil. Memórias Do Inst. Oswaldo Cruz 2001, 96, 175–178. [Google Scholar] [CrossRef]
- Garvin, M.C.; Marra, P.P.; Crain, S.K. Prevalence of Hematozoa in Overwintering American Redstarts (Setophaga ruticilla): No Evidence for Local Transmission. J. Wildl. Dis. 2004, 40, 115–118. [Google Scholar] [CrossRef]
- Allgayer, M.C.; Guedes, N.M.R.; Chiminazzo, C.; Cziulik, M.; Weimer, T.A. Clinical pathology and parasitologic evaluation of free-living nestlings of the hyacinth macaw (Anodorhynchus hyacinthinus). J. Wildl. Dis. 2009, 45, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Belo, N.O.; Pinheiro, R.T.; Reis, E.S.; Ricklefs, R.E.; Braga, É.M. Prevalence and Lineage Diversity of Avian Haemosporidians from Three Distinct Cerrado Habitats in Brazil. PLoS ONE 2011, 6, e17654. [Google Scholar] [CrossRef]
- Belo, N.O.; Rodríguez-Ferraro, A.; Braga, E.M.; Ricklefs, R.E. Diversity of avian haemosporidians in arid zones of northern Venezuela. Parasitology 2012, 139, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Rosales, R.; Silva-Iturriza, A. Hemosporidian Parasites in Forest Birds from Venezuela: Genetic Lineage Analyses. Avian Dis. 2012, 56, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Matta, N.E.; Pacheco, M.A.; Escalante, A.A.; Valkiūnas, G.; Ayerbe-Quiñones, F.; Acevedo-Cendales, L.D. Description and molecular characterization of Haemoproteus macrovacuolatus n. sp. (Haemosporida, Haemoproteidae), a morphologically unique blood parasite of black-bellied whistling duck (Dendrocygna autumnalis) from South America. Parasitol. Res. 2014, 113, 2991–3000. [Google Scholar] [CrossRef]
- Sallaberry-Pincheira, N.; Gonzalez-Acuña, D.; Herrera-Tello, Y.; Dantas, G.P.M.; Luna-Jorquera, G.; Frere, E.; Valdés-Velasquez, A.; Simeone, A.; Vianna, J.A. Molecular Epidemiology of Avian Malaria in Wild Breeding Colonies of Humboldt and Magellanic Penguins in South America. EcoHealth 2015, 12, 267–277. [Google Scholar] [CrossRef]
- Svensson-Coelho, M.; Silva, G.T.; Santos, S.S.; Miranda, L.S.; Araújo-Silva, L.E.; Ricklefs, R.E.; Miyaki, C.Y.; Maldonado-Coelho, M. Lower Detection Probability of Avian Plasmodium in Blood Compared to Other Tissues. J. Parasitol. 2016, 102, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Vanstreels, R.E.T.; Uhart, M.; Rago, V.; Hurtado, R.; Epiphanio, S.; Catão-Dias, J.L. Do blood parasites infect Magellanic penguins (Spheniscus magellanicus) in the wild? Prospective investigation and climatogeographic considerations. Parasitology 2017, 144, 698–705. [Google Scholar] [CrossRef]
- Werther, K.; Luzzi, M.d.C.; Gonçalves, L.R.; de Oliveira, J.P.; Alves Junior, J.R.F.; Machado, R.Z.; André, M.R. Arthropod-borne agents in wild Orinoco geese (Neochen jubata) in Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2017, 55, 30–41. [Google Scholar] [CrossRef]
- Ferreira-Junior, F.C.; Dutra, D.d.A.; Silveira, P.; Pacheco, R.C.; Witter, R.; Ramos, D.G.d.S.; Pacheco, M.A.; Escalante, A.A.; Braga, É.M. A new pathogen spillover from domestic to wild animals: Plasmodium juxtanucleare infects free-living passerines in Brazil. Parasitology 2018, 145, 1949–1958. [Google Scholar] [CrossRef]
- Tostes, R.; Dias, R.J.P.; Oliveira, L.; de Senra, M.V.X.; Massard, C.L.; D’Agosto, M. Molecular and Morphological Characterization of a Brazilian Lineage of Plasmodium (Novyella) Unalis in Turdus spp. (Passeriformes) of the Atlantic Forest, with Remarks on New Hosts and High Genetic Variation. J. Parasitol. 2018, 104, 70–78. [Google Scholar] [CrossRef]
- de Oliveira, L.; Cedrola, F.; Senra, M.V.X.; Scopel, K.K.G.; Martinele, I.; Tostes, R.; Dias, R.J.P.; D’Agosto, M. Polymorphism evidence in Plasmodium (Haemamoeba) lutzi Lucena, 1939 (Apicomplexa, Haemosporida) isolated from Brazilian wild birds. Parasitol. Int. 2019, 70, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, A.C.; Strefezzi, R.d.F.; Sacristán, C.; Kolesnikovas, C.K.M.; Martins, A.; Mayorga, L.F.S.P.; Vanstreels, R.E.T.; Catão-Dias, J.L. Comparative morphometric evaluation of hepatic hemosiderosis in wild Magellanic penguins (Spheniscus magellanicus) infected with different Plasmodium spp. Subgenera. Rev. Bras. De Parasitol. Veterinária 2019, 28, 68–79. [Google Scholar] [CrossRef]
- de Oliveira, L.; Barino, G.T.M.; Rossi, M.F.; D’Agosto, M.; Dias, R.J.P.; Santos, H.A. Morphological and molecular characterization of Haemoproteus coatneyi and Haemoproteus erythrogravidus (Haemosporida: Haemoproteidae) in Passeriformes in Brazil’s Atlantic Forest. Rev. Bras. De Parasitol. Vet. Braz. J. Vet. Parasitol. Orgao Do Col. Bras. De Parasitol. Vet. 2020, 29, e011520. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Dias, R.J.P.; Rossi, M.F.; D’Agosto, M.; Santos, H.A. Molecular diversity and coalescent species delimitation of avian haemosporidian parasites in an endemic bird species of South America. Parasitol. Res. 2020, 119, 4033–4047. [Google Scholar] [CrossRef]
- Matoso, R.V.; Cedrola, F.; Barino, G.T.M.; Dias, R.J.P.; Rossi, M.F.; D’Agosto, M. New morphological and molecular data for Haemoproteus (H.) paramultipigmentatus in the Atlantic Forest of Brazil. Parasitol. Int. 2021, 84, 102375. [Google Scholar] [CrossRef]
- Morel, A.P.; Webster, A.; Prusch, F.; Anicet, M.; Marsicano, G.; Trainini, G.; Stocker, J.; Giani, D.; Bandarra, P.M.; da Rocha, M.I.S.; et al. Molecular detection and phylogenetic relationship of Haemosporida parasites in free-ranging wild raptors from Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100521. [Google Scholar] [CrossRef]
- Alvarez-Londoño, J.; Cardona-Romero, M.; Martínez-Sánchez, E.T.; Ossa-López, P.A.; Pérez-Cárdenas, J.E.; Gonzalez, A.D.; Rivera-Páez, F.A.; Castaño-Villa, G.J. Avian haemosporidian (Haemosporida: Plasmodium and Haemoproteus) in the department of Arauca, Colombian Orinoquia region. Parasitol. Res. 2022, 121, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.R.; Vanstreels, R.E.T.; Serafini, P.P.; Fontana, C.S.; da Silva, T.W.; Chiarani, E.; Carvalho, A.M.; Ferreira Junior, F.C.; Braga, É.M.; Locatelli-Dittrich, R. Blood parasites of passerines in the Brazilian Pampas and their implications for a potential population supplementation program for the endangered Yellow Cardinal (Gubernatrix cristata). Parasitol. Res. 2022, 121, 3203–3215. [Google Scholar] [CrossRef]
- Lima, M.B.; Borges, A.; Wolf, M.; Santos, H.A.; Dias, R.J.P.; Rossi, M.F. First record of Trypanosoma (Ornithotrypanum) infecting Neotropical birds. Parasitol. Res. 2024, 123, 156. [Google Scholar] [CrossRef]
- Cadena-Ortiz, H.; Mantilla, J.S.; de Aguilar, J.R.; Flores, D.; Bahamonde, D.; Matta, N.E.; Bonaccorso, E. Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: Diversity and factors related to prevalence and parasitaemia. Parasitology 2019, 146, 765–773. [Google Scholar] [CrossRef]
- Moens, M.A.J.; Pérez-Tris, J. Discovering potential sources of emerging pathogens: South America is a reservoir of generalist avian blood parasites. Int. J. Parasitol. 2016, 46, 41–49. [Google Scholar] [CrossRef]
- Travis, E.K.; Vargas, F.H.; Merkel, J.; Gottdenker, N.; Miller, R.E.; Parker, P.G. Hematology, serum chemistry, and serology of galápagos penguins (Spheniscus mendiculus) in the galápagos islands, ecuador. J. Wildl. Dis. 2006, 42, 625–632. [Google Scholar] [CrossRef]
- de Freitas, R.V.M.; Barino, G.T.M.; Cedrola, F.; Dias, R.J.P.; D’Agosto, M.; Massard, C.L. Insights on the taxonomy of Haemoproteus parasites infecting cracid birds. Parasitol. Int. 2023, 94, 102730. [Google Scholar] [CrossRef]
- Ashford, R.W.; Palmer, T.T.; Ash, J.S.; Bray, R.S. Blood parasites of Ethiopian birds. 1. General survey. J. Wildl. Dis. 1976, 12, 409–426. [Google Scholar] [CrossRef]
- Ayeni, J.S.O.; Dipeolu, O.O.; Okaeme, A.N. Parasitic infections of the grey-breasted helmet guinea-fowl (Numida meleagris galeata) in Nigeria. Vet. Parasitol. 1983, 12, 59–63. [Google Scholar] [CrossRef]
- Earlé, R.A.; Horak, I.G.; Huchzermeyer, F.W.; Bennett, G.F.; Braack, L.E.; Penzhorn, B.L. The prevalence of blood parasites in helmeted guineafowls, Numida meleagris, in the Kruger National Park. Onderstepoort J. Vet. Res. 1991, 58, 145–147. [Google Scholar]
- Graczyk, T.K.; Brossy, J.J.; Plös, A.; Stoskopf, M.K. Avian malaria seroprevalence in Jackass penguins (Spheniscus demersus) in South Africa. J. Parasitol. 1995, 81, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Grim, K.C.; Van der Merwe, E.; Sullivan, M.; Parsons, N.; McCutchan, T.F.; Cranfield, M. Plasmodium juxtanucleare associated with mortality in black-footed penguins (Spheniscus demersus) admitted to a rehabilitation center. J. Zoo Wildl. Med. 2003, 34, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.F.; Ariey, F.; Greiner, E.C. Hematozoa of the avian family vangidae (the vangas). J. Parasitol. 2004, 90, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.F.; Greiner, E.C. Hematozoa of the avian family brachypteraciidae (the ground-rollers). J. Parasitol. 2004, 90, 1468–1472. [Google Scholar] [CrossRef]
- Savage, A.F.; Greiner, E.C. Haemoproteids of the avian family dicruridae (the drongos). J. Parasitol. 2005, 91, 131–134. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Sehgal, R.N.M.; Iezhova, T.A.; Smith, T.B. Further Observations on the Blood Parasites of Birds in Uganda. J. Wildl. Dis. 2005, 41, 580–587. [Google Scholar] [CrossRef][Green Version]
- Savage, A.F.; Ariey, F.; Greiner, E.C. Leucocytozoon atkinsoni n. sp. (Apicomplexa: Leucocytozoidae) from the avian family Timaliidae. Syst. Parasitol. 2006, 64, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Chasar, A.; Loiseau, C.; Valkiūnas, G.; Iezhova, T.; Smith, T.B.; Sehgal, R.N.M. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 2009, 18, 4121–4133. [Google Scholar] [CrossRef]
- Savage, A.F.; Robert, V.; Goodman, S.M.; Raharimanga, V.; Raherilalao, M.J.; Andrianarimisa, A.; Ariey, F.; Greiner, E.C. Blood Parasites in Birds from Madagascar. J. Wildl. Dis. 2009, 45, 907–920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parsons, N.J.; Gous, T.A.; Schaefer, A.M.; Vanstreels, R.E.T. Health evaluation of African penguins (Spheniscus demersus) in southern Africa. Onderstepoort J. Vet. Res. 2016, 83, a1147. [Google Scholar] [CrossRef] [PubMed]
- Boundenga, L.; Perkins, S.L.; Ollomo, B.; Rougeron, V.; Leroy, E.M.; Renaud, F.; Prugnolle, F. Haemosporidian Parasites of Reptiles and Birds from Gabon, Central Africa. J. Parasitol. 2017, 103, 330–337. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Eberhart-Phillips, L.J.; Cristina Carmona-Isunza, M.; Zefania, S.; Navarro, M.J.; Kruger, O.; Hoffman, J.I.; Székely, T.; Figuerola, J. Extremely low Plasmodium prevalence in wild plovers and coursers from Cape Verde and Madagascar. Malar. J. 2017, 16, 243. [Google Scholar] [CrossRef]
- Chaisi, M.E.; Osinubi, S.T.; Dalton, D.L.; Suleman, E. Occurrence and diversity of avian haemosporidia in Afrotropical landbirds. Int. J. Parasitol. Parasites Wildl. 2018, 8, 36–44. [Google Scholar] [CrossRef]
- Tchoumbou, M.A.; Mayi, M.P.A.; Malange, E.N.F.; Foncha, F.D.; Kowo, C.; Fru-Cho, J.; Tchuinkam, T.; Awah-Ndukum, J.; Dorazio, R.; Nota Anong, D.; et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. Int. J. Parasitol. 2020, 50, 63–73. [Google Scholar] [CrossRef]
- Musa, S.; Mackenstedt, U.; Woog, F.; Dinkel, A. Untangling the actual infection status: Detection of avian haemosporidian parasites of three Malagasy bird species using microscopy, multiplex PCR, and nested PCR methods. Parasitol. Res. 2022, 121, 2817–2829. [Google Scholar] [CrossRef]
- Musa, S. Mitochondrial Genome Amplification of Avian Haemosporidian Parasites from Single-Infected Wildlife Samples Using an Innovative Nested PCR Approach. Res. Sq. 2023, 122.12, 2967–2975. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Chagas, C.R.F.; Valkiūnas, G.; dos Anjos, C.C.; Parsons, N.J.; Roberts, D.G.; Snyman, A.; Hurtado, R.; Kirchgatter, K.; Ludynia, K.; et al. Haemoproteus jenniae (Haemoproteidae, Haemosporida) infects gulls (Larus spp.) in South Africa, with redescription of Haemoproteus skuae. Parasitology 2023, 150, 1286–1295. [Google Scholar] [CrossRef]
- Holz, P. Disseminated granulomas caused by protozoan megaloschizonts in superb lyrebirds (Menura novaehollandiae). Aust. Vet. J. 1997, 75, 672–673. [Google Scholar] [CrossRef] [PubMed]
- Peirce, M.A.; Lederer, R.; Adlard, R.D.; O’Donoghue, P.J. Pathology associated with endogenous development of haematozoa in birds from southeast Queensland. Avian Pathol. 2004, 33, 445–450. [Google Scholar] [CrossRef]
- Baillie, S.M.; Gudex-Cross, D.; Barraclough, R.K.; Blanchard, W.; Brunton, D.H. Patterns in avian malaria at founder and source populations of an endemic New Zealand passerine. Parasitol. Res. 2012, 111, 2077–2089. [Google Scholar] [CrossRef]
- Cannell, B.L.; Krasnec, K.V.; Campbell, K.; Jones, H.I.; Miller, R.D.; Stephens, N. The pathology and pathogenicity of a novel Haemoproteus spp. Infection in wild Little Penguins (Eudyptula minor). Vet. Parasitol. 2013, 197, 74–84. [Google Scholar] [CrossRef]
- Niebuhr, C.N.; Poulin, R.; Tompkins, D.M. Is Avian Malaria Playing a Role in Native Bird Declines in New Zealand? Testing Hypotheses along an Elevational Gradient. PLoS ONE 2016, 11, e0165918. [Google Scholar] [CrossRef]
- Gartrell, B.; Agnew, D.; Alley, M.; Carpenter, T.; Ha, H.J.; Howe, L.; Hunter, S.; McInnes, K.; Munday, R.; Roe, W.; et al. Investigation of a mortality cluster in wild adult yellow-eyed penguins (Megadyptes antipodes) at Otago Peninsula, New Zealand. Avian Pathol. 2017, 46, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Sijbranda, D.C.; Gartrell, B.D.; Grange, Z.L.; Howe, L. Use of a real-time PCR to explore the intensity of Plasmodium spp. Infections in native, endemic and introduced New Zealand birds. Parasitology 2017, 144, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Brice, B.; Nguyen, M.; Loh, R.; Greay, T.; Adlard, R.; Ryan, U.; Yang, R. Further characterisation of Leucocytozoon podargii in wild tawny frogmouths (Podargus strigoides) in Western Australia. Parasitol. Res. 2019, 118, 1833–1840. [Google Scholar] [CrossRef]
- Filion, A.; Deschamps, L.; Niebuhr, C.N.; Poulin, R. Anthropogenic landscape alteration promotes higher disease risk in wild New Zealand avian communities. PLoS ONE 2022, 17, e0265568. [Google Scholar] [CrossRef] [PubMed]
- Sousa, O.E.; Herman, C.M. Blood parasites of birds from Chiriqui and Panama Provinces in the Republic of Panama. J. Wildl. Dis. 1982, 18, 205–221. [Google Scholar] [CrossRef]
- Mora-Chavarría, E.; Umaña-Castro, R.; Abou-Madi, N.; Solano-González, S.; Retamosa-Izaguirre, M.; Jiménez-Soto, M.; Blanco-Peña, K. Health assessment of captive psittacine species in prerelease programs at costa rican rescue centers. J. Zoo Wildl. Med. 2017, 48, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Quillfeldt, P.; Martínez, J.; Hennicke, J.; Ludynia, K.; Gladbach, A.; Masello, J.F.; Riou, S.; Merino, S. Hemosporidian blood parasites in seabirds—A comparative genetic study of species from Antarctic to tropical habitats. Naturwissenschaften 2010, 97, 809–817. [Google Scholar] [CrossRef]
- Palinauskas, V.; Valkiūnas, G.; Bolshakov, C.V.; Bensch, S. Plasmodium relictum (lineage P-SGS1): Effects on experimentally infected passerine birds. Exp. Parasitol. 2008, 120, 372–380. [Google Scholar] [CrossRef]
- Senar, J.C. Keel and Tarsus Length May Provide a Good Predictor of Avian Body Size. ARDEA-WAGENINGEN. 1977. Available online: https://www.academia.edu/2608794/Keel_and_tarsus_length_may_provide_a_good_predictor_of_avian_body_size (accessed on 12 November 2024).
- Hicks, O.; Burthe, S.J.; Daunt, F.; Newell, M.; Butler, A.; Ito, M.; Sato, K.; Green, J.A. The energetic cost of parasitism in a wild population. Proc. Biol. Sci. 2018, 285, 20180489. [Google Scholar] [CrossRef]
- Schoepf, I.; Olson, S.; Moore, I.T.; Bonier, F. Experimental reduction of haemosporidian infection affects maternal reproductive investment, parental behaviour and offspring condition. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221978. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, I.A. The effect of dehydration on macro- and microrheological blood properties. Clin. Hemorheol. Microcirc. 2002, 26, 85–90. [Google Scholar]
- de Jonge, G.; Dos Santos, T.L.; Cruz, B.R.; Simionatto, M.; Bittencourt, J.I.M.; Krum, E.A.; Moss, M.F.; Borato, D.C.K. Interference of in vitro hemolysis complete blood count. J. Clin. Lab. Anal. 2018, 32, e22396. [Google Scholar] [CrossRef]
- Cadenas, E. Mechanisms of Oxygen Activation and Reactive Oxygen Species Detoxification. In Oxidative Stress and Antioxidant Defenses in Biology; Ahmad, S., Ed.; Springer: New York, NY, USA, 1995; pp. 1–61. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Sherman, I.W. Biochemistry of Plasmodium (malarial parasites). Microbiol. Rev. 1979, 43, 453–495. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Kirk, K. Transport of the essential nutrient isoleucine in human erythrocytes infected with the malaria parasite Plasmodium falciparum. Blood 2007, 109, 2217–2224. [Google Scholar] [CrossRef]
- Giorgi, M.S.; Arlettaz, R.; Christe, P.; Vogel, P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis). Proc. Biol. Sci. 2001, 268, 2071–2075. [Google Scholar] [CrossRef]
- Maturana, C.R.; de Oliveira, A.D.; Nadal, S.; Bilalli, B.; Serrat, F.Z.; Soley, M.E.; Igual, E.S.; Bosch, M.; Lluch, A.V.; Abelló, A.; et al. Advances and challenges in automated malaria diagnosis using digital microscopy imaging with artificial intelligence tools: A review. Front. Microbiol. 2022, 13, 1006659. [Google Scholar] [CrossRef]
- Kramer, M.H.; Harris, D.J. Avian Blood Collection. J. Exot. Pet Med. 2010, 19, 82–86. [Google Scholar] [CrossRef]
- Garnham, P.C.C. Locomotion in the Parasitic Protozoa. Biol. Rev. 1966, 41, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, S.I.; Schultz, J.J.; Atkinson, C.T. PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines. J. Parasitol. 2002, 88, 153–158. [Google Scholar] [CrossRef]
- Fallon, S.M.; Ricklefs, R.E.; Swanson, B.L.; Bermingham, E. Detecting avian malaria: An improved polymerase chain reaction diagnostic. J. Parasitol. 2003, 89, 1044–1047. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Križanauskienė, A.; Palinauskas, V.; Sehgal, R.N.M.; Bensch, S. A Comparative Analysis of Microscopy and PCR-Based Detection Methods for Blood Parasites. J. Parasitol. 2008, 94, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new pcr assay for simultaneous studies of leucocytozoon, plasmodium, and haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Piqueras, A.; Gómez-Muñoz, M.T.; Martín-Maldonado, B. Hemoparasites in Wild Birds: A Systematic Review of Their Ecology and Clinical Implications. Animals 2025, 15, 2570. https://doi.org/10.3390/ani15172570
Alvarado-Piqueras A, Gómez-Muñoz MT, Martín-Maldonado B. Hemoparasites in Wild Birds: A Systematic Review of Their Ecology and Clinical Implications. Animals. 2025; 15(17):2570. https://doi.org/10.3390/ani15172570
Chicago/Turabian StyleAlvarado-Piqueras, Alberto, María Teresa Gómez-Muñoz, and Bárbara Martín-Maldonado. 2025. "Hemoparasites in Wild Birds: A Systematic Review of Their Ecology and Clinical Implications" Animals 15, no. 17: 2570. https://doi.org/10.3390/ani15172570
APA StyleAlvarado-Piqueras, A., Gómez-Muñoz, M. T., & Martín-Maldonado, B. (2025). Hemoparasites in Wild Birds: A Systematic Review of Their Ecology and Clinical Implications. Animals, 15(17), 2570. https://doi.org/10.3390/ani15172570







