Development of IVF Porcine Embryos in Microwell Culture System
Simple Summary
Abstract
1. Introduction
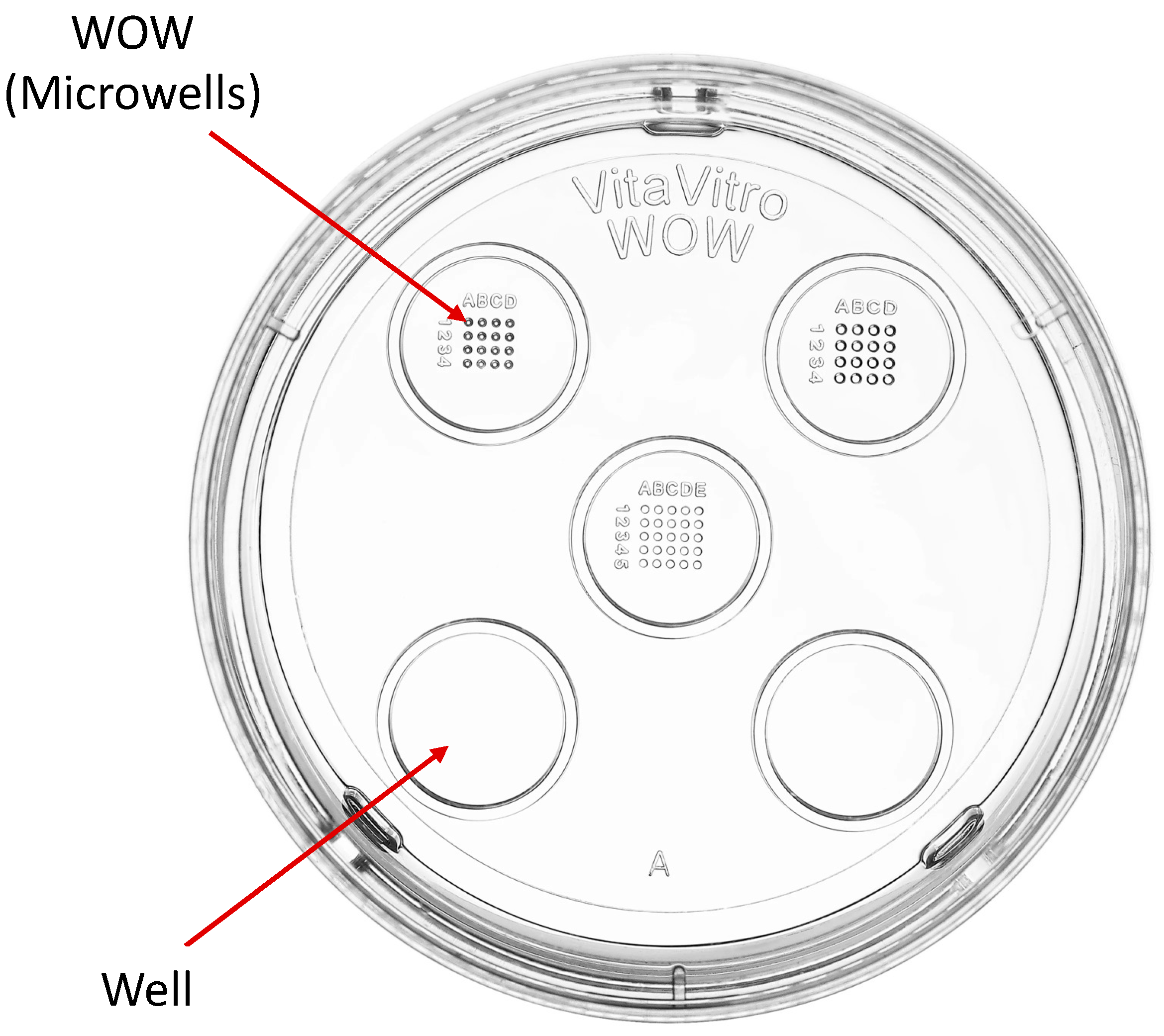
2. Materials and Methods
2.1. In Vitro Oocyte Maturation
2.2. In Vitro Fertilization (IVF)
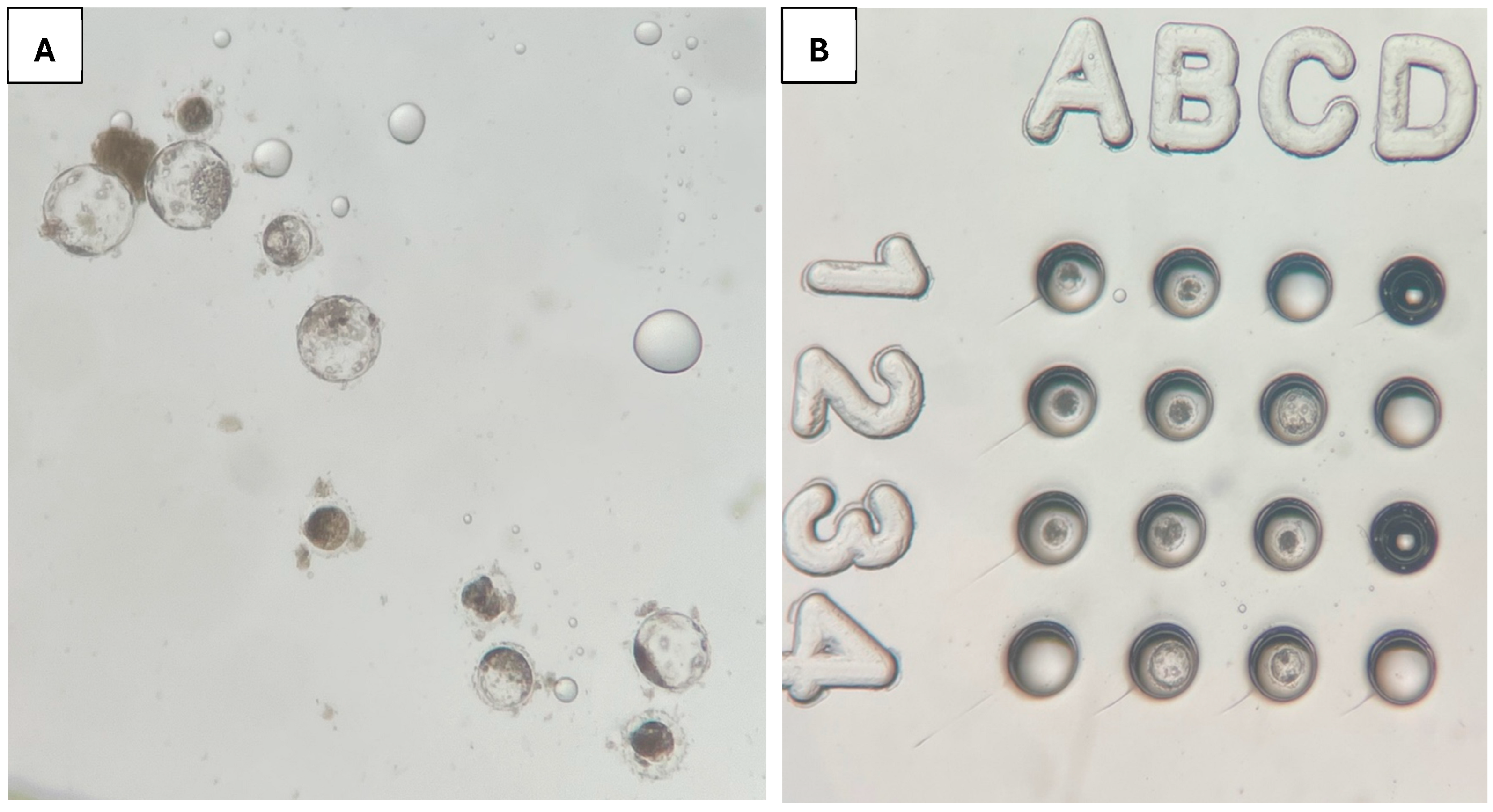
2.3. Embryo Culture
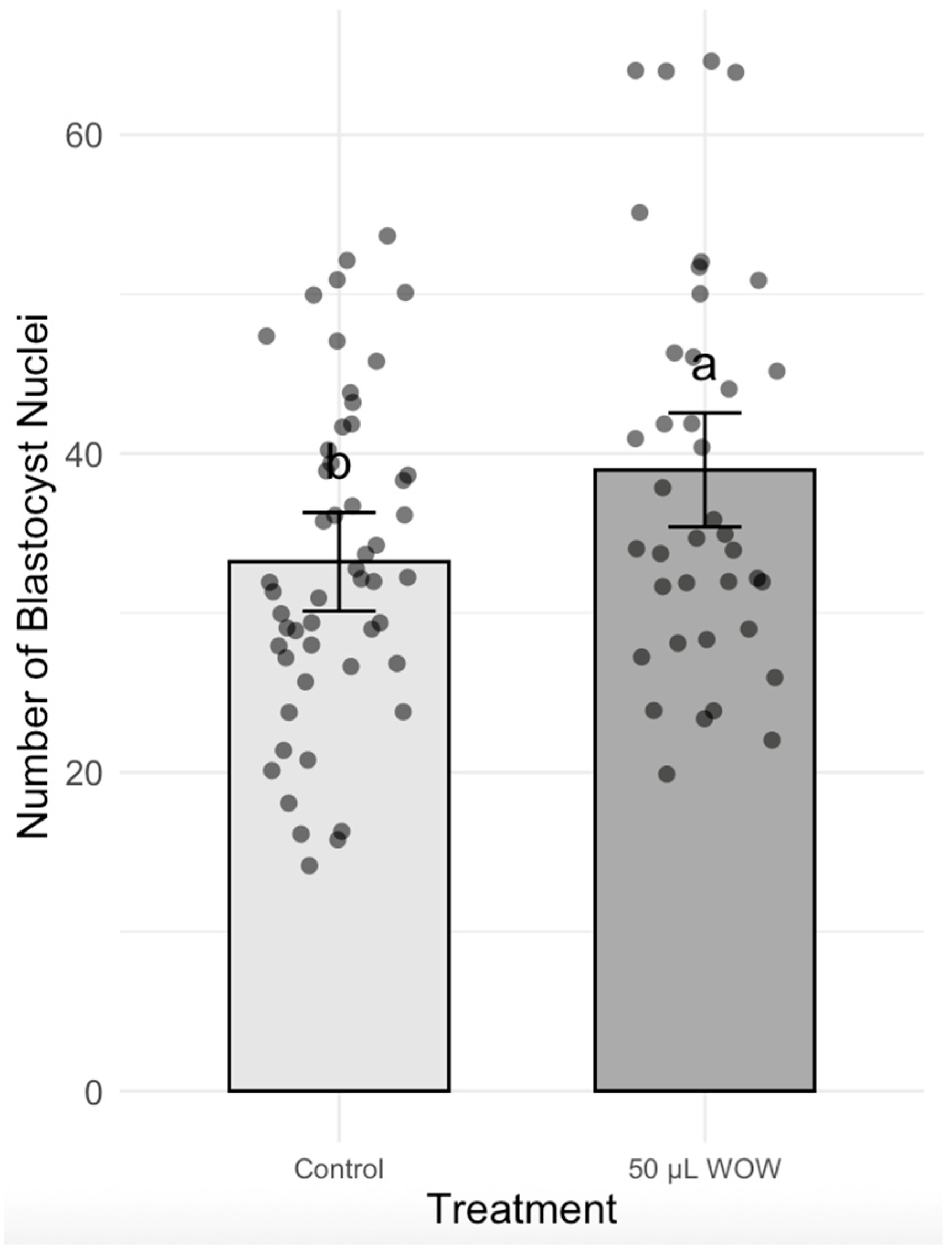
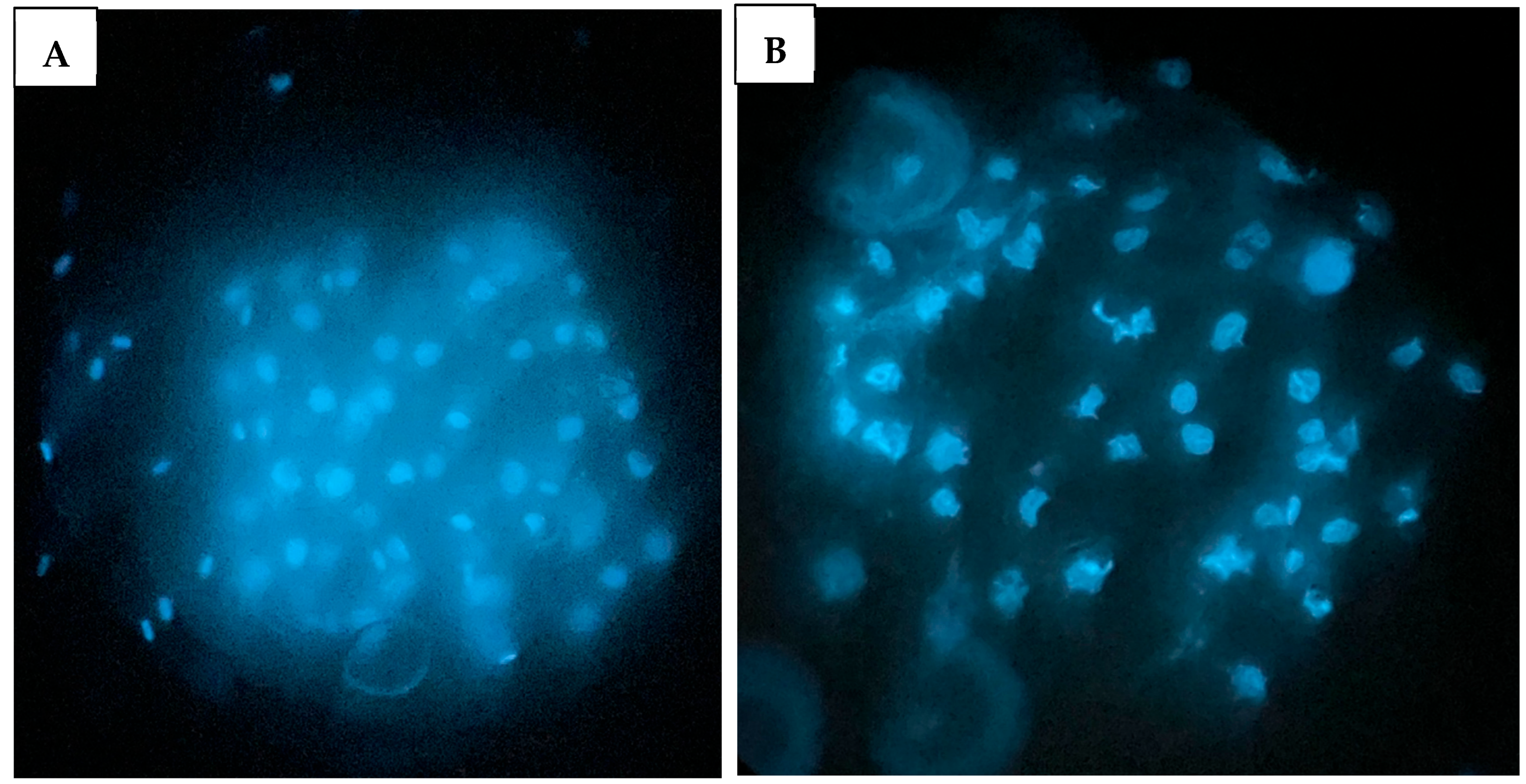
2.4. Nuclear Number of Blastocysts
2.5. Statistical Analysis
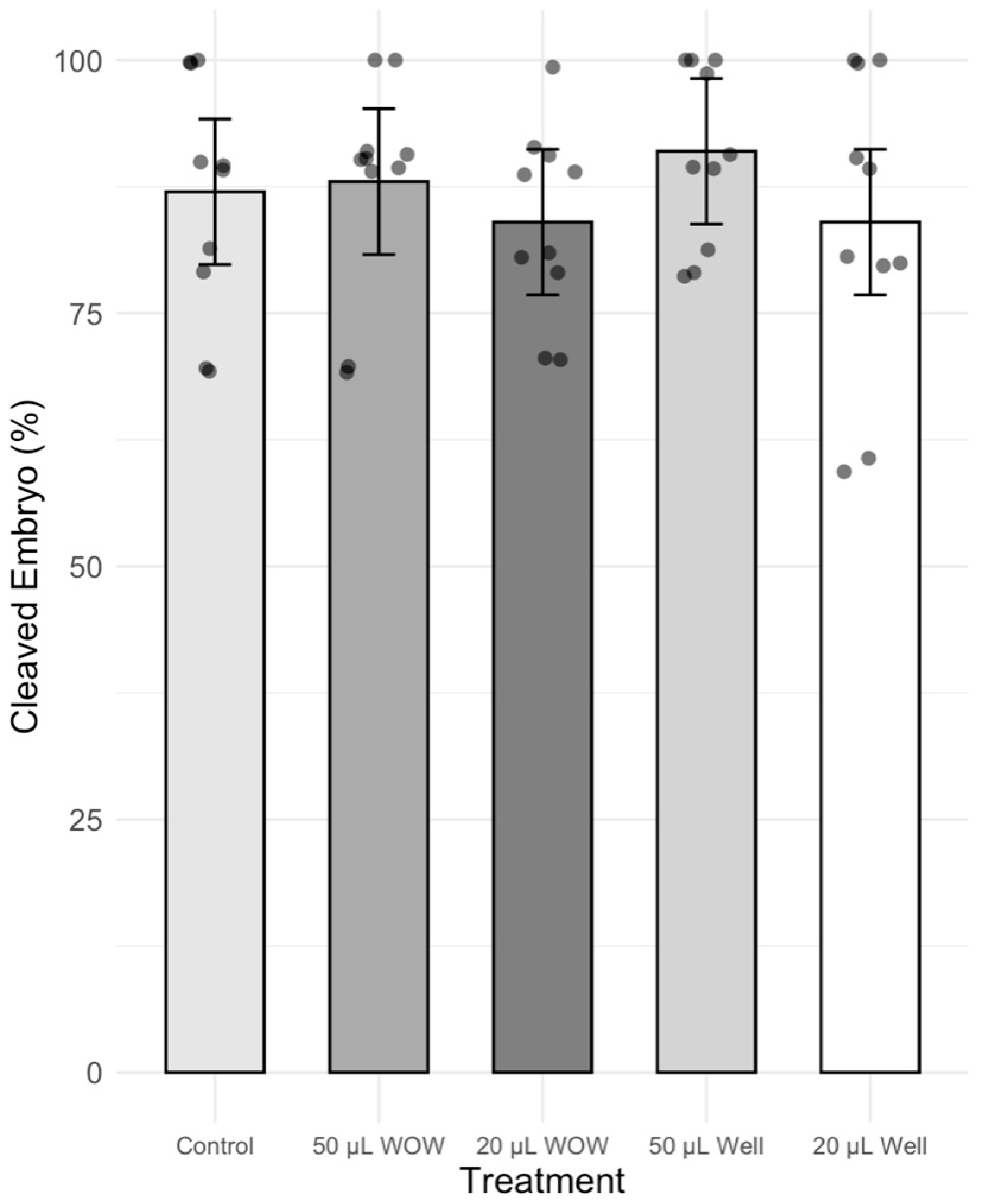
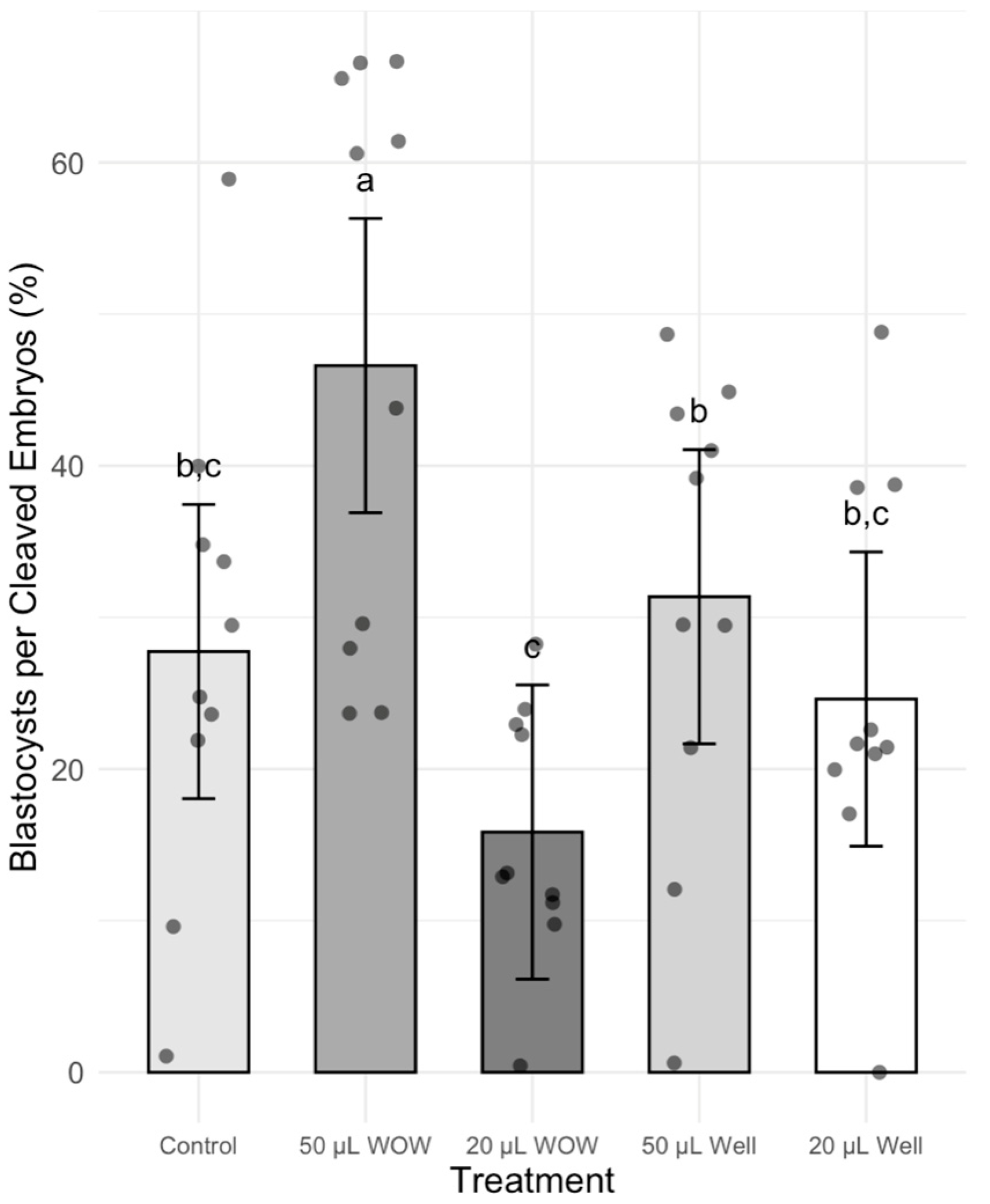
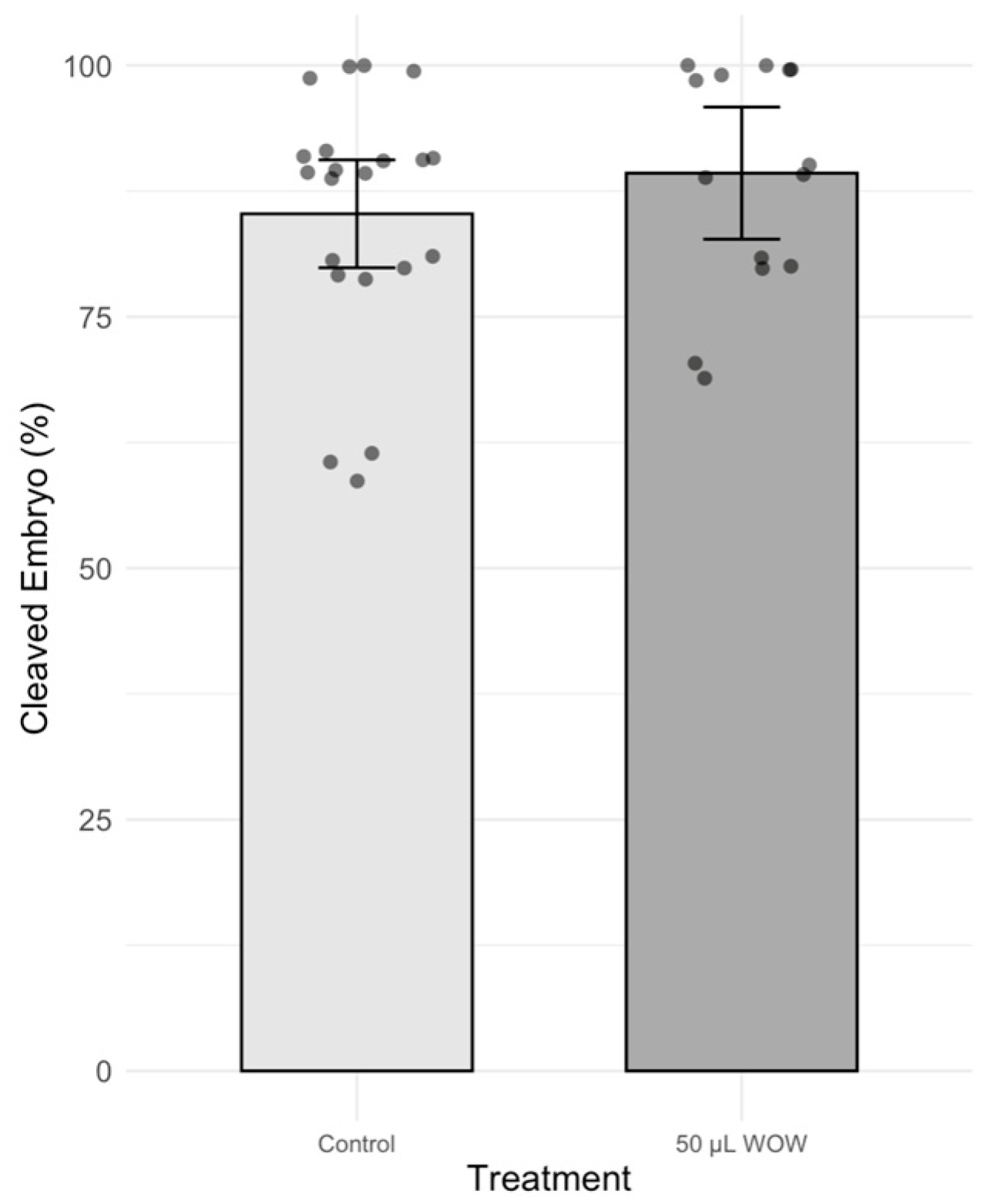
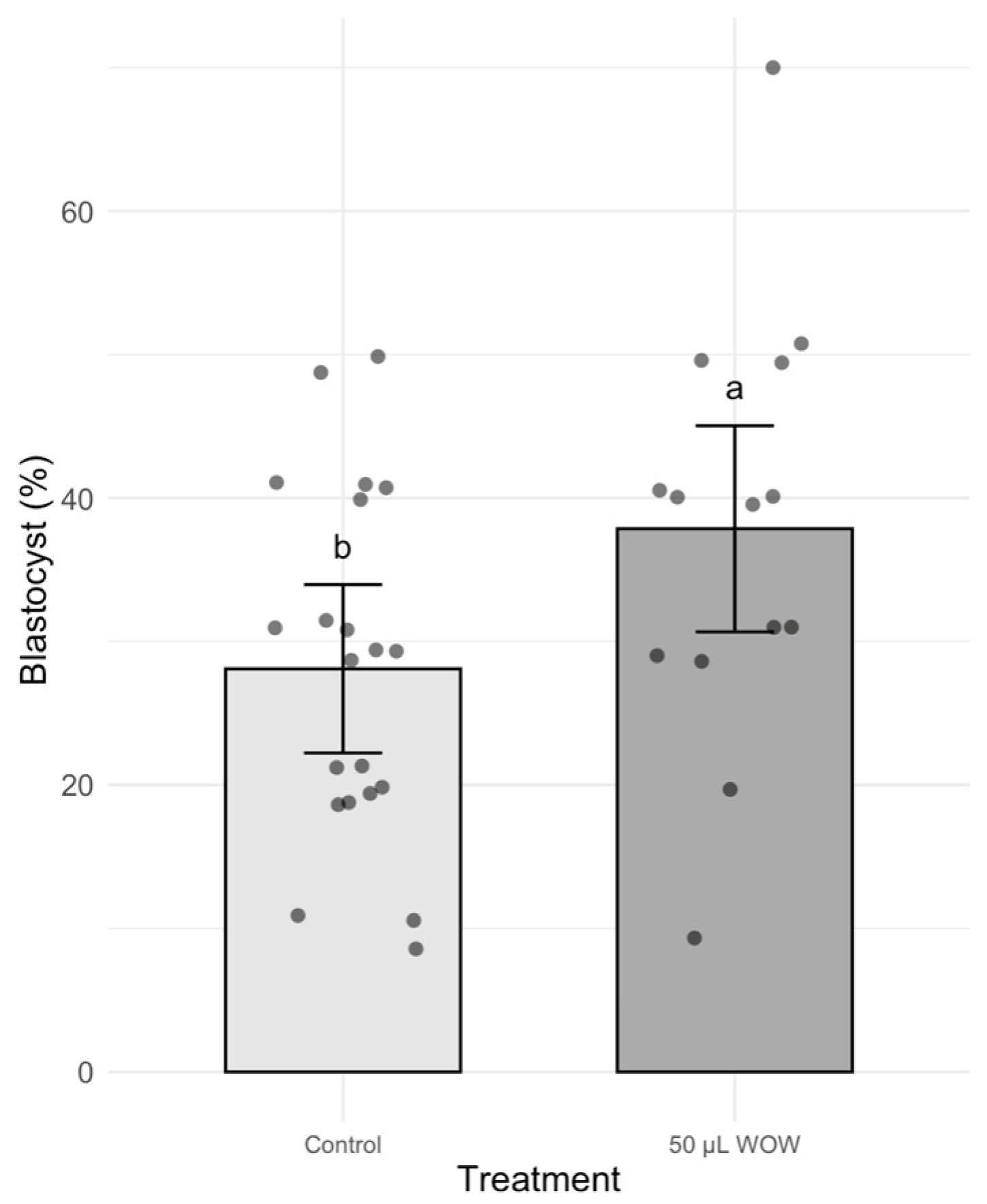
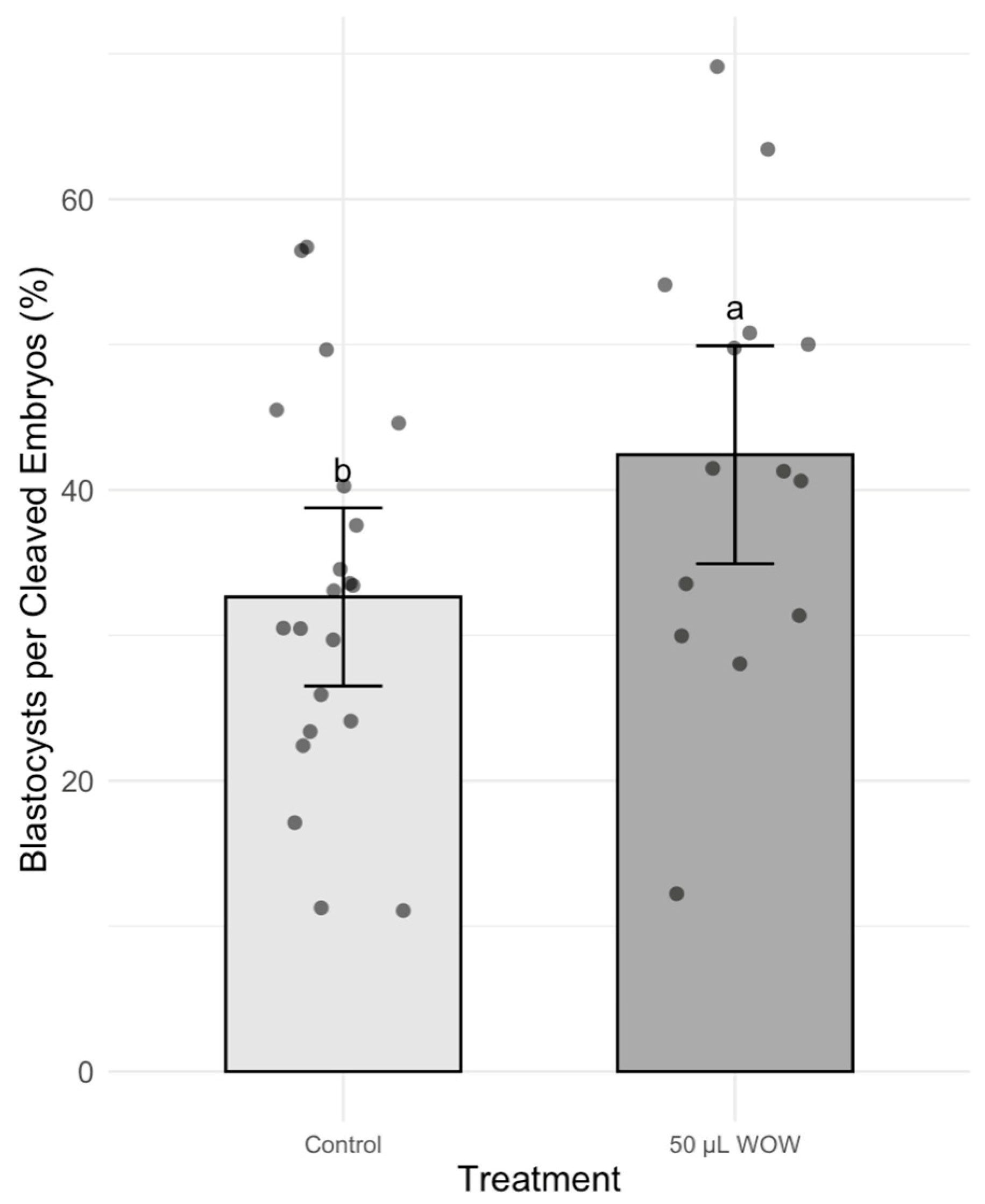
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walters, E.M.; Prather, R.S. Advancing Swine Models for Human Health and Diseases. Mo. Med. 2013, 110, 212. [Google Scholar]
- Walters, E.M.; Wells, K.D.; Bryda, E.C.; Schommer, S.; Prather, R.S. Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab Anim. 2017, 46, 167–172. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Macháty, Z.; Day, B.N.; Prather, R.S. Development of Early Porcine Embryos In Vitro and In Vivo. Biol. Reprod. 1998, 59, 451–455. [Google Scholar] [CrossRef]
- Devreker, F.; Englert, Y. In vitro development and metabolism of the human embryo up to the blastocyst stage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 51–56. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Heras, S.; De Sutter, P.; Deforce, D.; Peelman, L.; De Schauwer, C.; Van Soom, A. Autocrine embryotropins revisited: How do embryos communicate with each other in vitro when cultured in groups? Biol. Rev. 2017, 92, 505–520. [Google Scholar] [CrossRef]
- Looman, J.; Rodriguez, Z.; Waugh, L.; Hickerson, S.; Gibbons, J. Effects of bovine ova density and culture supplements on cleavage and blastocyst development rates of in vitro embryos. Clin. Theriogenology 2024, 16, 9801. [Google Scholar] [CrossRef]
- Vajta, G.; Korösi, T.; Du, Y.; Nakata, K.; Ieda, S.; Kuwayama, M.; Nagy, Z.P. The Well-of-the-Well system: An efficient approach to improve embryo development. Reprod. Biomed. Online 2008, 17, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Luddi, A.; Pavone, V.; Governini, L.; Capaldo, A.; Landi, C.; Ietta, F.; Paccagnini, E.; Morgante, G.; De Leo, V.; Piomboni, P. Emerging role of embryo secretome in the paracrine communication at the implantation site: A proof of concept. Fertil. Steril. 2021, 115, 1054–1062. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Qian, X.; Kuai, Y.; Xu, Y. The Inclusion Principles of Human Embryos in the WOW-Based Time-Lapse System: A Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 549216. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.A.; Lee, S.L.; Kim, J.G.; Kumar, B.M.; Balasubramanian, S.; Choe, S.Y.; Rho, G.-J. Development and quality of porcine embryos in different culture system and embryo-producing methods. Zygote 2007, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vajta, G.; Peura, T.T.; Holm, P.; Páldi, A.; Greve, T.; Trounson, A.O.; Callesen, H. New method for culture of zona-included or zona-free embryos: The Well of the Well (WOW) system. Mol. Reprod. Dev. 2000, 55, 256–264. [Google Scholar] [CrossRef]
- Taka, M.; Iwayama, H.; Fukui, Y. Effect of the well of the well (WOW) system on in vitro culture for porcine embryos after intracytoplasmic sperm injection. J. Reprod. Dev. 2005, 51, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Xu, C.L.; Wang, J.; Sun, Y.P.; Chian, R.C. Effect of culture medium volume and embryo density on early mouse embryonic development: Tracking the development of the individual embryo. J. Assist. Reprod. Genet. 2012, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chao, C.-H.; Jaeger, L.A.; Papp, A.B.; Machaty, Z.; Ward, M.A. Calcium oscillations in fertilized pig oocytes are associated with repetitive interactions between STIM1 and ORAI1. Biol. Reprod. 2018, 98, 510–519. [Google Scholar] [CrossRef]
- wow-humdish—VitaVitro Biotech n.d. Available online: https://www.vitavitro.com/wow-humdish/ (accessed on 20 June 2025).
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.K.; Iwamura, S. Birth of Piglets Derived from Porcine Zygotes Cultured in a Chemically Defined Medium. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef]
- de Andrade, A.F.C.; Balogun, K.; Machaty, Z.; Knox, R.V. Effects of supplemental antioxidants on in vitro fertility measures for cryopreserved boar spermatozoa. Theriogenology 2023, 200, 33–42. [Google Scholar] [CrossRef]
- Chen, P.R.; Uh, K.; Redel, B.K.; Reese, E.D.; Prather, R.S.; Lee, K. Production of Pigs from Porcine Embryos Generated in vitro. Front. Anim. Sci. 2022, 3, 826324. [Google Scholar] [CrossRef]
- Paria, B.C.; Dey, S.K. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth factors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–4760. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Gardner, D.K. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum. Reprod. 1992, 7, 558–562. [Google Scholar] [CrossRef]
- O’Neill, C. Evidence for the Requirement of Autocrine Growth Factors for Development of Mouse Preimplantation Embryos in Vitro. Biol. Reprod. 1997, 56, 229–237. [Google Scholar] [CrossRef]
- Díaz-Cueto, L.; Gerton, G.L. The Influence of Growth Factors on the Development of Preimplantation Mammalian Embryos. Arch. Med. Res. 2001, 32, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Biggers, J.D.; Anderson, E. Mitochondria and early development of the mouse. J. Exp. Zool. 1971, 176, 179–191. [Google Scholar] [CrossRef]
- Swegen, A.; Grupen, C.G.; Gibb, Z.; Baker, M.A.; de Ruijter-Villani, M.; Smith, N.D.; Stout, T.A.E.; Aitken, R.J. From Peptide Masses to Pregnancy Maintenance: A Comprehensive Proteomic Analysis of The Early Equine Embryo Secretome, Blastocoel Fluid, and Capsule. Proteomics 2017, 17, 1600433. [Google Scholar] [CrossRef] [PubMed]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef]
- Stokes, P.J.; Abeydeera, L.R.; Leese, H.J. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev. Biol. 2005, 284, 62–71. [Google Scholar] [CrossRef]
- Thibodeaux, J.K.; Del Vecchio, R.P.; Hansel, W. Role of platelet-derived growth factor in development of in vitro matured and in vitro fertilized bovine embryos. Reproduction 1993, 98, 61–66. [Google Scholar] [CrossRef]
- Cockburn, K.; Rossant, J. Making the blastocyst: Lessons from the mouse. J. Clin. Investig. 2010, 120, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Howles, C.M. Role of LH and FSH in ovarian function. Mol. Cell Endocrinol. 2000, 161, 25–30. [Google Scholar] [CrossRef]
- Stoddart, N.R.; Wild, A.E.; Fleming, T.P. Stimulation of development in vitro by platelet-activating factor receptor ligands released by mouse preimplantation embryos. Reproduction 1996, 108, 47–53. [Google Scholar] [CrossRef]
- Wiley, L.M.; Yamami, S.; Van Muyden, D. Effect of potassium concentration, type of protein supplement, and embryo density on mouse preimplantation development in vitro. Fertil. Steril. 1986, 45, 111–119. [Google Scholar] [CrossRef]
- O’Doherty, E.M.; Wade, M.G.; Hill, J.L.; Boland, M.P. The role of oocyte numbers in culture on development of bovine oocytes in an in vitro system. Theriogenology 1997, 1, 296. [Google Scholar] [CrossRef]
- Larson, M.A.; Kubisch, H.M. The effects of group size on development and interferon-τ secretion by in-vitro fertilized and cultured bovine blastocysts. Hum. Reprod. 1999, 14, 2075–2079. [Google Scholar] [CrossRef]
- Khurana, N.K.; Niemann, H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000, 54, 741–756. [Google Scholar] [CrossRef]
- Donnay, I.; Van Langendonckt, A.; Auquier, P.; Grisart, B.; Vansteenbrugge, A.; Massip, A.; Dessy, F. Effects of co-culture and embryo number on the in vitro development of bovine embryos. Theriogenology 1997, 47, 1549–1561. [Google Scholar] [CrossRef]
- Duarte De Oliveira, A.T.; Felix Lopes, R.F.; Rodrigues, J.L. Gene expression and developmental competence of bovine embryos produced in vitro under varying embryo density conditions. Theriogenology 2005, 64, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Krylov, V.; Kren, R.; Fulka, J. Development of pig embryos after electro-activation and in vitro fertilization in pzm-3 or pzm supplemented with fetal bovine serum. J. Reprod. Dev. 2006, 52, 91–98. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Houghton, F.D. Hypoxia and Reproductive Health: Hypoxic regulation of preimplantation embryos: Lessons from human embryonic stem cells. Reproduction 2021, 161, F41–F51. [Google Scholar] [CrossRef]
- Kleijkers, S.H.M.; Van Montfoort, A.P.A.; Bekers, O.; Coonen, E.; Derhaag, J.G.; Evers, J.L.H.; Dumoulin, J.C. Ammonium accumulation in commercially available embryo culture media and protein supplements during storage at 2–8 °C and during incubation at 37 °C. Hum. Reprod. 2016, 31, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Krisher, R.L. Effect of ammonium during in vitro maturation on oocyte nuclear maturation and subsequent embryonic development in pigs. Anim. Reprod. Sci. 2010, 117, 302–307. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogun, K.; Machaty, Z. Development of IVF Porcine Embryos in Microwell Culture System. Animals 2025, 15, 2528. https://doi.org/10.3390/ani15172528
Balogun K, Machaty Z. Development of IVF Porcine Embryos in Microwell Culture System. Animals. 2025; 15(17):2528. https://doi.org/10.3390/ani15172528
Chicago/Turabian StyleBalogun, Kayode, and Zoltan Machaty. 2025. "Development of IVF Porcine Embryos in Microwell Culture System" Animals 15, no. 17: 2528. https://doi.org/10.3390/ani15172528
APA StyleBalogun, K., & Machaty, Z. (2025). Development of IVF Porcine Embryos in Microwell Culture System. Animals, 15(17), 2528. https://doi.org/10.3390/ani15172528







