Human Adenovirus 36 Antibodies in Horses with Different Metabolic Statuses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Permissions
2.2. Studied Animals and Blood Sampling
2.3. Study Group
2.4. Virus Neutralization Assay
2.5. Biochemical Analysis of Tested Serum
2.6. Statistical Analysis
3. Results
3.1. Virus Neutralization Assay
3.2. Biochemical Analysis of Tested Serum
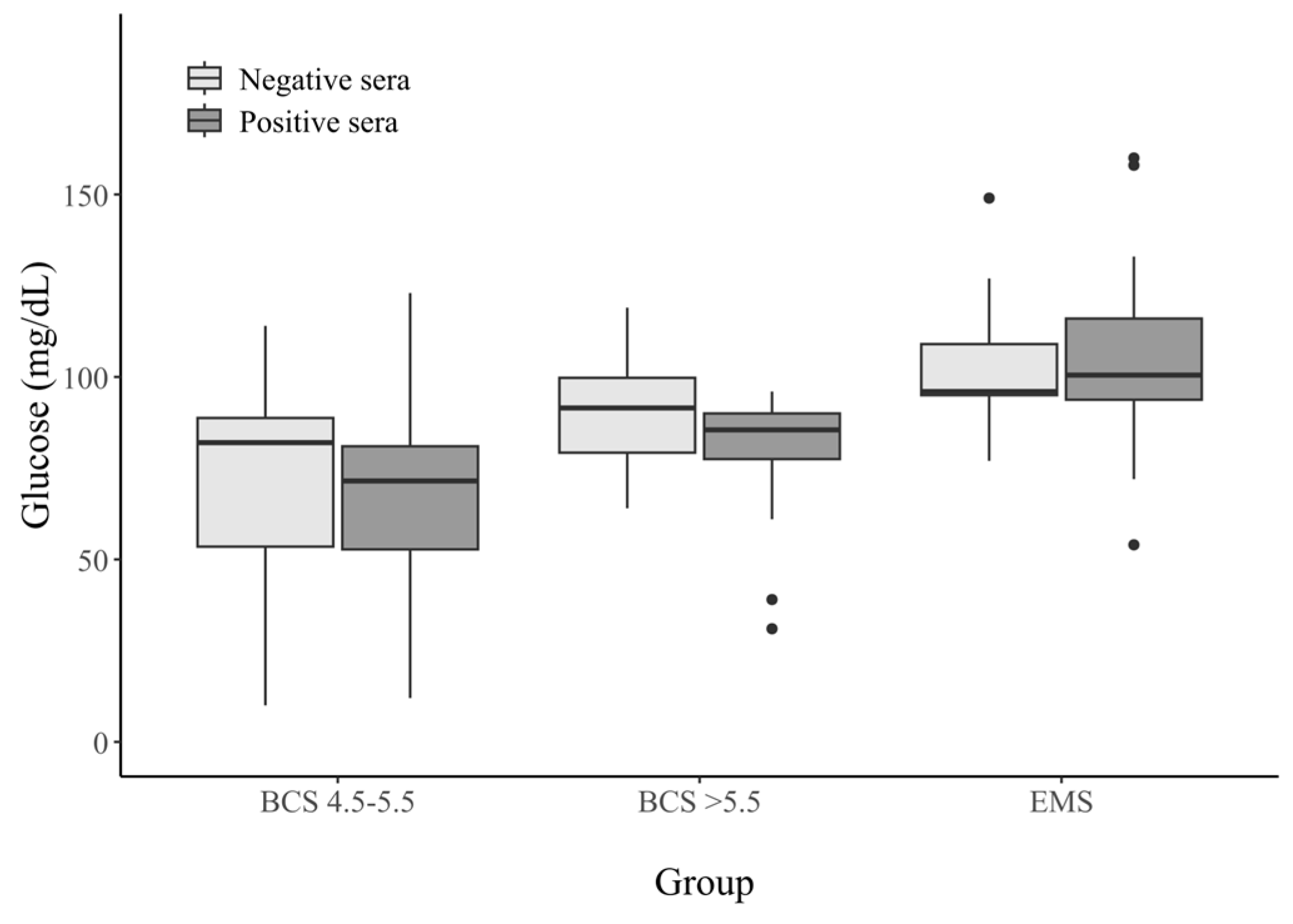
3.2.1. Glucose Concentration in Horse Serum
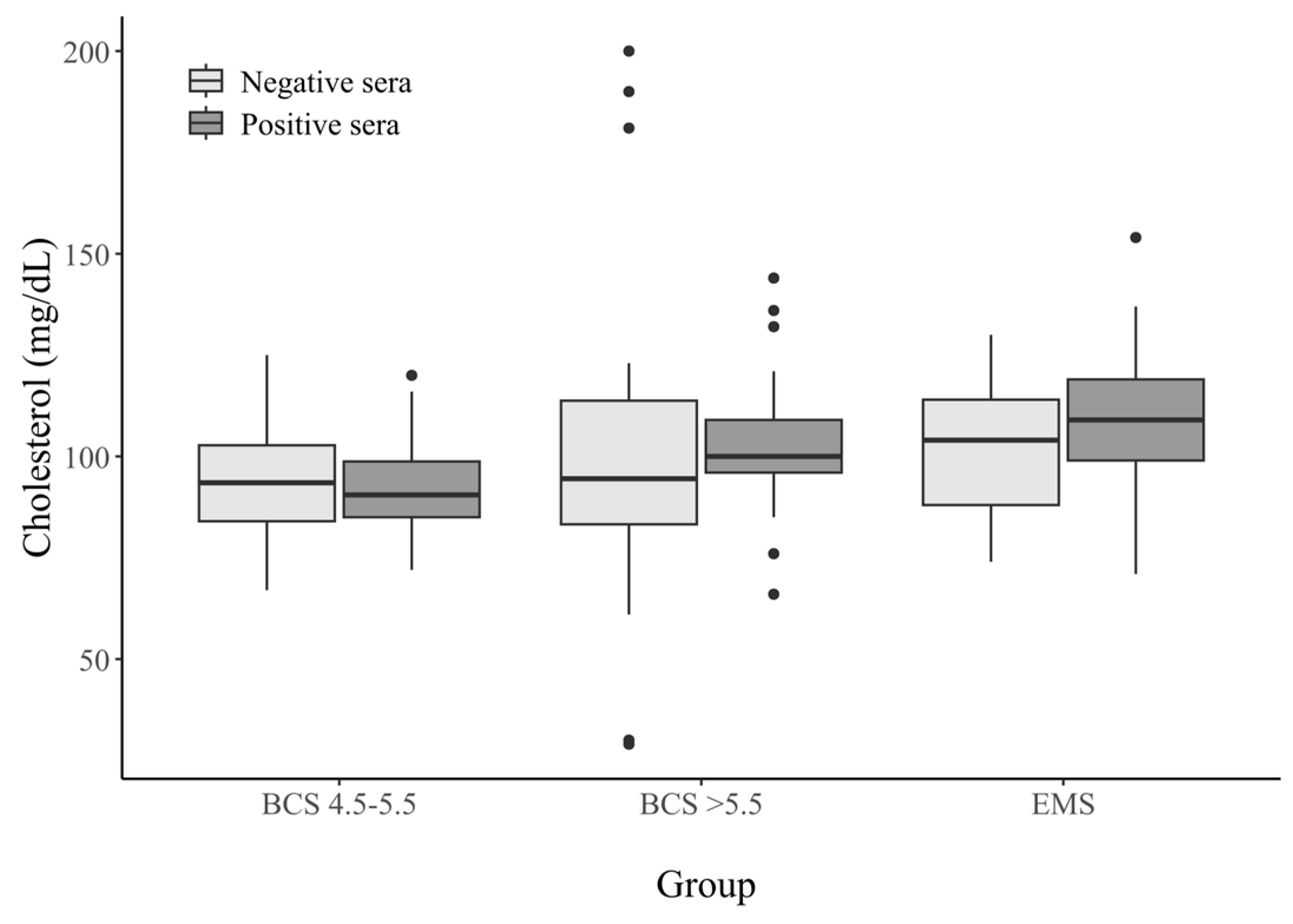
3.2.2. Cholesterol Concentration in Horse Serum
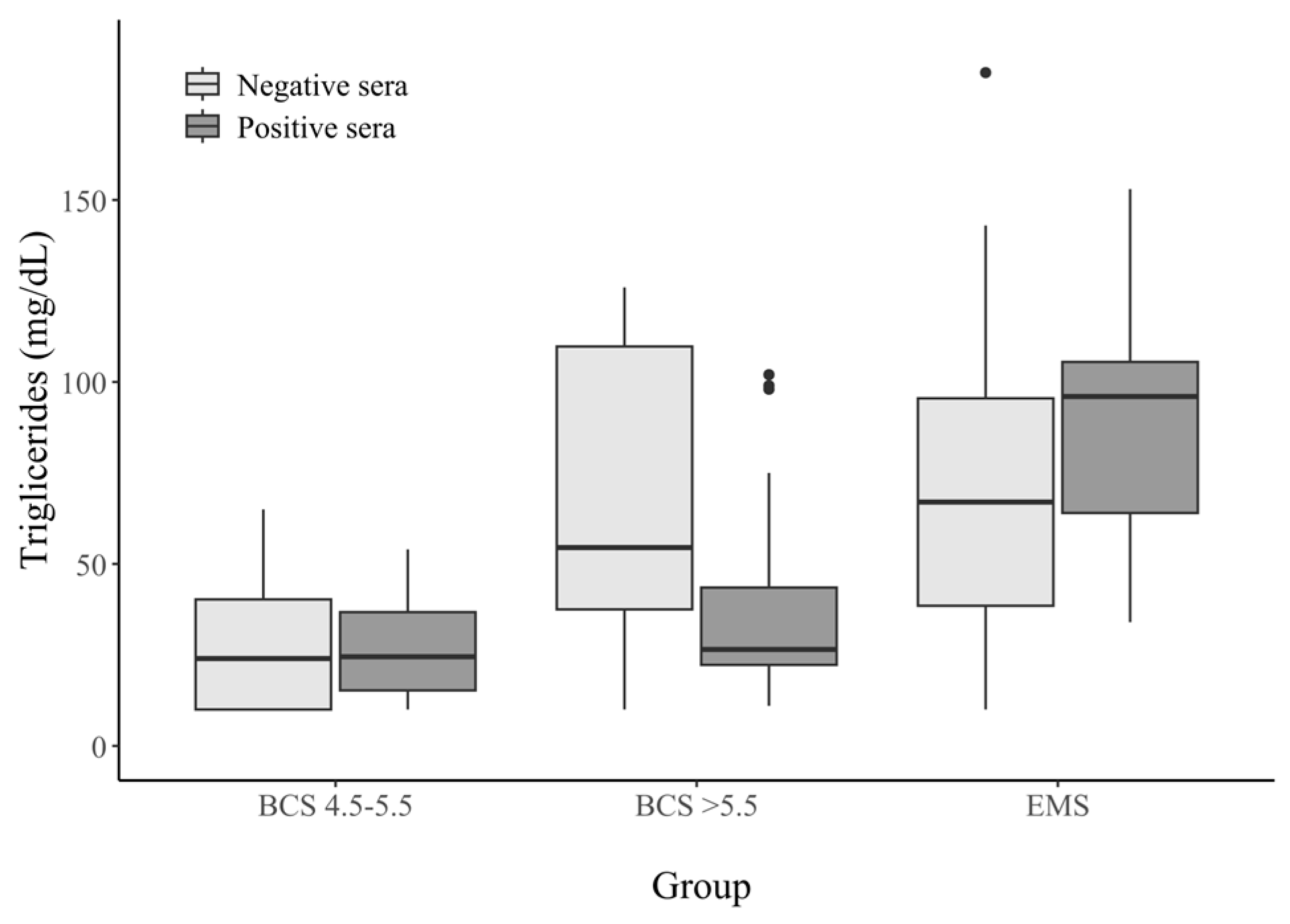
3.2.3. Triglycerides Concentration in Horse Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nam, J.H.; Na, H.N.; Atkinson, R.L.; Dhurandhar, N.V. Genomic stability of adipogenic human adenovirus 36. Int. J. Obes. 2014, 38, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Adenoviridae~ViralZone. Available online: https://viralzone.expasy.org/4 (accessed on 7 March 2025).
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Kulkarni, P.; Ajinkya, S.M.; Sherikar, A. Effect of adenovirus infection on adiposity in chicken. Vet. Microbiol. 1992, 31, 101–107. [Google Scholar] [CrossRef]
- Mitra, A.K.; Clarke, K. Viral obesity: Fact or fiction? Obes. Rev. 2010, 11, 289–296. [Google Scholar] [CrossRef]
- Whigham, L.D.; Israel, B.A.; Atkinson, R.L. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R190–R194. [Google Scholar] [CrossRef]
- Da, J.; Fernandes, S.; Schuelter-Trevisol, F.; Carolina, A.; Cancelier, L.; Gonçalves, E.; Silva, H.C.; Gomes De Sousa, D.; Atkinson, R.L.; Trevisol, J.D. Adenovirus 36 prevalence and association with human obesity: A systematic review. Int. J. Obes. 2021, 45, 1342–1356. [Google Scholar] [CrossRef]
- de Lacerda Suplicy, H.; Bornschein, A.; de Lacerda, H.; Rua, S.; Leão, A. Infeccions as the etiology for obesity Infecções na etiologia da obesidade Descritores Infecções humanas por vírus; obesidade. Arq. Bras. Endocrinol. Metabol. 2009, 53, 159–164. [Google Scholar]
- Chwirot, A.; Migdał, P.; Florek, M.; Stygar, D.; Kublicka, A.; Michalczyk, K.; Napierkowska, S.; Uchańska, O.; Matczuk, A.; Rączkowski, W.; et al. Dogs are a susceptible species to human adenovirus 36 infection: New insights into the host range of the virus causing infectious obesity. Vet. Microbiol. 2025, 302, 110369. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; Gneiss, L. Adenovirus 36 and Obesity: An Overview. Viruses 1980, 7, 3719–3740. [Google Scholar] [CrossRef]
- Dhurandhar, N.V. Insulin sparing action of adenovirus 36 and its E4orf1 protein. J. Diabetes Its Complicat. 2013, 27, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Shin, A.C.; Yu, M.; Yang, H.M.O.; Rathod, M.; Jen, K.-L.C.; Mohan Kumar, S.; Mohan Kumar, P.S.; Markward, N.; Dhurandhar, N.V. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity 2006, 14, 1905–1913. [Google Scholar] [CrossRef]
- Pasarica, M.; Dhurandhar, N.V. Infectobesity: Obesity of Infectious Origin. Adv. Food Nutr. Res. 2007, 52, 61–102. [Google Scholar] [PubMed]
- Dhurandhar, N.V.; Israel, B.A.; Kolesar, J.M.; Mayhew, G.; Cook, M.E.; Atkinson, R.L. Transmissibility of adenovirus-induced adiposity in a chicken model. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 990–996. [Google Scholar] [CrossRef]
- Almgren, M.; Atkinson, R.L.; Hilding, A.; He, J.; Brismar, K.; Schalling, M.; Ostenson, C.G.; Lavebratt, C. Human adenovirus-36 is uncommon in type 2 diabetes and is associated with increased insulin sensitivity in adults in Sweden. Ann. Med. 2014, 46, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Frank, N. Equine metabolic syndrome. Vet. Clin. N. Am. Equine Pract. 2011, 27, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Cefalu, W.T.; Zhang, X.H.; Yu, Y.; Qin, J.; Son, L.; Rogers, P.M.; Mashtalir, N.; Bordelon, J.R.; Ye, J.; et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes 2008, 57, 1805–1813. [Google Scholar] [CrossRef]
- Rogers, P.M.; Mashtalir, N.; Rathod, M.A.; Dubuisson, O.; Wang, Z.; Dasuri, K.; Babin, S.; Gupta, A.; Markward, N.; Cefalu, W.T.; et al. Metabolically favorable remodeling of human adipose tissue by human adenovirus type 36. Diabetes 2008, 57, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Dubuisson, O.; Rubicz, R.; Liu, N.; Allison, D.B.; Curran, J.E.; Comuzzie, A.G.; Blangero, J.; Leach, C.T.; Göring, H.; et al. Long-term changes in adiposity and glycemic control are associated with past adenovirus infection. Diabetes Care. 2013, 36, 701–707. [Google Scholar] [CrossRef]
- Marjani, A.; Khatami, A.; Saadati, H.; Asghari, M.; Razizadeh, M.H.; Abbasi, A.; Zarei, M.; Beikzadeh, L.; Soleimani, A. Association of adenovirus 36 infection and obesity; An updated meta-analysis of community-based studies. Rev. Med. Virol. 2022, 32, e2255. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Israel, B.A.; Kolesar, J.M.; Mayhew, G.F.; Cook, M.E.; Atkinson, R.L. Increased adiposity in animals due to a human virus. Int. J. Obes. 2000, 24, 989–996. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Whigham, L.D.; Abbott, D.H.; Schultz-Darken, N.J.; Israel, B.A.; Bradley, S.M.; Kemnitz, J.W.; Allison, D.B.; Atkinson, R.L. Nutritional Immunology Human Adenovirus Ad-36 Promotes Weight Gain in Male Rhesus and Marmoset Monkeys 1,2. J. Nutr. 2002, 132, 3155–3160. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Dhurandhar, N.V.; Allison, D.B.; Bowen, R.L.; A Israel, B.; Albu, J.B.; Augustus, A.S. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int. J. Obes. 2005, 29, 281–286. [Google Scholar] [CrossRef]
- Na, H.N.; Nam, J.H. Adenovirus 36 as an obesity agent maintains the obesity state by increasing MCP-1 and inducing inflammation. J. Infect. Dis. 2012, 205, 914–922. [Google Scholar] [CrossRef]
- Elzinga, S.; Wood, P.; Adams, A.A. Plasma lipidomic and inflammatory cytokine profiles of horses with equine metabolic syndrome. J. Equine Vet. Sci. 2016, 40, 4955. [Google Scholar] [CrossRef]
- Matia-Garcia, I.; Ocampo-Galeana, J.A.; Muñoz-Valle, J.F.; Soñanez-Organis, J.G.; González, R.A.; Guzmán-Guzmán, I.P.; Marino-Ortega, L.A.; Parra-Rojas, I. An Observational Study Suggests That Natural HAdV-36 Infection Decreases Blood Glucose Levels without Affecting Insulin Levels in Obese Young Subjects. Viruses 2024, 16, 922. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Dhurandhar, E.J.; Ingram, D.K.; Vaughan, K.; Mattison, J.A. Natural infection of human adenovirus 36 in rhesus monkeys is associated with a reduction in fasting glucose 36. J. Diabetes. 2014, 6, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Alcocer, J.; García-Benavides, L.; Muñoz-Valle, J.F.; de la Cruz-Mosso, U.; González, R.A.; Luquín, S.; Alarcon-Romero, L.D.; Marino-Ortega, L.A.; Matia-Garcia, I.; Parra-Rojas, I. Presence of adenovirus-36 DNA in adipose tissue of women: Relationship with adipocyte morphology and the expression of C/EBPβ and HIF-1α. Diabetes Metab. Syndr. Obes. 2021, 14, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; Cangemi, R.; Mariani, S.; Casella, G.; de Cesare, A.; Trovato, F.M.; Garozzo, A.; Gnessi, L. Adenovirus 36 DNA in human adipose tissue. Int. J. Obes. 2015, 39, 761–1764. [Google Scholar] [CrossRef]
- Shirani, F.; Teimoori, A.; McAinch, A.J.; Rashno, M.; Latifi, S.M.; Karandish, M. Human adenovirus 36 improves insulin sensitivity and lipid profiles and increases inflammatory markers in Wistar rats. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 980–984. [Google Scholar] [CrossRef]
- Kapila, M.; Khosla, P.; Dhurandhar, N.V. Novel short-term effects of adenovirus Ad-36 on hamster lipoproteins. Int J Obes Relat Metab Disord. 2004, 28, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Hong, Y.M.; Ye, M.B.; Park, S.; Kim, I.B.; Nam, J.H. Adenovirus 36 attenuates weight loss from exercise but improves glycemic control by increasing mitochondrial activity in the liver. PLoS ONE 2014, 9, e114534. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Keen, J.; McGowan, C. Equine metabolic syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Calam, R.R.; Bessman, J.D.; Ernst, D.J.; Smith, S.S.; Szamosi, D.I.; Warunek, D.J.; Wiseman, J.D. Procedures for the Handling and Processing of Blood Specimens: Approved Guideline, 3rd ed.; NCCLS Document H18-A3; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2004; Volume 24, No. 38. [Google Scholar]
| SN Antibody Titer | BCS 4.5–5.5 (n = 52) | BCS > 5.5 (n = 52) | EMS (n = 47) | |
|---|---|---|---|---|
| 0 | 19 | 22 | 16 | Seronegative (n = 79, 52.3%) |
| 1:2 | 5 | 3 | 0 | |
| 1:4 | 6 | 2 | 6 | |
| 1:8 | 8 | 4 | 6 | Seropositive (n = 72, 47.7%) |
| 1:16 | 8 | 3 | 7 | |
| 1:32 | 1 | 9 | 4 | |
| 1:64 | 4 | 6 | 5 | |
| 1:128 | 0 | 2 | 1 | |
| 1:256 | 1 | 1 | 0 |
| Glucose (mg/dL) | BCS 4.5–5.5 | BCS > 5.5 | EMS | |||
|---|---|---|---|---|---|---|
| Seropositive Sera (n = 22) | Seronegative Sera (n = 30) | Seropositive Sera (n = 25) | Seronegative Sera (n = 27) | Seropositive Sera (n = 25) | Seronegative Sera (n = 21) | |
| 0–50 | 5 (22.7%) | 7 (23.3%) | 2 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 51–64 | 3 (13.6%) | 2 (6.6%) | 1 (4%) | 2 (7.4%) | 1 (4%) | 0 (0%) |
| 65–84 | 9 (40.9%) | 11 (36.6%) | 7 (28%) | 9 (33.3%) | 2 (8%) | 2 (9.5%) |
| 85–100 | 3 (13.6%) | 7 (23.3%) | 15 (60%) | 11 (40.7%) | 9 (36%) | 9 (42.8%) |
| 101–125 | 2 (9%) | 3 (10%) | 0 (0%) | 5 (18.5%) | 9 (36%) | 8 (38%) |
| 126–150 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 2 (9.5%) |
| >151 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (12%) | 0 (0%) |
| Cholesterol (mg/dL) | BCS 4.5–5.5 | BCS > 5.5 | EMS | |||
|---|---|---|---|---|---|---|
| Seropositive Sera (n = 22) | Seronegative Sera (n = 30) | Seropositive Sera (n = 25) | Seronegative Sera (n = 27) | Seropositive Sera (n = 25) | Seronegative Sera (n = 21) | |
| 0–50 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (7.4%) | 0 (0%) | 3 (14.2%) |
| 51–80 | 5 (22.7%) | 7 (23.3%) | 1 (4%) | 5 (18.5%) | 1 (4%) | 2 (9.5%) |
| 81–90 | 6 (27.2%) | 6 (20%) | 2 (8%) | 6 (22.2%) | 2 (8%) | 5 (23.8%) |
| 91–110 | 9 (40.9%) | 14 (46.6%) | 16 (64%) | 6 (22.2%) | 16 (64%) | 5 (23.8%) |
| >111 | 0 (0%) | 3 (10%) | 6 (24%) | 8 (29.6%) | 6 (24%) | 12 (44.4%) |
| Triglycerides (mg/dL) | BCS 4.5–5.5 | BCS > 5.5 | EMS | |||
|---|---|---|---|---|---|---|
| Seropositive Sera (n = 22) | Seronegative Sera (n = 30) | Seropositive Sera (n = 25) | Seronegative Sera (n = 27) | Seropositive Sera (n = 25) | Seronegative Sera (n = 21) | |
| 0–11 | 4 (18.1%) | 9 (30%) | 1 (4%) | 3 (11.1%) | 0 (0%) | 1 (4.7%) |
| 12–30 | 8 (36.3%) | 9 (30%) | 15 (60%) | 2 (7.4%) | 0 (0%) | 3 (14.2%) |
| 31–50 | 7 (31.8%) | 10 (33.3%) | 5 (20%) | 5 (18.5%) | 4 (16%) | 3 (14.2%) |
| 50–68 | 1 (4.5%) | 2 (6.6%) | 1 (4%) | 5 (18.5%) | 4 (16%) | 4 (19%) |
| >69 | 2 (9%) | 0 (0%) | 3 (12%) | 12 (44.4%) | 17 (68%) | 8 (38%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwirot, A.; Niedźwiedź, A.; Stygar, D.; Siwińska, N.; Paszkowska, M.; Niżański, W.; Napierkowska, S.; Migdał, P.; Kublicka, A.; Marynowska, M.; et al. Human Adenovirus 36 Antibodies in Horses with Different Metabolic Statuses. Animals 2025, 15, 2527. https://doi.org/10.3390/ani15172527
Chwirot A, Niedźwiedź A, Stygar D, Siwińska N, Paszkowska M, Niżański W, Napierkowska S, Migdał P, Kublicka A, Marynowska M, et al. Human Adenovirus 36 Antibodies in Horses with Different Metabolic Statuses. Animals. 2025; 15(17):2527. https://doi.org/10.3390/ani15172527
Chicago/Turabian StyleChwirot, Aleksandra, Artur Niedźwiedź, Dominika Stygar, Natalia Siwińska, Marzena Paszkowska, Wojciech Niżański, Skarlet Napierkowska, Paweł Migdał, Agata Kublicka, Maja Marynowska, and et al. 2025. "Human Adenovirus 36 Antibodies in Horses with Different Metabolic Statuses" Animals 15, no. 17: 2527. https://doi.org/10.3390/ani15172527
APA StyleChwirot, A., Niedźwiedź, A., Stygar, D., Siwińska, N., Paszkowska, M., Niżański, W., Napierkowska, S., Migdał, P., Kublicka, A., Marynowska, M., Matczuk, A., Fuller, D., & Bażanów, B. (2025). Human Adenovirus 36 Antibodies in Horses with Different Metabolic Statuses. Animals, 15(17), 2527. https://doi.org/10.3390/ani15172527







