Genetic Variants in the ATF6 Gene and Their Relationship with Milk-Quality Traits in Yaks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Milk Composition Analysis
2.2. Genotyping
2.3. Statistical Analysis
3. Results
3.1. Alignment to the Reference Genome
3.2. Association Analysis of Milk Traits and Genotypes of SNPs in Yak
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, Q.; Wang, L.; Wang, K.; Yang, Y.; Ma, T.; Wang, Z.; Zhang, X.; Ni, Z.; Hou, F.; Long, R.; et al. Yak whole-genome resequencing reveals domestication signatures and prehistoric population expansions. Nat. Commun. 2015, 6, 10283. [Google Scholar] [CrossRef]
- Li, A.; Liu, C.; Han, X.; Zheng, J.; Zhang, G.; Qi, X.; Du, P.; Liu, L. Tibetan Plateau yak milk: A comprehensive review of nutritional values, health benefits, and processing technology. Food Chem. X 2023, 20, 100919. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Zheng, X.; Guo, J.; Duan, H.; Zhou, S.; Yan, W. Yak Milk: Nutritional Value, Functional Activity, and Current Applications. Foods 2023, 12, 2090. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Xing, H. Lipidomic study of yak milk reveals potential biomarkers for the design of infant formulas. J. Dairy Sci. 2025, 108, 3234–3246. [Google Scholar] [CrossRef]
- Kalwar, Q.; Ma, X.; Xi, B.; Korejo, R.A.; Bhuptani, D.K.; Chu, M.; Yan, P. Yak milk and its health benefits: A comprehensive review. Front. Vet. Sci. 2023, 10, 1213039. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Wang, X.; Qi, X.; Zhang, L.; Xin, Y.; Yang, Z.; Fan, R.; Li, Y.; Liu, L.; et al. A systemic review of yak milk and its products on the Qinghai-Tibet Plateau: Unique products, chemical composition, biological activities, and microbial composition. Trends Food Sci. Technol. 2024, 154, 104792. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Tang, D.; Xi, B.; Li, W.; Chen, Z.; Bao, Y.; Dingkao, R.; Gao, Y.; Wang, P.; et al. Exploring the link between microbial community structure and flavour compounds of traditional fermented yak milk in Gannan region. Food Chem. 2024, 435, 137553. [Google Scholar] [CrossRef]
- Ma, X.; Yang, G.; Zhang, J.; Ma, R.; Shen, J.; Feng, F.; Yu, D.; Huang, C.; Ma, X.; La, Y.; et al. Association between Single Nucleotide Polymorphisms of PRKD1 and KCNQ3 Gene and Milk Quality Traits in Gannan Yak (Bos grunniens). Foods 2024, 13, 781. [Google Scholar] [CrossRef]
- Feng, F.; Yang, G.; Ma, X.; Zhang, J.; Huang, C.; Ma, X.; La, Y.; Yan, P.; Zhandui, P.; Liang, C. Polymorphisms within the PRKG1 Gene of Gannan Yaks and Their Association with Milk Quality Characteristics. Foods 2024, 13, 1913. [Google Scholar] [CrossRef]
- Shi, L.; Lv, X.; Liu, L.; Yang, Y.; Ma, Z.; Han, B.; Sun, D. A post-GWAS confirming effects of PRKG1 gene on milk fatty acids in a Chinese Holstein dairy population. BMC Genet. 2019, 20, 53. [Google Scholar] [CrossRef]
- Schneider, H.; Krizanac, A.M.; Falker-Gieske, C.; Heise, J.; Tetens, J.; Thaller, G.; Bennewitz, J. Genomic dissection of the correlation between milk yield and various health traits using functional and evolutionary information about imputed sequence variants of 34,497 German Holstein cows. BMC Genom. 2024, 25, 265. [Google Scholar] [CrossRef]
- Adachi, Y.; Yamamoto, K.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 2008, 33, 75–89. [Google Scholar] [CrossRef]
- Yonekura, S.; Tsuchiya, M.; Tokutake, Y.; Mizusawa, M.; Nakano, M.; Miyaji, M.; Ishizaki, H.; Haga, S. The unfolded protein response is involved in both differentiation and apoptosis of bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 3568–3578. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zhen, Z.; Yu, Y.; Qiu, Y.; Xiang, W. GRP78 regulates milk biosynthesis and the proliferation of bovinemammaryepithelial cells through the mTOR signaling pathway. Cell. Mol. Biol. Lett. 2019, 24, 57. [Google Scholar] [CrossRef]
- Sharmin, M.M.; Mizusawa, M.; Hayashi, S.; Arai, W.; Sakata, S.; Yonekura, S. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8643–8654. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, W.; Cheng, Y.; Wu, Y.; Wu, H.; He, M.; Chen, S.; Man, C.; Gao, H.; Du, L.; et al. Development and verification of a 10K liquid chip for Hainan black goat based on genotyping by pinpoint sequencing of liquid captured targets. BMC Genom. Data 2024, 25, 44. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-efficient, Visualization-enhanced, and Parallel-accelerated Tool for Genome-wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Dong, A.; Wang, J.; Li, Q.; He, S.; Maubois, J.-L. Protein composition of yak milk. Dairy Sci. Technol. 2010, 90, 111–117. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Li, Q.; Wang, J.; Cheng, J.; Xue, J.; Shi, J. The chemical composition and nitrogen distribution of Chinese yak (Maiwa) milk. Int. J. Mol. Sci. 2011, 12, 4885–4895. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Q.; Zhao, B.; Chen, X.; Song, X. Functional Peptides from Yak Milk Casein: Biological Activities and Structural Characteristics. Int. J. Mol. Sci. 2024, 25, 9072. [Google Scholar] [CrossRef]
- Wang, T.; Ma, X.; Feng, F.; Zheng, F.; Zheng, Q.; Zhang, J.; Zhang, M.; Ma, C.; Deng, J.; Guo, X.; et al. Study on Single Nucleotide Polymorphism of LAP3 Gene and Its Correlation with Dairy Quality Traits of Gannan Yak. Foods 2024, 13, 2953. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Noida, India, 1996. [Google Scholar]
- Dai, W.; White, R.; Liu, J.; Liu, H. Organelles coordinate milk production and secretion during lactation: Insights into mammary pathologies. Prog. Lipid Res. 2022, 86, 101159. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, R.; Zhang, P.; Han, X.; Guo, F.J. Transmission of ER stress response by ATF6 promotes endochondral bone growth. J. Orthop. Surg. Res. 2015, 10, 141. [Google Scholar] [CrossRef]
- Invernizzi, G.; Naeem, A.; Loor, J.J. Short communication: Endoplasmic reticulum stress gene network expression in bovine mammary tissue during the lactation cycle. J. Dairy Sci. 2012, 95, 2562–2566. [Google Scholar] [CrossRef]
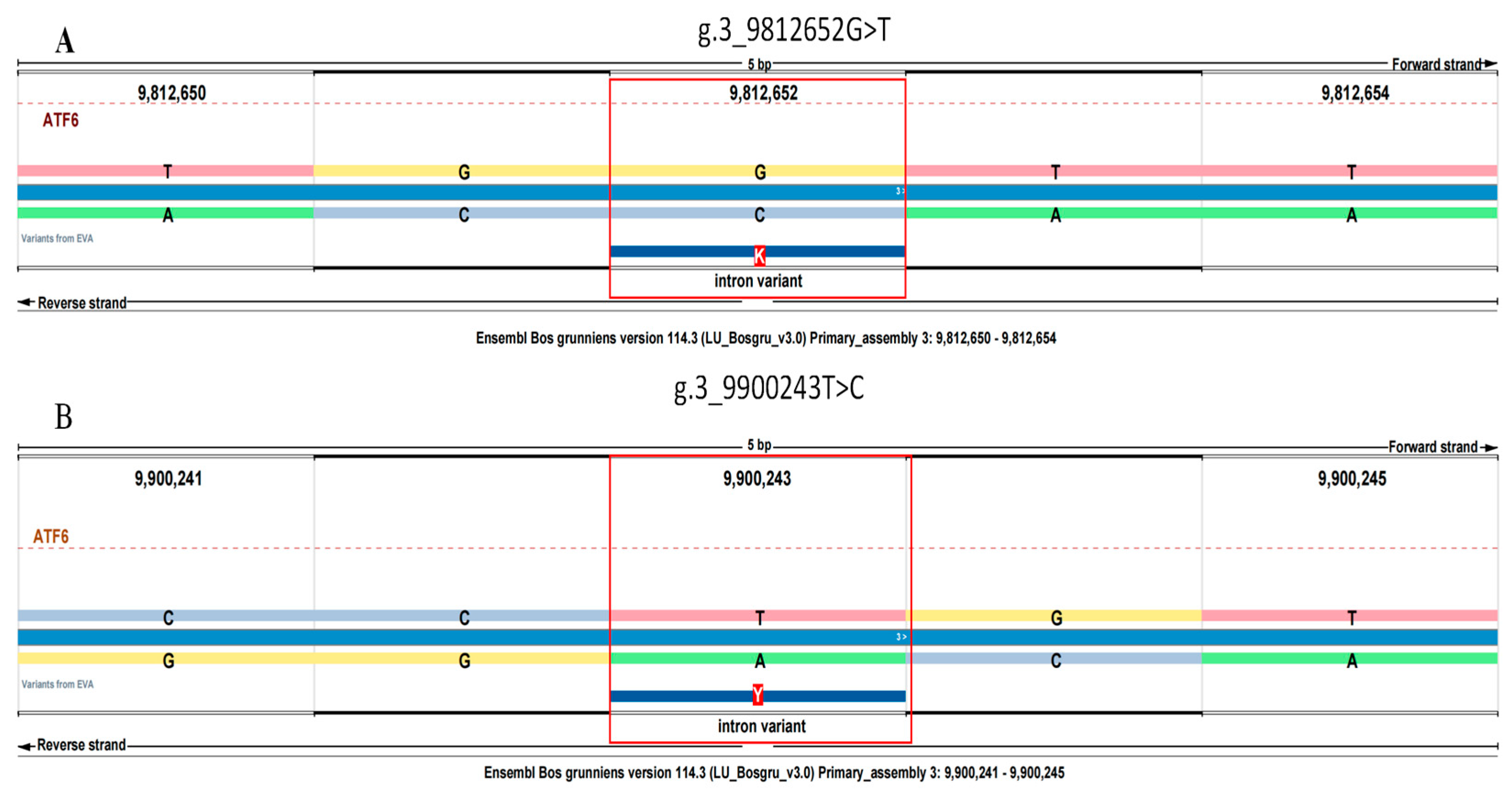
| SNPs | Position | Genotypic Frequencies | Allelic Frequencies | PIC | He | Ne | HW p Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| g. 3_9812652G>T | Intron | GG | GT | TT | G | T | 0.135 | 0.145 | 1.17 | 0.3380 |
| 0.853 | 0.137 | 0.010 | 0.921 | 0.079 | ||||||
| g. 3_9900243T>C | Intron | CC | CT | TT | C | T | 0.245 | 0.286 | 1.40 | 0.0312 |
| 0.702 | 0.250 | 0.048 | 0.827 | 0.173 | ||||||
| g. 3_9812652G>T | ||||||||
| Genotype | Casein/% | Protein/% | Acidity/°T | SNF/% | Lactose/% | Urea/μM | CitrAcid/°T | Fat/% |
| GG | 4.23 ± 0.025 | 5.18 ± 0.039 | 12.91 ± 0.106 | 11.40 ± 0.033 | 4.90 ± 0.014 | 0.03 ± 0.001 | 0.22 ± 0.002 | 5.47 ± 0.165 |
| GT | 4.53 ± 0.063 | 5.58 ± 0.096 | 13.81 ± 0.264 | 11.74 ± 0.083 | 4.86 ± 0.034 | 0.03 ± 0.002 | 0.22 ± 0.006 | 6.01 ± 0.412 |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | 0.293 | 0.290 | 0.298 | 0.230 |
| g. 3_9900243T > C | ||||||||
| Genotype | Casein/% | Protein/% | Acidity/°T | SNF/% | Lactose/% | Urea/μM | CitrAcid/°T | Fat/% |
| CC | 4.21 ± 0.026 | 5.15 ± 0.040 | 12.85 ± 0.108 | 11.39 ± 0.036 | 4.90 ± 0.014 | 0.03 ± 0.001 | 0.23 ± 0.003 | 5.51 ± 0.180 |
| CT | 4.34 ± 0.043 | 5.32 ± 0.067 | 13.24 ± 0.183 | 11.54 ± 0.060 | 4.92 ± 0.024 | 0.03 ± 0.001 | 0.21 ± 0.004 | 5.44 ± 0.304 |
| p-value | <0.01 | 0.027 | 0.070 | 0.031 | 0.516 | 0.728 | 0.017 | 0.855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Guo, X.; La, Y.; Wu, X.; Chu, M.; Bao, P.; Yan, P.; Liang, C. Genetic Variants in the ATF6 Gene and Their Relationship with Milk-Quality Traits in Yaks. Animals 2025, 15, 2524. https://doi.org/10.3390/ani15172524
Ma X, Guo X, La Y, Wu X, Chu M, Bao P, Yan P, Liang C. Genetic Variants in the ATF6 Gene and Their Relationship with Milk-Quality Traits in Yaks. Animals. 2025; 15(17):2524. https://doi.org/10.3390/ani15172524
Chicago/Turabian StyleMa, Xiaoming, Xian Guo, Yongfu La, Xiaoyun Wu, Min Chu, Pengjia Bao, Ping Yan, and Chunnian Liang. 2025. "Genetic Variants in the ATF6 Gene and Their Relationship with Milk-Quality Traits in Yaks" Animals 15, no. 17: 2524. https://doi.org/10.3390/ani15172524
APA StyleMa, X., Guo, X., La, Y., Wu, X., Chu, M., Bao, P., Yan, P., & Liang, C. (2025). Genetic Variants in the ATF6 Gene and Their Relationship with Milk-Quality Traits in Yaks. Animals, 15(17), 2524. https://doi.org/10.3390/ani15172524





