Environmental Application of a Bacteriophage Cocktail Reduces Antibiotic-Resistant Escherichia coli in Poultry Litter Without Disrupting Gut Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteriophages
2.2. Bacteriophage Propagation and Cocktail Preparation
2.3. Experimental Design and Animal Housing
2.4. Treatment and Sampling Protocol
2.5. Cecal Content Analysis
2.6. Productive Performance
2.7. Statistical Analysis
3. Results
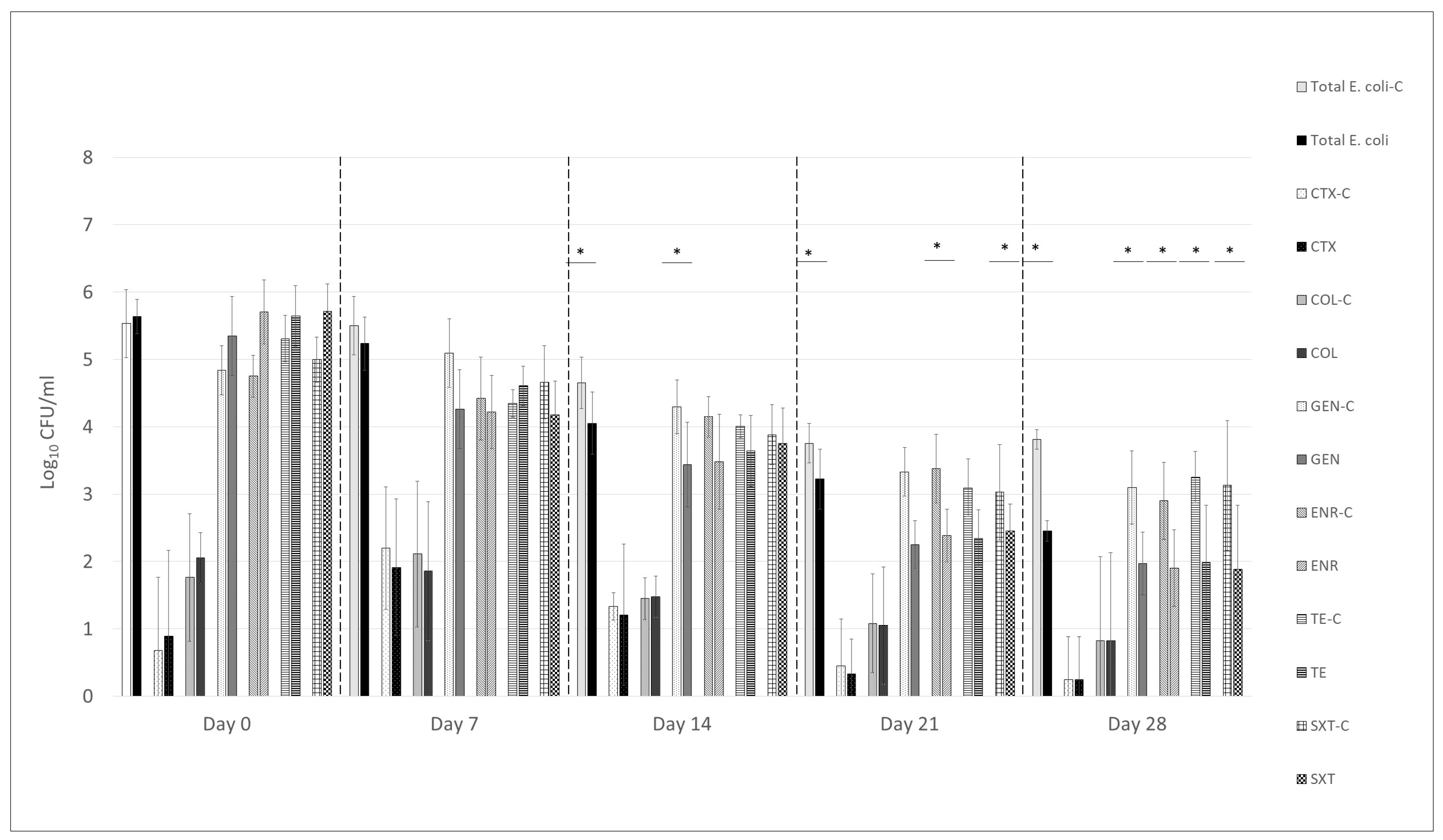
3.1. Effect of UPWr_E Phage Cocktail on Total and Antimicrobial-Resistant Escherichia coli in Litter
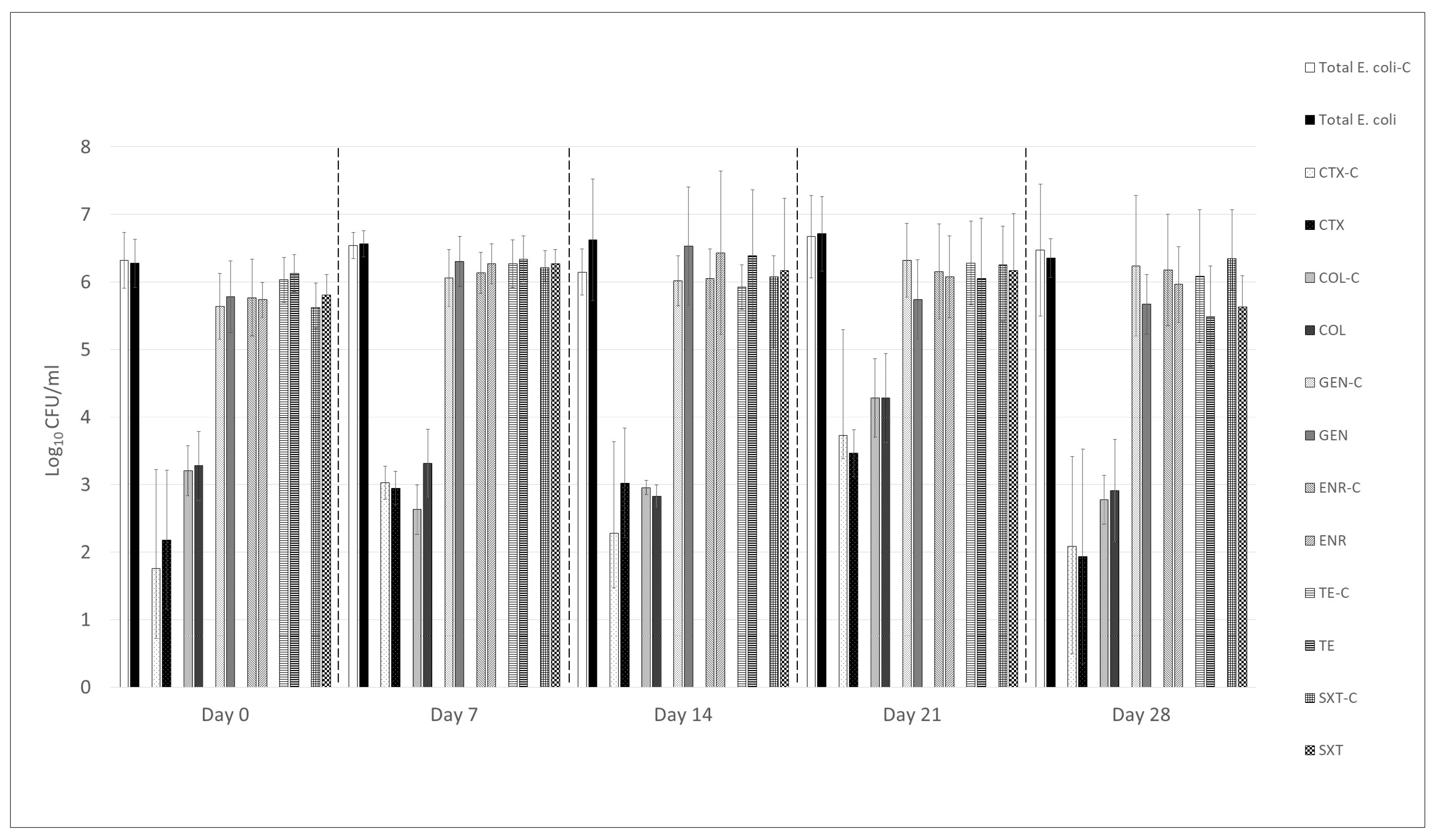
3.2. Effect of UPWr_E Phage Cocktail on Total and Antimicrobial-Resistant Escherichia coli in Feces
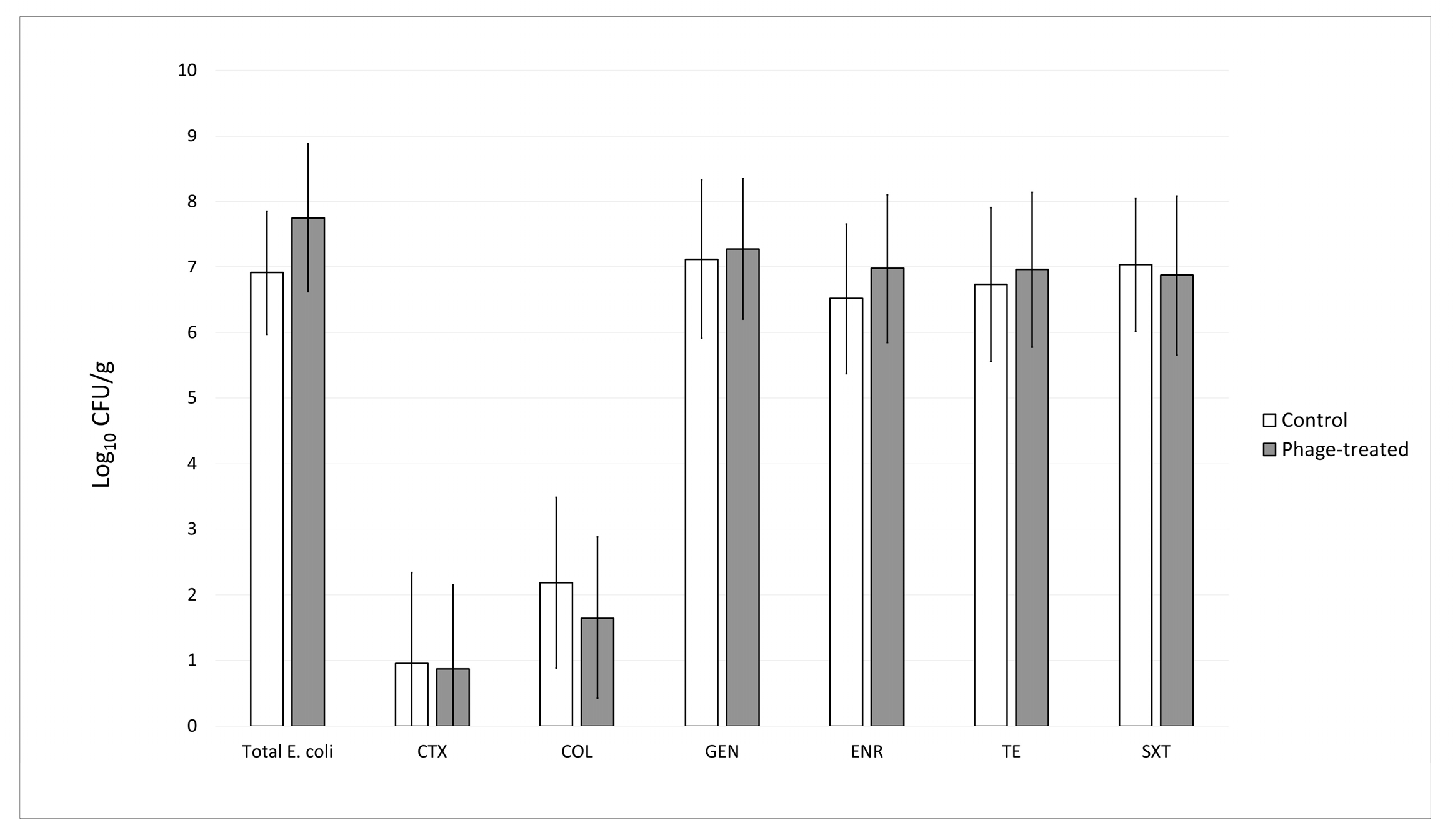
3.3. Evaluation of the Impact of the UPWr_E Bacteriophage Cocktail on Total and Antimicrobial-Resistant Escherichia coli in the Cecal Contents of Broiler Chickens
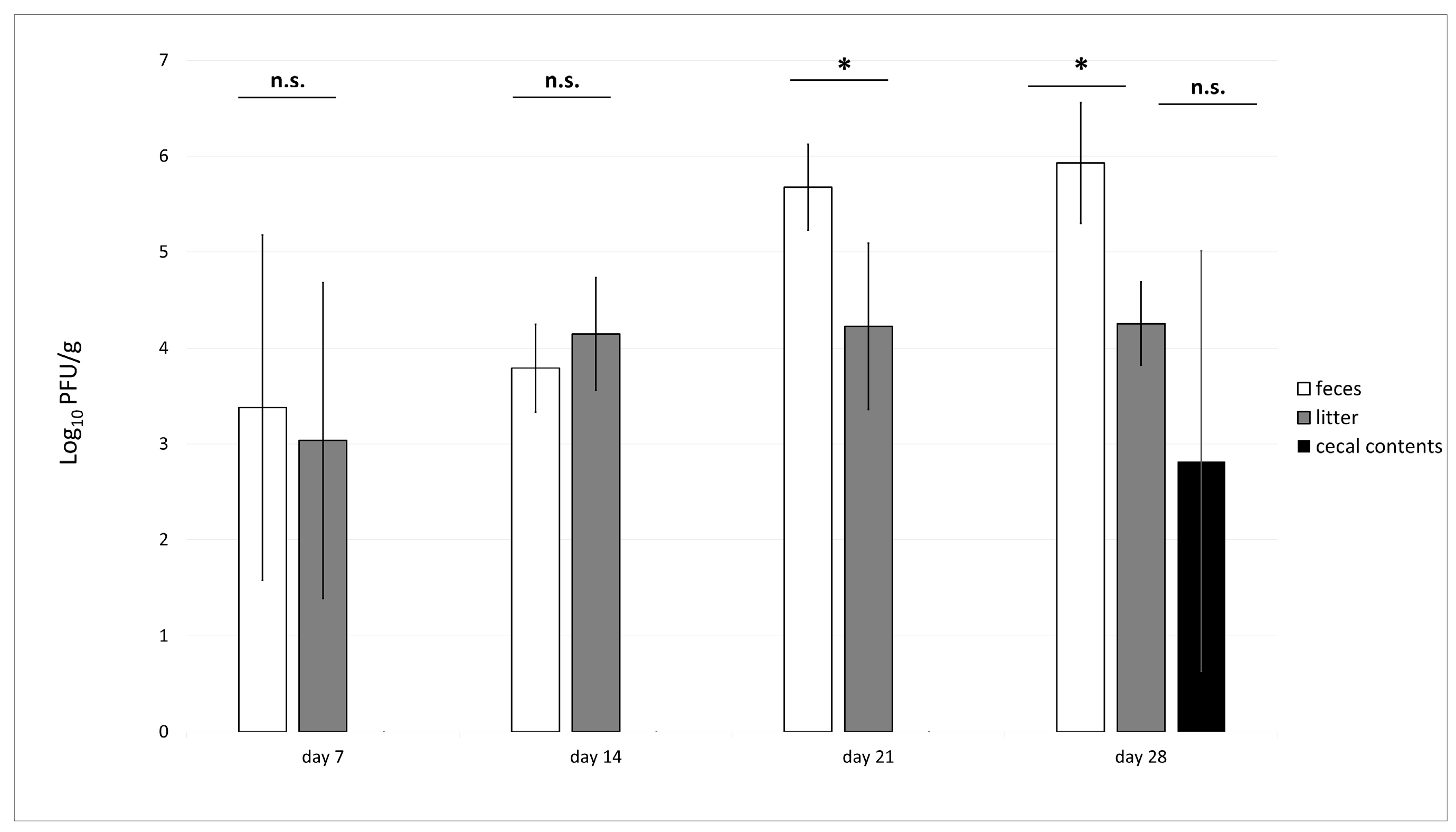
3.4. Phage Recovery from Litter, Feces and Cecal Samples
3.5. Growth Performance Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CFU | Colony forming unit |
| APEC | Avian pathogenic Escherichia coli |
| EFSA | European Food Safety Authority |
| OD | Optical density |
| PFU | Plaque forming units |
| PBS | Phosphate-buffer saline |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| CLSI | Clinical and Laboratory Standards Institute |
| FCR | Feed conversion ratio |
| MOI | Multiplicity of infection |
References
- Korczyński, M.; Kupczyński, R.; Świniarska, M.; Konkol, D.; Opaliński, S. Fortification of animal foodstuffs. In Food Biofortification Technologies; Saeid, A., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 275–313. [Google Scholar]
- OECD Agriculture Statistics. OECD iLibrary. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/data/oecd-agriculture-statistics_agr-data-en (accessed on 20 February 2025).
- Agunos, A.; Léger, D.; Carson, C. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can. Vet. J. 2012, 53, 1289–1300. [Google Scholar] [PubMed]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Herskin, M.; et al. Assessment of animal diseases caused by bacteria resistant to antimicrobials: Poultry. EFSA J. 2021, 19, e07114. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.; Veldman, K.; Wasyl, D.; Guerra, B.; et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019, 17, e05709. [Google Scholar] [PubMed]
- Dorado-García, A.; Mevius, D.J.; Jacobs, J.J.; Van Geijlswijk, I.M.; Mouton, J.W.; Wagenaar, J.A.; Heederik, D.J. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in the Netherlands. J. Antimicrob. Chemother. 2016, 71, 3607–3619. [Google Scholar] [CrossRef]
- Hesp, A.; Veldman, K.; van der Goot, J.; Mevius, D.; van Schaik, G. Monitoring antimicrobial resistance trends in commensal Escherichia coli from livestock, the Netherlands, 1998 to 2016. Euro Surveill. 2019, 24, 1800438. [Google Scholar] [CrossRef]
- Hesp, A.; van Schaik, G.; Wiegel, J.; Heuvelink, A.; Mevius, D.; Veldman, K. Antimicrobial resistance monitoring in commensal and clinical Escherichia coli from broiler chickens: Differences and similarities. Prev. Vet. Med. 2022, 204, 105663. [Google Scholar] [CrossRef]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Lundborg, C.S.; Lien, L.T.Q. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Gómez-Gómez, C.; Blanco-Picazo, P.; Brown-Jaque, M.; Quirós, P.; Rodríguez-Rubio, L.; Cerdà-Cuellar, M.; Muniesa, M. Infectious phage particles packaging antibiotic resistance genes found in meat products and chicken feces. Sci. Rep. 2019, 9, 13281. [Google Scholar] [CrossRef]
- Lopes, E.D.S.; Ferreira Santaren, K.C.; Araujo de Souza, L.C.; Parente, C.E.T.; Picão, R.C.; Jurelevicius, D.A.; Seldin, L. Cross-environmental cycling of antimicrobial resistance in agricultural areas fertilized with poultry litter: A one health approach. Environ. Pollut. 2024, 363, 125177. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.D.S.; de Souza, L.C.A.; Santaren, K.C.F.; Parente, C.E.T.; Seldin, L. Microbiome and resistome in poultry litter-fertilized and unfertilized agricultural soils. Antibiotics 2025, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.A.; Huff, W.E.; Huff, G.R.; Rath, N.C.; Zhou, Z.Y.; Donoghue, A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult. Sci. 2014, 93, 2788–2792. [Google Scholar] [CrossRef]
- Gamez, I.J.; Brashears, M.M.; Nightingale, K. Impact of VAM-S Bacteriophage Solution on the environmental microbiome in poultry litter systems from commercial operations. Poult. Sci. 2025, 105, 117. [Google Scholar]
- Śliwka, P.; Moreno, D.S.; Korzeniowski, P.; Milcarz, A.; Kuczkowski, M.; Kolenda, R.; Kozioł, S.; Narajczyk, M.; Roesler, U.; Tomaszewska-Hetman, L.; et al. Avian pathogenic Escherichia coli-targeting phages for biofilm biocontrol in the poultry industry. Vet. Microbiol. 2025, 301, 110363. [Google Scholar] [CrossRef]
- Moreno, D.S.; Kuczkowski, M.; Korzeniowski, M.; Grzymajło, K.; Woźniak-Biel, A.; Śliwka, P.; Rywińska, A.; Kuźmińska-Bajor, M. Application of UPWr_E124 phage cocktail for effective reduction of avian pathogenic Escherichia coli in mice and broiler chickens. Vet. Microbiol. 2025, 302, 110398. [Google Scholar] [CrossRef]
- Adams, M.H. Methods of study of bacterial viruses. In Bacteriophages; Adams, M.H., Ed.; Interscience: New York, NY, USA, 1959; pp. 443–522. [Google Scholar]
- EUCAST. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistance of Clinical and/or Epidemiological Importance, Version 2.01, European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2017.
- Oxendine, A.; Walsh, A.A.; Young, T.; Dixon, B.; Hoke, A.; Rogers, E.E.; Lee, M.D.; Maurer, J.J. Conditions necessary for the transfer of antimicrobial resistance in poultry litter. Antibiotics 2023, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- King, G.M.J.P.; Brooks, S.; Brown, C.; Gerba, G.A.; O’Connor, I.L. Land Application of Organic Residuals: Public Health Threat or Environmental Benefit; American Society for Microbiology: Washington, DC, USA, 2011. [Google Scholar]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Munro, F.; Moore, A. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 2005, 71, 1231–1236. [Google Scholar] [CrossRef]
- Pornsukarom, S.; Thakur, S. Horizontal dissemination of antimicrobial resistance determinants in multiple Salmonella serotypes following isolation from the commercial swine operation environment after manure application. Appl. Environ. Microbiol. 2017, 83, e01503-17. [Google Scholar] [CrossRef]
- Mourand, G.; Le Devendec, L.; Delannoy, S.; Fach, P.; Keita, A.; Amelot, M.; Jaunet, H.; Dia, M.E.H.; Kempf, I. Variations of the Escherichia coli population in the digestive tract of broilers. Avian Pathol. 2020, 49, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production systems and important antimicrobial resistant-pathogenic bacteria in poultry: A review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Bass, L.; Liebert, C.A.; Lee, M.D.; Summers, A.O.; White, D.G.; Thayer, S.G.; Maurer, J.J. Incidence and characterization of integrons, genetic elements mediating multiple-drug resistance, in avian Escherichia coli. Antimicrob. Agents Chemother. 1999, 43, 2925–2929. [Google Scholar] [CrossRef]
- Goldstein, C.; Lee, M.D.; Sanchez, S.; Hudson, C.; Phillips, B.; Register, B.; Grady, M.; Liebert, C.; Summers, A.; White, D.G.; et al. Incidence of Class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob. Agents Chemother. 2001, 45, 723–726. [Google Scholar] [CrossRef]
- Nandi, S.; Maurer, J.J.; Hofacre, C.; Summers, A.O. Gram-positive bacteria are a major reservoir of Class 1 antibiotic resistance integrons in poultry litter. Proc. Natl. Acad. Sci. USA 2004, 101, 7118–7122. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H. Phage products for fighting antimicrobial resistance. Microorganisms 2022, 10, 1324. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Kittler, S.; Mengden, R.; Korf, I.H.E.; Bierbrodt, A.; Wittmann, J.; Plötz, M.; Jung, A.; Lehnherr, T.; Rohde, C.; Lehnherr, H.; et al. Impact of bacteriophage-supplemented drinking water on the E. coli population in the chicken gut. Pathogens 2020, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Phage therapy: Various perspectives on how to improve the art. Methods Mol. Biol. 2018, 1734, 113–127. [Google Scholar]
- Hagens, S.; Loessner, M.J. Bacteriophage for biocontrol of foodborne pathogens: Calculations and considerations. Curr. Pharm. Biotechnol. 2010, 11, 58–68. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry gut health—Microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Control | Phage-Treated | SEM | p Value |
|---|---|---|---|---|
| Body weight (g) | 2490 | 2606 | 71.52 | 0.444 |
| Feed intake (g) | 3592 | 3720 | 75.06 | 0.421 |
| Feed conversion ratio | 1.445 | 1.431 | 0.012 | 0.586 |
| Mortality (%) | 8.333 | 4.170 | - | 0.103 |
| European Production Efficiency Factor | 377 | 417 | 15.12 | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźmińska-Bajor, M.; Kuczkowski, M.; Konkol, D.; Korczyński, M.; Rakicka-Pustułka, M.; Kozioł, S.; Tomaszewska-Hetman, L.; Rywińska, A. Environmental Application of a Bacteriophage Cocktail Reduces Antibiotic-Resistant Escherichia coli in Poultry Litter Without Disrupting Gut Microbiota. Animals 2025, 15, 2525. https://doi.org/10.3390/ani15172525
Kuźmińska-Bajor M, Kuczkowski M, Konkol D, Korczyński M, Rakicka-Pustułka M, Kozioł S, Tomaszewska-Hetman L, Rywińska A. Environmental Application of a Bacteriophage Cocktail Reduces Antibiotic-Resistant Escherichia coli in Poultry Litter Without Disrupting Gut Microbiota. Animals. 2025; 15(17):2525. https://doi.org/10.3390/ani15172525
Chicago/Turabian StyleKuźmińska-Bajor, Marta, Maciej Kuczkowski, Damian Konkol, Mariusz Korczyński, Magdalena Rakicka-Pustułka, Sylwia Kozioł, Ludwika Tomaszewska-Hetman, and Anita Rywińska. 2025. "Environmental Application of a Bacteriophage Cocktail Reduces Antibiotic-Resistant Escherichia coli in Poultry Litter Without Disrupting Gut Microbiota" Animals 15, no. 17: 2525. https://doi.org/10.3390/ani15172525
APA StyleKuźmińska-Bajor, M., Kuczkowski, M., Konkol, D., Korczyński, M., Rakicka-Pustułka, M., Kozioł, S., Tomaszewska-Hetman, L., & Rywińska, A. (2025). Environmental Application of a Bacteriophage Cocktail Reduces Antibiotic-Resistant Escherichia coli in Poultry Litter Without Disrupting Gut Microbiota. Animals, 15(17), 2525. https://doi.org/10.3390/ani15172525






