Simple Summary
Truncatelloidea are the richest in species group of fresh- and brackish water snails, still poorly studied. The Anatolia harbours many truncatelloid species, often endangered by human activity and climate warming, especially dangerous for these snails inhabiting springs and adapted to cold water. But these snails are still hardly known. Despite the close geographic relationship between Asia Minor and Europe, some Anatolian groups have no closer relationships with the European ones; rather, relationships with the Asiatic fauna must be considered. We have studied one of such groups, finding six species new to science, and four of them representing two new genera; we describe these snails. Minute dimensions, resulting in simplified anatomy, as well as wide variation in the morphology, cause serious problems with the reconstruction of evolutionary (phylogenetic) relationships between these snails, and even with species distinction. Thus, the application of molecular data—DNA sequences—has become obligatory to solve this problem. However, sometimes the molecular data, and especially the results of their analysis, are not congruent with the morphological ones, and this is the case we are describing. This once more stresses the necessity of an integrative, holistic approach in the study of animal evolution.
Abstract
Previous studies identified a clade of the Hydrobiidae endemic to Anatolia, with no closer relationships with the European snails, despite close relations between the fauna of these two regions. Three genera—each monotypic: Sheitanok Schütt et Şeşen, 1991, Anadoludamnicola Şahin, Koca et Yıldırım, 2012, and Kozanium Odabaşı et Falniowski, 2025—belonged to this clade. Our study describes the Hydrobiidae collected at five springs in Anatolia. Shell morphology and biometry, radulae, soft parts pigmentation, female reproductive organs, and penes are described and illustrated. Cytochrome oxidase subunit I (COI) sequences have been used to infer phylogenetic relationships and species distinctness of these snails. An integrative taxonomic approach resulted in the distinction of six species new to science. One of them belonged to the genus Sheitanok, another one to Anadoludamnicola. The other four species are clustered into two genera not described thus far, and the offered names are Dicle and Adaniya. It is noteworthy that three species recognized within one of these genera were clearly demarcated both molecularly and morphologically (morphological differences were higher than typical of the hydrobiid congeners). However, two of the three techniques of species delimitation based on the molecular sequences did not confirm their distinctness. This stresses the necessity of a holistic approach in the truncatelloid taxonomy.
1. Introduction
The freshwater truncatelloid fauna of Turkey (Türkiye) has been studied by some researchers [1,2,3,4,5,6,7]. Nonetheless, our current knowledge of the hydrobiid fauna inhabiting Türkiye is notably limited in comparison with what is known about it in other Mediterranean countries [8]. Türkiye encompasses three of the biogeographical regions of the Palaearctic [9]—Anatolian, Mediterranean, and Black Sea—resulting in a rich biodiversity. Considering the minute size of the hydrobiid snails, which are easily overlooked and rather hard to study, their contribution to this biodiversity should still be significantly underestimated.
To date, more than 70 hydrobiid taxa have been reported from Türkiye, the majority of which have a pattern of divergence from the west towards the southeast [5,10,11,12]. Many hydrobiid genera known to occur in Türkiye have a circum-Mediterranean distribution, such as Pseudamnicola Paulucci, 1878, and Grossuana Radoman, 1983, which probably reflects the Messinian Salinity Crisis. Others, however, are endemic to Anatolia (i.e., Turcorientalia Radoman, 1973; Erosiconcha Delicado et Gürlek, 2021; Cariohydrobia Delicado et Gürlek, 2021; Anadoludamnicola Şahin, Koca et Yıldırım, 2012) [5,8], being possibly later immigrants from Asia.
Odabaşı et al. [13] described a new monotypic genus, Kozanium Odabaşı et Falniowski, 2025, with its type species, Kozanium torosum Odabaşı et Falniowski, 2025, from Dağılcak Spring, Dağılcak Nature Park, Kozan district of Adana province. Kozanium molecularly clustered within the entirely Anatolian clade, with Sheitanok Schütt et Şeşen, 1991, type species S. amidicus Schütt et Şeşen, 1991 (represented by Sheitanok sp. in their molecular phylogeny: sequence of Delicado et al. [14]), and Anadoludamnicola Şahin, Koca et Yıldırım, 2012, represented by the type species A. gloeri Şahin, Koca et Yıldırım, 2012. Recently, we have collected truncatelloidean gastropods at five new localities in SE Anatolia. They represent six taxa new to science, all of them clustered within this entirely Anatolian clade. The study describes these new taxa.
Our study should extend the knowledge about the Turkish microsnail fauna, their diversity and habitats, stressing the urgent need for its conservation.
2. Materials and Methods
The snails have been collected by sieve at five sampling locations, which are springs or spring-fed streams (Table 1, Figure 1 and Figure 2) in the south and southeast of Türkiye.

Table 1.
Sampling locations of snails used in this study.

Figure 1.
Sampling locations used in this study (red dots: 1–5, see Table 1). Some reference locations (black dots) are also shown: A–type locality of Sheitanok amidicus [15]; B,C–other localities of Sheitanok amidicus [15]; D–Sheitanok sp. [14]; E–type locality of Anadoludamnicola gloeri [16]; F–Anadoludamnicola gloeri [14].

Figure 2.
Sampled localities: (A,B)—Spring in the Finik Castle Ruins (locality 4 and 5, respectively); (C)—Sarız Çayı (locality 1); (D)—Küçükçamurlu Spring (locality 2); (E)—Küp Şelalesi waterfall, and the stream (F)—Spring flowing into the Doğan Çayı near the Küp Şelalesi waterfall (locality 3).
Locality 1 is situated in the upper region of the Seyhan River basin. The sampling point is a wide stream called the Sarız Çayı near Yamanlı Village in the Tufanbeyli District of the Adana Province (Figure 2C). A thermal power station is located very close to the sampling point. The stream is wide but shallow and fast-flowing, and the water is rather turbid. The sampling location’s bank has sparse vegetation and has been heightened using natural riverbed soil. Locality 2 (Figure 2D) is characterized by its small stream, which is fast-flowing, clear, and cold. It is located at a high elevation in the mountainous region and flows through the village of Küçükçamurlu in the Göksun district of the Kahramanmaraş province. The riverbed is composed of stones and pebbles, with a small amount of macrophytes. The presence of small springs along the riverbed is noteworthy, and these can be seen as they line the river, creating a unique natural landscape. Locality 3 (Figure 2E,F), the Küp Şelalesi waterfall flows into the Doğan Çayı stream near Küp village in the Aladağ district of the Adana province. The material was collected from a large spring situated on the elevated western bank of the stream. This spring then discharges into the stream. Locality 4 (Figure 2A,B) and 5, Finik Castle Ruins, located in Güçlükonak town of Şırnak province, along the coast of the Dicle River. It is known that the castle was constructed in the Asur times by natural limestone (http://www.guclukonak.gov.tr/finik-surlari, accessed on 2 June 2025). The springs in the castle flow into the river, which has sparse vegetation along its banks. Dicle bilgini and Sheitanok esmaae have been sampled in the same spring.
Samples were collected by hand using tweezers from the macrolithic habitat at the sampling points (Table 1, Figure 2). For the molecular study, snails were washed twice in 80% ethanol and left to stand in this solution for ca. 12 h, after which the solution was changed twice in 24 h. Finally, after a few days, the 80% solution was exchanged for a 96% solution of ethanol, and the material was stored at −20 °C. The shells were photographed with a Canon EOS 50D digital camera (Ōita, Japan), under a Nikon SMZ18 microscope (Tokyo, Japan) with a dark field. The dissections were conducted under a Nikon SMZ18 microscope with a dark field, equipped with a Nikon DS-5 digital camera, whose captured images were used to draw anatomical structures with a graphic tablet. Morphometric parameters of the shell were measured by one person using a Nikon DS-5 digital camera (Tokyo, Japan) and ImageJ v. 1.53g image analysis software [17]. Seven biometric parameters of the shell were measured (Figure 3), following the scheme of Falniowski et al. [18]. The type materials are deposited at the Limnology Museum of Çanakkale Onsekiz Mart University (COMULM), Çanakkale, Türkiye.

Figure 3.
Diagram showing the measurement of the shell. Measured variables: a—shell height; b—body whorl width; c—aperture height; d—spire height; e—aperture width; α—apex angle measured between the lines tangential to the spire; β—angle between the body whorl suture and the line perpendicular to the columella.
Statistical multivariate analysis of biometrical variables and molecular Operational Taxonomic Units (mOTU) p-distances was conducted in Canoco 5.15 [19,20]. The p-distances between the mOTUs were subject to a Principal Coordinates Analysis (PCoA) using all axes with positive eigenvalues. The biometrical data table was analyzed using PCA (Principal Components Analysis), and a direct ordination method was performed, namely RDA (Redundancy Analysis), with the species as the explanatory variable. Response data (biometrical descriptors) were log-transformed by the relation Y′ = log(1 + Y) and centered by variables. Significance was tested with the Monte Carlo permutation test, using 999 unrestricted permutations and the level of alpha = 0.05. Polygon envelopes have illustrated the grouping of individuals, with each species being ascribed a different color. Simple and conditional term effects have tested the capacity of species to explain differences in biometrical values. To avoid errors in multiple testing, we also used adjusted p-values based on the false discovery rate. In the diagrams, the species are coded by the first three letters of the genus, followed by the first three letters of the species. Each code also has a mOTU ascribed, as follows: Dicbil = Dicle bilgini (mOTU A), Adatuf = Adaniya tufanbeyi (mOTU B), Adaozb = Adaniya ozbeki (mOTU C), Adacun = Adaniya cuneytsolaki (mOTU D), Sheesm = Sheitanok esmaae (mOTU E), Anaayh = Anadoludamnicola ayhani (mOTU H), Koztor = Kozanium torosum (mOTU I). There are some mOTU without biometry or species’ names coded as follows: Anadoludamnicola gloeri = mOTU G and code Anaglo (found in site F), and Sheitanok sp. = mOTU F and code Shesp (found in site D).
The shells and opercula were cleaned with an ultrasonic cleaner. The penes were photographed under a Motic microscope (Xiamen, China) with a dark field. The radulae were extracted with Clorox™ (Oakland, California, US), applying the techniques described by Falniowski [21], and examined and photographed using a HITACHI S-4700 scanning electron microscope (Tokyo, Japan).
DNA was extracted from whole specimens; tissues were hydrated in tris-EDTA (TE) buffer (3 × 10 min); then, total genomic DNA was extracted with the Sherlock extraction kit (A&A Biotechnology, Gdańsk, Poland), and the final product was dissolved in 20 μL of TE buffer. The extracted DNA was stored at −80 °C at the Department of Malacology, Institute of Zoology and Biomedical Research, Jagiellonian University in Kraków (Poland). Mitochondrial cytochrome oxidase subunit I (COI) was sequenced. Details of PCR conditions, primers used, and sequencing followed Szarowska et al. [22]. In the phylogenetic analysis, sequences from GenBank were used (Table 2). For analysis, we selected the sequences of snails most closely related to the newly described species, since the analysis of the biodiversity of Anatolia was not our aim. The sequences and our preliminary phylogenetic analyses were selected based on the Barcode of Life Data System [23]. All sequences were initially aligned in MUSCLE [24] implemented in MEGA 7 [25] and then checked in Bioedit 7.1.3.0 [26]. Genetic uncorrected p-distances were calculated in MEGA 7, with transitions and transversions included and uniform rates among sites. The estimation of the proportion of invariant sites and the saturation tests for entire datasets [27,28] were performed with DAMBE [29]. The data were analysed using approaches based on Bayesian inference (BI) and maximum likelihood (ML). For RAxML analysis, the jModelTest2 via the CIPRES Science Gateway [30] was used to find the best-fitting model; the model TrN+I+G was chosen. The ML analysis was conducted in RAxML-NG v. 0.8.0 [31] via the web service available at https://raxml-ng.vital-it.ch/, (accessed on 17 June 2025) with ten random and ten parsimony-started trees. In the BI analysis, the TrN+I+G model of nucleotide substitution was also applied. The analyses were run using MrBayes v.3.2.7a [32] with default priors. Two simultaneous analyses were performed, each with 10,000,000 generations, with one cold chain and three heated chains, starting from random trees and sampling the trees every 1000 generations. The first 25% of the trees were discarded as burn-in. The analyses were summarized as a 50% majority-rule consensus tree. Convergence was checked in Tracer v.1.7.1 [33]; in all cases, the Effective Sample Size exceeded 200. FigTree v.1.4.4 [34] was used to visualise the trees. Three species delimitation methods were performed: Poisson Tree Processes (PTP) [35], Automatic Barcode Gap Discovery (ABGD) [36], and Assemble Species by Automatic Partitioning (ASAP) [37]. The PTP approach was run using the web server (https://species.h-its.org/ptp/), accessed on 17 June 2025, with 100,000 MCMC generations, 100 thinning, and 0.1 burn-in. We used the RAXML output phylogenetic tree. The ABGD approach used the web server (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html, accessed on 17 June 2025) with the default parameters. The ASAP approach was run using the web server (https://bioinfo.mnhn.fr/abi/public/asap/, accessed on 17 June 2025), according to simple distance (p-distances). The Fastachar application [38] and DeSignate [39] were used to determine the Molecular Diagnositic Characters (MDCs) for COI. Two types of characters were accepted: at binary positions (where the character state in the query group is different from the uniform character state of the reference group) and at asymmetric positions (where the character state in the query group is not different from the uniform character state of the reference group). Molecular diagnoses for the genus were verified against all genera within a large clade (mOTU A–mOTU I). For individual species, molecular diagnoses were determined between species within a given genus. The letters used in the MDCs list denote the nucleotide position in our sequence set, and the letter in parentheses denotes the specific nucleotide characteristic for the particular group.

Table 2.
COI sequences used for phylogenetic inference.
3. Results
3.1. Molecular Part
We obtained 12 new sequences of COI (467 bp, GenBank accession numbers PX127888-PX127899). The test for the substitution saturation analysis showed an ISS (0.75) significantly smaller than the critical ISS value (ISSC: 0.94), indicating that sequences are not saturated and thus useful in phylogenetic reconstruction. In all analyses, the topologies of the resulting phylograms were identical in both the ML and BI phylogram analyses; thus, we present the phylogram computed with RAxML.
In our molecular tree (Figure 4), all the collected gastropod taxa clustered within the well-supported (bootstrap support 90%, BP 0.94) clade, which consisted entirely of Anatolian taxa. Within this clade, there were two subclades (supports either 72 or 74%): one of them included Sheitanok Schütt et Şeşen, 1991, with Sheitanok sp. [14], Anadoludamnicola Şahin, Koca et Yıldırım, 2012, and Kozanium Odabaşı et FalniowskiOTU, 2025. One of our sequences clusters as a sister taxon with Sheitanok sp., another one with Anadoludamnicola gloeri Şahin, Koca et Yıldırım, 2012. All three techniques of species delimitation confirmed the distinctness of our two taxa. The other subclade consisted only of our new sequences, grouped in two clusters of presumably genus level, whose distinctness was confirmed by all three techniques. In the second of them, including the sequences from three localities, both ASAP and ABGD species delimitation techniques resulted in no species-level delimitation. On the other hand, bPTP delimited three species. The p-distances are given in Table 3.
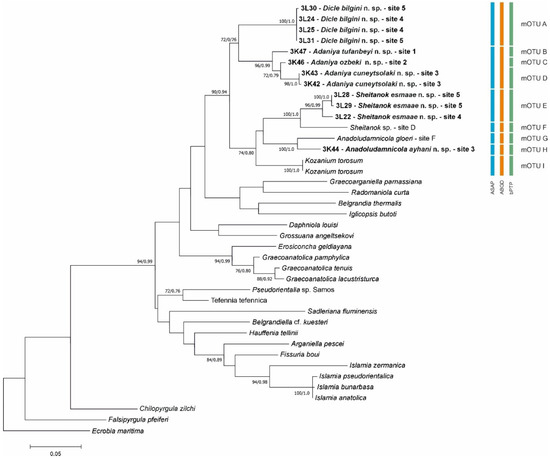
Figure 4.
Maximum likelihood tree based on COI sequences. Bootstrap and BP for the nodes are given. Results of species delimitation are also shown. Bold indicates new sequences.

Table 3.
Uncorrected p-distances between mOTUs shown in Figure 4. Numbers in italics and bold indicate distances within mOTU. mOTUs means molecular Operational Taxonomic Units.
3.2. Shell Biometry

Table 4.
Shell measurements in mm. Measured variables: a—shell height; b—body whorl width; c—aperture height; d—spire height; e—aperture width; α—apex angle measured between the lines tangential to the spire; β—angle between the body whorl suture and the line perpendicular to the columella.
The PCoA diagram on mOTU is depicted in Figure 5. Considering proximity as an indicator of similarity, and in this case, reduced p-distance, we can distinguish broadly four groups: closely placed G and H (Anaglo and Anaayh), then in the lower left quarter, a closely related D and C (Adaozb and Adacun), followed by B (Adatuf), and further distanced from A (Dicbil). E and F share the same space in the lower right quarter (Shesp. and Sheesm), while I (Koztar) is placed isolated from all the others. The PCA of individual biometric variables classified by species is depicted in Figure 6. Most species have distinguished positions based on biometrical variables of their individuals, while some slight overlap (only at the edges of their variability) is also registered. Sheiesm, Adaozb, and Adatuf do not overlap at all, whereas the others are more or less slightly closer and partially overlap. If the species is taken as a predictor, the corresponding RDA is shown in Figure 7 (relations between biometrical variables constrained by the species in an RDA ordination diagram) and Figure 8 (classified RDA individuals diagram based on relations between biometrical descriptors predicted by the species). The RDA shows a highly significant and strong relation between the species and biometrical descriptors (test on all axes pseudo-F = 35.9, p = 0.001, adjusted explained variation is 80.72%). The biometrical variable alpha is maximized in Sheesm, followed by Anaayh, while the rest of the morphological descriptors are positively correlated and maximized in Anatuf. All species are well dispersed across the ordination space, hinting towards a dissimilarity and certain contrasts in terms of biometrical variables between the taxa. The relations can be traced finely in Figure 8.

Figure 5.
Principal Coordinate Analysis (PCoA) on the genetic p-distances between the mOTUs.

Figure 6.
Principal Component Analysis (PCA) of individuals’ biometrical variables classified by species.

Figure 7.
The relation between biometrical variables constrained by the species in a Redundancy Analysis (RDA) ordination diagram.

Figure 8.
Classified RDA diagram of individuals based on relations between biometrical descriptors predicted by the species.
We have also tested the effects of species on biometrical variability in individuals. The results are shown in Table 5.

Table 5.
The effects of species on biometrical variability in individuals.
The simple term effects (as if each species acts alone) show significant values for Adatuf, Sheesm, Adaozb, and Anaayh, while the last three species have an insignificant effect (these being also those which show a certain overlap in Figure 8). In sharp contrast, if conditional term effects are considered (the effect of each predictor after accounting for the effect of the formerly selected predictor(s)), all predictors prove to be significant. There are mostly significant differences in biometrical variability between species, but a certain overlap is also noticed at least between some taxa. The limitation is due to the reduced number of individuals analyzed in this study.
3.3. Systematic Part
Truncatelloidea Gray, 1840
Hydrobiidae W. Stimpson, 1865
Horatiinae D.W. Taylor, 1966
Genus DicleFalniowski and Odabaşı, n. gen.
urn:lsid:zoobank.org:act:9CE9C090-DBBD-44CE-922E-896ABC007376
Type species Dicle bilgini Falniowski and Odabaşı, n. sp. by monophyly.
Diagnosis
Shell ovate-conic, broad, with spire moderately high, whorls slightly convex, body whorl massive, aperture broadly ovoid, parietal lip broad and complete, umbilicus moderately broad; female reproductive organs with one, distal receptaculum seminis and cylindrical rather bulky bursa copulatrix; penis simple, gradually tapering or broadly triangular, with a small outgrowth on its left side, its termination blunt with small papilla, vas deferens visible inside. Binary MDCs: 54(G), 234(C), 294(C), 376(C), 378(T); asymmetric MDCs: 9(A), 21(A), 195(C), 198(G), 225(C), 273(A), 429(G), 444(T), 462(A).
Etymology
The genus is named after the Dicle River, also known as the Tigris, which, together with the Euphrates, forms the Upper Mesopotamia region in southeastern Türkiye.
Dicle bilgini Falniowski and Odabaşı, n. sp.
urn:lsid:zoobank.org:act:86D8F90A-2B55-40CA-B556-68041CA04ACC
GenBank no. for COI: PX127896-PX127899
Type locality: Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, Türkiye (locality 4, Figure 2A,B).
Holotype: (Figure 9A) Ethanol-fixed specimen, Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, Türkiye (locality 4), leg. İhsan Ekin, 06.04.2025. Voucher number COMULM-G 302.

Figure 9.
Shells of the studied taxa. (A–E)—Dicle bilgini n. sp.: (A)—holotype, (B)—3L30, (C)—3L24, (D)—3L25, (E)—3L31; (F,G)—Adaniya tufanbeyi n. sp.: (F)–holotype, (G)—3K47; (H,I)—Adaniya ozbeki n. sp.: (H)—holotype, (I)—3K46; (J–L)—Adaniya cuneytsolaki n. sp.: (J)—holotype, (K)—3K42, (L)—3K43; (M–R)—Sheitanok esmaae n. sp.: (M–O)—holotype, (P)—3L28, (Q)—3L29, (R)—3L22; (S,T)—Anadoludamnicola ayhani n. sp.: (S)—holotype, (T)—3K44. Scale bar: 1 mm.
Paratypes: 73 ethanol-fixed specimens, Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak-Türkiye, Voucher number COMULM-G 303.
Diagnosis As for the genus
Description
Shell (Figure 9A,E)
Up to 1.87 mm high, ovate-conic, broad, with a moderately high spire, about four whorls, and a spire height of 22–24% of the shell height. Teleoconch whorls slightly convex, growing regularly in diameter, suture shallow but well-marked, body whorl massive. Aperture broadly ovoid, outer lip simple, parietal lip broad and complete, umbilicus moderately broad. Teleoconch moderately thick-walled, its wall translucent, with delicate growth lines, periostracum whitish or yellowish. Shell measurements: Table 4. Operculum paucispiral, oval, smooth on both surfaces.
Radula (Figure 10H). The central tooth has short and blunt cusps according to the following formula:
(5)4–1–4(5)
1–1

Figure 10.
Radulae of the studied taxa. (A,B)—Anadoludamnicola ayhani n. sp. (locality 3); (C,D)—Adaniya ozbeki n. sp (locality 2); (E)—Sheitanok esmaae n. sp. (locality 4); (F)—Adaniya tufanbeyi n. sp. (locality 1); (G)—Adaniya cuneytsolaki n. sp. (locality 3); (H)—Dicle bilgini n. sp. (locality 4). Scale bar: (A,B,E)—20 μm; (G)—30 μm; (H)—40 μm, (C,D,F)—50 μm.
The median cusp about three times longer than the adjacent ones, a deep sinus at the frontal side of the cutting edge, and the basal tongue is narrowly V-shaped. The lateral tooth with 2–1–2(3) blunt cusps; the biggest one is slightly longer than the adjacent ones, massive, and with a rounded tip. The inner marginal tooth with 22–24 long and sharp cusps, and the outer marginal one with about 18 sharp cusps.
Morphology and anatomy of soft parts
The head, tentacles, and mantle are intensely pigmented. There is no ctenidium. The female reproductive organs with one distal receptaculum seminis and a cylindrical, bulky bursa copulatrix. The penis (Figure 11A–F) simple, gradually tapering or broadly triangular, with a small outgrowth on its left side; its termination blunt with a small papilla, and the vas deferens visible inside.

Figure 11.
Penes of the studied taxa. (A–F)—Dicle bilgini n. sp. (locality 4); (G–J)—Adaniya tufanbeyi n. sp. (locality 1); (K–N)—Adaniya ozbeki n. sp. (locality 2). Scale bar: 200 μm.
Etymology
The specific epithet bilgini refers to Fikret Bílgín, estimable malacologist deeply devoted to the study of the Turkish molluscs.
Known distribution
Also in the Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak-Türkiye (locality 5), about 500 m from the type locality.
Differential diagnosis
The radula in Dicle bilgini with a central tooth with fewer cusps on the cutting edge and less prominent basal cusps than in Kozanium, as well as in Adaniya n. gen. Unlike in Sheitanok, the shell is not valvatoid. Anadaluamnicola differs in the lack of basal cusps. In the female reproductive organs of Dicle bilgini, the bursa copulatrix is cylindrical, unlike in the other genera within this clade. The penis, unlike Anudaluamnicola, is never strap-like, and unlike Sheitanok, without a big terminal papilla, is covered with a thin layer of chitin. In Adaniya, there is either no/vestigial or big and broad outgrowth on its left side.
Genus Adaniya Odabaşı and Falniowski gen. nov.
urn:lsid:zoobank.org:act:0C9EFD28-94E2-4BD2-A689-1D726C953567
Type species Adaniya tufanbeyi Jaszczyńska and Odabaşı n. sp.
Diagnosis
Shell ovate-conic or trochiform, with spire low or moderately low, body whorl massive, aperture broadly ovoid, parietal lip broad and complete, umbilicus moderately broad; or broad; female reproductive organs with one, distal receptaculum seminis and moderately big or small bursa copulatrix; penis broadly triangular, simple or with a lobe on its left side. Binary MDCs: 288(C), 345(C), 351(T), 363(G), 423(G); asymmetric MDCs: 90(A).
Etymology
This genus is named after Adaniya, the name by which the region was known during the time of the Hittite civilization in Anatolia.
Differential diagnosis
Unlike in Sheitanok, the shell is not valvatoid. Unlike Anudaloamnicola, there are basal cusps on the central tooth in the radula. The penis, unlike Anudaluamnicola, is never strap-like, and unlike Sheitanok, it lacks a big terminal papilla covered with a thin layer of chitin. Bursa copulatrix and especially receptaculum seminis are smaller than those in Kozanium.
Adaniya tufanbeyi Jaszczyńska and Odabaşı n. sp.
urn:lsid:zoobank.org:act:0774F308-2E86-4539-B719-8A91E51AC1A0
GenBank no. for COI: PX127892
Type locality: Sarız Çayı, Near Yamanlı Village, Tufanbeyli District, Adana Province, Türkiye (locality 1, Figure 2C)
Holotype: (Figure 9F) Ethanol-fixed specimen, Sarız Çayı, Near Yamanlı Village, Tufanbeyli District, Adana Province, Türkiye, leg. Deniz Anıl Odabaşı, 19.10.2024. Voucher number COMULM-G 304.
Paratypes: 29 ethanol-fixed specimens, Sarız Çayı, Near Yamanlı Village, Tufanbeyli District, Adana Province, collection of the COMULM (Voucher number COMULM-G 305), Türkiye.
Diagnosis
A representative of Adaniya with an ovate-conical, broad shell; a central tooth with a massive cutting edge and about ten minute cusps; two pairs of basal cusps; a bursa copulatoria whose duct is not sharply demarcated from the bursa; and a penis broadly triangular, with or without a very small outgrowth on its left side. Binary MDCs: 21(T), 84(T), 88(T), 99(G), 117(T), 177(T), 255(G), 258(A), 300(A), 402(A).
Description
Shell (Figure 9F,G)
Up to 3.12 mm high, ovate-conic, broad, with a moderately high spire, about four whorls, and a spire height of 26–28% of the shell height. Teleoconch whorls slightly convex, growing regularly in diameter, suture shallow but well-marked, body whorl massive. Aperture broadly ovoid, near circular in shape, outer lip simple, parietal lip narrow and complete, umbilicus slit-like. Teleoconch rather thick-walled, growth lines nearly invisible, periostracum yellowish. Shell measurements: Table 4. Operculum paucispiral, oval, smooth on both surfaces.
Radula (Figure 10F) The central tooth has short and blunt cusps according to the following formula:
(10)9–1–9(10)
2–2
The median cusp broad and massive, about three times longer than the small, sharp adjacent cusps. A deep sinus present on the front side of the cutting edge, and the basal tongue narrowly V-shaped. The lateral tooth with 6–1–5 cusps; the largest cusp twice as long as the adjacent cusps and massive with a rounded tip. The inner marginal tooth with 34–36 long, sharp cusps; the outer marginal tooth with about 22 sharp cusps.
Morphology and anatomy of soft parts
The head and mantle intensely pigmented black. The female reproductive organs (Figure 12A) with short, broad pallial glands; a narrow loop of the oviduct; a large distal receptaculum seminis with a short duct; and a small bursa copulatrix gradually narrowing into a long duct. The penis (Figure 11G–J) broadly triangular and simple. sometimes with a very small outgrowth on its left side. Its distal end blunt with a papilla and a visible vas deferens.

Figure 12.
Female reproductive organs. (A)—Adaniya tufanbeyi n. sp. (locality 1); (B)—Adaniya ozbeki n. sp. (locality 2); (C)—Adaniya cuneytsolaki n. sp. (locality 3); (D)—Anadoludamnicola ayhani n. sp. (locality 3). Abbreviations: bc—bursa copulatrix; cbc—duct of bursa copulatrix; ga—albuminoid gland; gn—nidamental gland; gp—gonoporus; ov—oviduct; ovl—loop of (renal) oviduct; rs—receptaculum seminis. Scale bar: 1 mm.
Etymology
The specific epithet tufanbeyi refers to Osman Tufan Bey, who is renowned for his invaluable service to the Cilician region (today the Adana and Osmaniye provinces) during the Turkish struggle for independence. He displayed great courage and bravery in the face of adversity.
Known distribution: Type locality only.
Differential diagnosis
Shell much bigger than in A. cuneytsolaki and with a higher spire than in A. ozbeki. The dens centralis in the radula, unlike A. cuneytsolaki, with a massive cutting edge similar to that in A. ozbeki, but with fewer minute cusps and one, not two pairs of basal cusps. The bursa copulatrix sac-shaped without a sharply demarcated duct, unlike the other two species. The penis similar to that in A. cuneytsolaki but different from that in A. ozbeki in lacking a big triangular nonglandular lobe on its left side.
Adaniya ozbekiHofmanandOdabaşı n. sp.
urn:lsid:zoobank.org:act:91BCA77D-0B27-40E6-AD60-7945B26CF166
GenBank no. for COI: PX127891
Type locality: Küçükçamurlu Spring, Göksun District, Kahramanmaraş Province, Türkiye (locality 2, Figure 2D).
Holotype: (Figure 9H) Ethanol-fixed specimen, Küçükçamurlu Spring, Göksun District, Kahramanmaraş Province, Türkiye (locality 2). leg. Deniz Anıl Odabaşı, 19.10.2024. Voucher number COMULM-G 306
Paratypes: 19 ethanol-fixed specimens, Küçükçamurlu Spring, Göksun District, Kahramanmaraş Province, collection of the COMULM (Voucher number COMULM-G 307), Türkiye.
Diagnosis
A representative of Adaniya with a trochiform and broad shell, a central tooth with a massive cutting edge, and about six minute cusps, and one pair of basal cusps. The bursa copulatrix big and oval with a short, broad duct. The penis with a wide, triangular, nonglandular outgrowth on the medial section of the left. Binary MDCs: 144(A), 147(G), 150(G).
Description
Shell (Figure 9H,I)
Up to 2.44 mm high, trochiform, and very broad. It has a low spire with about four whorls, and the spire is 12–17% of the shell’s height. The teleoconch whorls slightly convex and growing abruptly in diameter. The suture shallow and slightly marked. The body whorl very massive. Aperture broadly ovoid, near circular in shape, outer lip simple, parietal lip broad and complete, umbilicus rather broad. Teleoconch rather thick-walled, growth lines nearly invisible, periostracum yellowish. Shell measurements: Table 4. Operculum paucispiral, oval, smooth on both surfaces.
Radula (Figure 10C,D) The central tooth has short and blunt cusps according to the following formula:
(6)5–1–5(6)
1–1
The median cusp broad and massive, about four times longer than the small, blunt adjacent ones. A deep sinus present on the front side of the cutting edge, and the basal tongue narrowly V-shaped. The lateral tooth with 6–1–5 cusps; the largest cusp twice as long as the adjacent cusps and is massive with a rounded tip. The inner marginal tooth with 32–34 long, sharp cusps; the outer marginal tooth has about 23 sharp cusps.
Morphology and anatomy of soft parts
The head and mantle intensely pigmented black. The female reproductive organs (Figure 12B) short pallial glands, a large oval bursa copulatrix with a short, broad duct, a large, spherical distal receptaculum seminis, and a long, narrow loop of the renal oviduct. The penis (Figure 11K–N) broadly triangular with a wide, nonglandular outgrowth on the left side of the medial section. The distal end of the penis blunt with a papilla and a visible vas deferens, and some brown pigment spots on its dorsal side.
Etymology
The specific epithet ozbeki refers to Prof. dr Murat Özbek, who is one of the world’s leading experts on freshwater amphipods in Türkiye.
Known distribution: Type locality only.
Differential diagnosis
Shell much bigger than in A. cuneytsolaki and with a lower spire than in A. tufanbeyi. Radula differs from A. tufanbeyi in the central tooth with six (instead of ten) and smaller cusps along a massive cutting edge, and one, not two pairs of basal cusps; in A. cuneytsolaki, the cutting edge consists of fewer but much bigger cusps. Bursa copulatrix with a well-demarcated duct, unlike in A. tufanbeyi, and shorter and bulkier than in A. cuneytsolaki. Penis with a wide triangular, nonglandular outgrowth on the left part in its medial section, unlike A. tufanbeyi and A. cuneytsolaki.
Adaniya cuneytsolakiOdabaşı and Jaszczyńska n. sp.
urn:lsid:zoobank.org:act:1833F87E-957B-45B3-9B72-8AD8BEC90B8C
GenBank no. for COI: PX127888-PX127889
Type locality: Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, Türkiye (locality 3, Figure 2E,F).
Holotype: (Figure 9J) Ethanol-fixed specimen, Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, Türkiye (locality 3), leg. Deniz Anıl Odabaşı, 14.10.2024. Voucher number COMULM-G 308.
Paratypes: Five ethanol-fixed specimens, Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, collection of the COMULM (Voucher number COMULM-G 309), Türkiye.
Diagnosis
A representative of Adaniya that has a small, ovate-conical, and narrow shell; a central tooth with a non-massive cutting edge and long, sharp cusps; a sac-shaped bursa copulatrix with a short, broad duct; and a penis without any outgrowths. Binary MDCs: 66(C), 135(G), 148 (T), 198(C), 225(T), 232(T), 252(A), 414(C).
Description
Shell (Figure 9J–L)
Up to 1.83 mm high, ovate-conic, narrow, with a low or moderately high spire, with about 3½ whorls, spire height 18–26% of the shell height. Teleoconch whorls slightly convex, growing regularly in diameter, sometimes abruptly (Figure 9K), resulting in a massive body whorl, suture shallow and not well-marked. Aperture broadly ovoid, or near circular in shape, more or less dragged away from body whorl, resulting in umbilicus in a form of broad trough, outer lip simple and thick, parietal lip broad and complete. Teleoconch moderately thick-walled, growth lines slightly visible, periostracum whitish or yellowish. Shell measurements: Table 4. Operculum paucispiral, elongate-ellipsoidal, smooth on both surfaces.
Radula (Figure 10G) The central tooth has long and sharp cusps according to the following formula:
(5)4–1–4(5) or 4–1–4
1–1 1–1
The median cusp less than twice the length of the adjacent cusps, a deep sinus on the front side of the cutting edge, and a narrow, V-shaped basal tongue. The lateral tooth with four to five sharp cusps. The largest cusp twice as long as the adjacent ones and broad with a sharp tip. The inner marginal tooth with about 26 long, sharp cusps; the outer marginal tooth with about 20 sharp cusps.
Morphology and anatomy of soft parts
The head and mantle intensely pigmented black. The female reproductive organs (Figure 12C) with short pallial glands, a big, elongated, sac-shaped bursa copulatrix with a short, broad duct, a moderately big, sac-shaped distal receptaculum seminis, and a short loop of the renal oviduct. The penis (Figure 13A,B) simple and broadly triangular without any outgrowths, terminated with a papilla, and the vas deferens visible inside.

Figure 13.
Penes of the studied taxa. (A,B)—Adaniya cuneytsolaki n. sp. (locality 3); (C–I)—Sheitanok esmaae n. sp. (locality 4); (J,K)—Anadoludamnicola ayhani n. sp. (locality 3). Scale bar: 200 μm and 100 μm (for (D) only).
Etymology
The specific epithet cuneytsolaki refers to Prof. and Dr. Cüneyt Nadir Solak, a friendly person and one of Türkiye’s leading experts on diatoms.
Known distribution: Type locality only.
Differential diagnosis
The shell much smaller and slimmer than in A. tufanbeyi and A. ozbeki. Radula differs from those of these two species in that the cutting edge of the central tooth has a few big, sharp cusps. Bursa copulatrix differs from A. tufanbeyi in having a well-discernible duct and from A. ozbeki in being longer and bulkier. Penis without any outgrowth, similar to that in A. tufanbeyi, but unlike A. ozbeki.
Genus SheitanokSchütt & Şeşen, 1991
Type species Sheitanok amidicus Schütt & Şeşen, 1991
Sheitanok esmaaeEkin & Odabaşı, n. sp, 2025
urn:lsid:zoobank.org:act:5F56C1BC-5A56-4F41-A438-55A2EEF94FCF
GenBank no. for COI: PX127893-PX127895
Type locality: Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, Türkiye (locality 4, Figure 2A,B)
Holotype: (Figure 9M–O) Ethanol-fixed specimen, Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, Türkiye (locality 5), leg. Ihsan Ekin, 06.04.2025. Voucher number COMULM-G 310.
Paratypes: 33 ethanol-fixed specimens, Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, collection of the COMULM (Voucher number COMULM-G 311), Türkiye.
Diagnosis
A representative of the genus Sheitanok with a less wide umbilicus and aperture of the shell than in the type species. Binary MDCs: 12(C), 15(T), 36 (C), 40(C), 51(A), 75(C), 78(A), 96(A), 153(T), 168(T), 169(T), 171(G), 172(C), 177(T), 198(C), 201(A), 219(A), 225(G), 241(T), 270(C), 282(C), 285(A), 339(T), 396(A), 408(T), 411(T), 420(T), 429(C).
Description
Shell (Figure 9M–R)
Up to 1.27 mm high, valvatiform (depressed trochiform), broad, with spire extremely low, with about 3½ whorls, spire height 9–10% of the shell height. Teleoconch whorls flat and grow regularly in diameter. Suture shallow and not well-marked. The umbilicus moderately broad, completely open, and circular in shape. The body whorl broad and massive. The aperture narrowly ovoid with an angle at its upper side. The outer lip sinuate, and the parietal lip broad and complete. The teleoconch moderately thick-walled and translucent with slightly visible growth lines. The periostracum whitish or yellowish. Shell measurements: Table 4. Operculum paucispiral, oval with an angle, orange, smooth on both surfaces.
Radula (Figure 10E) The central tooth with long and blunt cusps according to the following formula:
5–1–5
(2)1–1(2)
The median cusp narrow and less than twice the length of the adjacent cusps. A deep sinus present on the front side of the cutting edge, and the basal tongue narrowly V-shaped. The lateral tooth with five blunt cusps, the largest of which slightly longer than the others and with a blunt tip. The inner marginal tooth with 24–26 long cusps, and the outer marginal tooth with about 21 cusps.
Morphology and anatomy of soft parts
Head and tentacles delicately pigmented, big eyespots, mantle intensely pigmented, ctenidium small with about nine filaments. Bursa copulatrix narrow and tubular in shape, with a relatively short duct and one distal seminal receptacle. Penis (Figure 13C–I) gradually tapering, simple, without outgrowths, with a big terminal papilla covered with a thin layer of chitin.
Etymology
The specific epithet esmaae refers to the late mother of Prof. and Dr. İhsan Ekin, Esma Ekin.
Known distribution: Also in the Spring in Finik Harabeleri (Finik Ruins), Eskiyapı, Güçlükonak, Şırnak, Türkiye (locality 4).
Differential diagnosis
Shell with a less wide umbilicus and a less wide aperture than in S. amidicus. Female reproductive organs similar to those described and illustrated by Schütt and Şeşen [15] for that species. Penis is also similar, as long as a schematic drawing of Schütt and Şeşen [15] is considered.
Remarks
Sheitanok amidicus was described from “Quelle im Ort Tüllük, 30 km N Diyarbakir an der Straße nach Elazlg” [15], at a straight-line distance of 190 km from our locality. The locality of Delicado et al. [14]’s Sheitanok sp.—“Buhur in Mardin province” does not exist; it is most probably Batur, a village in Dageçit Town in Mardin province—is 70 km from the type locality of Sheitanok amidicus and 180 km from our locality. Our sequences are evidently distinct from the ones of Delicado et al. [14], representing two distinct species. However, the species assignment of Sheitanok at both localities remains doubtful, as long as the specimens from the type locality remain unsequenced.
Genus Anadoludamnicola Şahin, Koca & Yıldırım, 2012
The type species A. gloeri Şahin, Koca & Yıldırım, 2012
Anadoludamnicola ayhaniOdabaşı n. sp.
urn:lsid:zoobank.org:act:4F4ABD8C-5A2F-4BAF-9F72-E7B76CCCE334
GenBank no. for COI: PX127890
Type locality: Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, Türkiye (locality 3, Figure 2E,F).
Holotype: (Figure 9S) Ethanol-fixed specimen, Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, Türkiye (locality 3), leg. Deniz Anıl Odabaşı, 14.10.2024. Voucher number COMULM-G 312.
Paratypes: Five ethanol-fixed specimens, Küp Şelalesi, Doğan Çayı, Aladağ District, Adana Province, collection of the COMULM (Voucher number COMULM-G 313), Türkiye.
Diagnosis
Representative of the genus Anudaluamnicola with no basal cusps on the central tooth. Binary MDCs: 9(C), 15(A), 18 (T), 69(C), 159(T), 163(T), 165(G), 169(C), 171(T), 172(T), 186(T), 195(G), 207(T), 222(C), 249(T), 265(G), 267(T), 268(C), 273(C), 289(C), 300(C), 330(C), 393(G), 405(T), 414(C), 432(T), 439(C), 462(T).
Description
Shell (Figure 9S,T)
Up to 1.66 mm high, ovate-conic, broad, with a rather low spire, about four whorls, and a spire height of 18–19% of the shell height. Teleoconch whorls nearly flat, growing regularly in diameter, with a shallow, not well-marked suture, and the body whorl massive. Aperture broadly ovoid, outer lip simple, parietal lip complete, narrow or broad, umbilicus broad. Teleoconch rather thick-walled, growth lines delicate, periostracum whitish or yellowish. Shell measurements: Table 4. Operculum paucispiral, oval, smooth on both surfaces.
Radula (Figure 10A,B)
The central tooth typically taenioglossate, with long and blunt cusps on the cutting edge, following the formula (6)5–1–5(6), but without the basal cusps. The median cusp narrow and less than twice the length of the adjacent cusps. A deep sinus present on the front side of the cutting edge, and the basal tongue narrowly V-shaped. The lateral tooth with (5)4–1–5 cusps; the largest cusp nearly twice as long as the adjacent cusps and narrow. The inner marginal tooth with 30–32 long cusps, and the outer marginal tooth about 16 cusps.
Morphology and anatomy of soft parts
The female reproductive organs (Figure 12D) with short pallial gland complexes, a large, spherical bursa copulatrix with a distinct, short duct on a medium-sized, sac-shaped distal receptaculum seminis, and a wide loop of the renal oviduct. The penis (Figure 13J, K) strap-like, narrow, and simple with a pointed tip and a spot of brown pigment on its dorsal side, the vas deferens visible inside.
Etymology
The specific epithet ayhani refers to the father of one of the authors, Ayhan Odabaşı.
Known distribution
Known from the type locality only.
Differential diagnosis
The radula of Anadoludamnicola ayhani has no basal cusps reported for A. gloeri by Şahin et al. [16]. The bursa copulatrix spherical, not flattened, and the receptaculum seminis shorter than in A. gloeri. The penis of A. ayhani in general resembles the one figured and described by Şahin et al. [16] for A. gloeri, but their description and schematic drawing make an impossibly more detailed comparison.
Remarks
Anadoludamnicola gloeri was described from İnek Pınarı just above the Tohma Çayı (Akçadağ, Malatya), at a straight-line distance of 240 km from our locality. The locality of Delicado et al. [14], Spring in Yarıkkaya, Tunceli, was 160 km from the type locality, and 390 km from our locality with Anadoludamnicola. Our sequences are evidently distinct from the ones of Delicado et al. [14], representing two distinct species. However, the species assignment of Anadoludamnicola at both localities remains doubtful, as long as the specimens from the type locality remain unsequenced.
Table 6 summarizes the diagnostic features of the newly described species.

Table 6.
Comparative table summarizing diagnostic features of the novel described species.
4. Discussion
There is a rich literature demonstrating the need, or even necessity, of molecular data for species distinction in the Truncatelloidea (see Falniowski [54] for review), despite the fact that the molecular- and morphology-based phylogeny may differ among all the organisms [55]. In our case, there is no conflict in the resulting phylogeny, but in the species delimitation. Two of the three species delimitation techniques suggested the Adaniya genus to be a monotypic one, but the morphology of the shell (also biometry), radulae, female reproductive organs, and penes clearly demonstrated the distinctness of these three species. It is noteworthy that such morphological distinctness between congeners is rather rare in the Truncatelloidea [54]. The algorithmic techniques of species delimitation based on sequence data are very helpful but cannot be uncritically used—their data interpretation should be careful, and a holistic, integrative approach should be strongly recommended [14]. Similar conclusions were presented by Duminil and Di Michele for plants [56], Tödter et al. for Hydrozoa [57], and Lefébure et al. for Crustacea [58].
The basal cusps, typical of most of the Truncatelloid taxa, we have not found in Anadoludamnicola. Prominent basal cusps (one pair) were figured by Şahin et al. [16]. Basal cusps are absent in some groups of the Truncatelloidea, like the Emmericiidae [59].
All the species described above belong to a hydrobiid clade entirely restricted to Anatolia (or, perhaps, West Asia), having no close relatives in Europe, as already noted by Odabaşı et al. [13]. As stated, the case already seems interesting, since the isolation of Anatolia from the Balkans was neither continuous over time, nor efficient, and most of the hydrobiid genera are represented in both areas [1,22,60,61,62,63]. On the other hand, according to Gürlek et al. [5], as many as 60% of the Anatolian prosobranch species are endemic. However, the distinctiveness of many hydrobioid species remains doubtful [54].
Neubauer and Wesselingh [63] reported a high percentage of the endemic gastropods in the early Pleistocene, but of these, about half of the species were endemic to the Aegean–Anatolian region, including also mainland Greece. In their study on freshwater crabs, Ghanavi et al. [64] reported that their genetic diversity was represented by East Mediterranean and Levant groups, the latter in East Anatolia, where the type locality of our new species is located. They inferred the centre of distribution for the Potamon Savigny, 1816 crabs to lie in their Levant region, and this also may be the case for our gastropod clade. According to Kosswig [65], there was a migration of Asiatic as well as Indo-African (from India) freshwater fauna to Anatolia during the Pliocene. In the Pleistocene, Anatolia served as a refugium during glaciations.
5. Conclusions
The discovery of six new species to science, part of which are classified to two new genera, following the collection of snails in five springs, clearly demonstrates the poor state of knowledge about the fauna of minute gastropods in Turkey. Our study also confirms, once more, the necessity of applying molecular data—DNA sequences—not only to infer phylogenetic relationships, but also to discriminate species. However, as we have demonstrated, the molecular discrimination alone may be misleading, and the morphological characters should always be considered in taxonomical decisions on the species level.
Author Contributions
Conceptualization, A.J., S.H., and A.F.; Methodology, A.J., S.H., and I.S.; Formal analysis, S.H. and I.S.; Investigation, A.J. and S.H.; Resources, D.A.O. and İ.E.; Writing—original draft, A.J., S.H., I.S., and A.F.; Writing—review and editing, A.J., S.H., D.A.O., İ.E., I.S., and A.F.; Visualization, A.J.; Funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the grant N18/DBS/000012 from the Institute of Zoology and Biomedical Research, Jagiellonian University.
Institutional Review Board Statement
This research was conducted with the permission of the Ministry of Agriculture and Forestry, General Directorate of National Parks and Nature Conservation (E-21264211-288.04-17247402, dated 23 December 2024).
Informed Consent Statement
Not applicable in this case.
Data Availability Statement
All the sequences are deposited in GenBank accession numbers PX127888-PX127899.
Acknowledgments
We are grateful for the assistance of Anna Łatkiewicz (Laboratory of FE Scanning Microscopy and Microanalysis, Institute of Geological Sciences, Jagiellonian University, Krakow, Poland) for her help with the SEM. We also extend our sincere gratitude to the Faculty of Marine Sciences and Technology at Çanakkale Onsekiz Mart University for providing laboratory support. The authors are grateful to the editor and the three reviewers for their substantial improvement of the present article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Radoman, P. Hydrobioidea a Superfamily of Prosobranchia (Gastropoda). I Systematics; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1983; Volume 57, pp. 1–256. [Google Scholar]
- Yildirim, M.Z.; Koca, S.B.; Kebapçi, U. Supplement to the Prosobranchia (Mollusca: Gastropoda) Fauna of Fresh and Brackish Waters of Turkey. Turk. J. Zool. 2006, 30, 197–204. [Google Scholar]
- Çaĝlan, D.C.; Yildirim, M.Z.; Szarowska, M.; Falniowski, A. Phylogenetic position of Tefennia Schütt et Yildirim, 2003 (Caenogastropoda: Rissooidea). Folia Malacol. 2012, 20, 271–277. [Google Scholar] [CrossRef]
- Gürlek, M.E. Three new truncatelloidean gastropod species from Turkey (Caenogastropoda: Littorinimorpha). Turk. J. Zool. 2017, 41, 991–997. [Google Scholar] [CrossRef]
- Gürlek, M.E.; Şahin Koşal, S.; Dokumcu, N.; Yildirim, M.Z. Checklist of the Freshwater Mollusca of Turkey (Mollusca: Gastropoda, Bivalvia). Fresenius Environ. Bull. 2019, 28, 2992–3013. Available online: https://www.researchgate.net/publication/332422818 (accessed on 17 May 2025).
- Odabaşı, S.; Odabaşı, D.A.; Acar, S. New species of freshwater molluscs from Gökçeada (northeastern Aegean Sea), Turkey (Gastropoda: Hydrobiidae, Bythinellidae). Arch. Molluskenkd. 2019, 148, 185–195. [Google Scholar] [CrossRef]
- Ekin, I. Endemic microsnail Sheitanok amidicus (Caenogastropoda: Hydrobiidae) additional locations, distribution, shell morphology, and near-threatened. J. Conchol. 2023, 44, 431–438. [Google Scholar]
- Yıldırım, M. The Prosobranchia (Gastropoda: Mollusca) species of Turkey and their zoogeographic distribution 1. Fresh and brackish water. Turk. J. Zool. 1999, 23, 877–900. [Google Scholar]
- Mittermeier, R.A.; Gil, P.R.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, J.C.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Amsterdam University Press: Amsterdam, The Netherlands, 2004; ISBN 9781840642070. [Google Scholar]
- Delicado, D.; Gürlek, M.E. Taxonomic transfer of two species of hydrobiid snails from western Anatolia (Caenogastropoda, Truncatelloidea) to two new genera, based on molecular and morphological evidence. Arch. Molluskenkd. 2021, 150, 119–131. [Google Scholar] [CrossRef]
- Odabaşı, D.A.; Odabaşı, S.; Deniz, O. New Grossuana (Littorinimorpha: Hydrobiidae) species from Mount Kazdağı, northwestern Turkey. Zool. Middle East 2022, 68, 145–155. [Google Scholar] [CrossRef]
- Odabaşı, D.A.; Mercan, D.; Odabaşı, S.; Arslan, N. A new species of Pseudorientalia (Gastropoda: Hydrobiidae) from midwestern Turkiye with notes on Pseudorientalia natolica (Küster, 1853). Zootaxa 2024, 5415, 585–592. [Google Scholar] [CrossRef]
- Odabaşı, D.A.; Falniowski, A.; Hofman, S.; Jaszczyńska, A. A new member of an entirely Anatolian clade of the Horatiinae D.W. Taylor, 1966, Hydrobiidae W. Stimpson, 1865. Molluscan Res. 2025, 45, 233–242. [Google Scholar] [CrossRef]
- Delicado, D.; Hauffe, T.; Wilke, T. Fifth mass extinction event triggered the diversification of the largest family of freshwater gastropods (Caenogastropoda: Truncatelloidea: Hydrobiidae). Cladistics 2024, 40, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Schütt, H.; Şeşen, R. Eine besondere Quellschnecke aus Ostanatolien (Prosobranchia: Hydrobiidae). Arch. Molluskenkd. 1991, 120, 175–178. [Google Scholar] [CrossRef]
- Şahin, S.K.; Koca, S.B.; Yildirim, M.Z. New genera Anatolidamnicola and Sivasi (Gastropoda: Hydrobiidae) from Sivas and Malatya (Turkey). Acta Zoo. Bulg. 2012, 64, 341–346. Available online: https://www.researchgate.net/publication/289105219 (accessed on 12 June 2025).
- Rueden, D.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, e529. [Google Scholar] [CrossRef]
- Falniowski, A.; Grego, J.; Rysiewska, A.; Osikowski, A.; Hofman, S. A new genus and species of Hydrobiidae Stimpson, 1865 (Caenogastropoda, Truncatelloidea) from Peloponnese, Greece. ZooKeys 2021, 1037, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.1x; Microcomputer Power: Ithaca, NY, USA, 2018; 536p. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Falniowski, A. Anatomical characters and SEM structure of radula and shell in the species-level taxonomy of freshwater prosobranchs (Mollusca: Gastropoda: Prosobranchia): A comparative usefulness study. Folia Malacol. 1990, 4, 53–142+78. [Google Scholar] [CrossRef]
- Szarowska, M.; Osikowski, A.; Hofman, S.; Falniowski, A. Pseudamnicola Paulucci, 1878 (Caenogastropoda: Truncatelloidea) from the Aegean Islands: A long or short story? Org. Divers. Evol. 2016, 16, 121–139. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. bold: The barcode of life data system (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Xia, X. Data Analysis in Molecular Biology and Evolution; Kluwer Academic Publishers: Boston, MA, USA, 2000. [Google Scholar]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, A.W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML NG: A fast, scalable and user friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, A.A. Posterior summarisation in Bayesian phylogenetics using Tracer1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 21 March 2025).
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2011, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Merckelbach, L.; Borges, L. Make every species count: FastaChar software for rapid determination of molecular diagnostic characters to describe species. Mol. Ecol. Resour. 2020, 20, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Hütter, T.; Ganser, M.H.; Kocher, M.; Halkic, M.; Agatha, S.; Augsten, N. DeSignate: Detecting signature characters in gene sequence alignments for taxon diagnoses. BMC Bioinform. 2020, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Delicado, D.; Pešić, V.; Ramos, M. Arganiella Giusti & Pezzoli, 1980 (Caenogastropoda: Truncatelloidea: Hydrobiidae): A widespread genus or several narrow-range endemic genera? Eur. J. Taxon. 2021, 750, 140–155. [Google Scholar] [CrossRef]
- Wilke, T.; Davis, G.M.; Falniowski, A.; Giusti, F.; Bodon, M.; Szarowska, M. Molecular systematics of Hydrobiidae (Mollusca: Gastropoda: Rissooidea): Testing monophyly and phylogenetic relationships. Proc. Acad. Nat. Sci. Phila. 2001, 151, 1–21. [Google Scholar] [CrossRef]
- Osikowski, A.; Hofman, S.; Rysiewska, A.; Sket, B.; Prevorčnik, S.; Falniowski, A. A case of biodiversity overestimation in the Balkan Belgrandiella A. J. Wagner, 1927 (Caenogastropoda: Hydrobiidae): Molecular divergence not paralleled by high morphological variation. J. Nat. Hist. 2018, 52, 323–344. [Google Scholar] [CrossRef]
- Szarowska, M.; Hofman, S.; Osikowski, A.; Falniowski, A. Daphniola Radoman, 1973 (Caenogastropoda: Truncatelloidea) at east Aegean islands. Folia Malacol. 2014, 22, 269–275. [Google Scholar] [CrossRef]
- Osikowski, A.; Hofman, S.; Georgiev, D.; Kalcheva, S.; Falniowski, A. Aquatic snails Ecrobia maritima (Milaschewitsch, 1916) and E. ventrosa (Montagu, 1803) (Caenogastropoda: Hydrobiidae) in the east Mediterranean and Black Sea. Ann. Zool. 2016, 66, 477–486. [Google Scholar] [CrossRef]
- Wilke, T.; Albrecht, C.; Anistratenko, V.V.; Sahin, S.K.; Yildirim, M.Z. Testing biogeographical hypotheses in space and time: Faunal relationships of the putative ancient Lake Eğirdir in Asia Minor. J. Biogeogr. 2007, 34, 1807–1821. [Google Scholar] [CrossRef]
- Hofman, S.; Grego, J.; Fehér, Z.; Erőss, Z.P.; Rysiewska, A.; Osikowski, A.; Falniowski, A. New data on the valvatiform-shelled Hydrobiidae (Caenogastropoda, Truncatelloidea) from southern Greece. ZooKeys 2021, 1062, 31–47. [Google Scholar] [CrossRef]
- Falniowski, A.; Georgiev, D.; Osikowski, A.; Hofman, S. Radiation of Grossuana Radoman, 1973 (Caenogastropoda: Truncatelloidea) in the Balkans. J. Molluscan Stud. 2016, 82, 305–313. [Google Scholar] [CrossRef]
- Rysiewska, A.; Prevorčnik, S.; Osikowski, A.; Hofman, S.; Beran, L.; Falniowski, A. Phylogenetic relationships in Kerkia and introgression between Hauffenia and Kerkia (Caenogastropoda: Hydrobiidae). J. Zool. Syst. Evol. Res. 2017, 55, 106–117. [Google Scholar] [CrossRef]
- Falniowski, A.; Lewarne, B.; Rysiewska, A.; Osikowski, A.; Hofman, S. Crenobiont, stygophile and stygobiont molluscs in the hydrographic area of the Trebišnjica River Basin. ZooKeys 2021, 1047, 61–89. [Google Scholar] [CrossRef] [PubMed]
- Beran, L.; Osikowski, A.; Hofman, S.; Falniowski, A. Islamia zermanica (Radoman, 1973) (Caenogastropoda: Hydrobidae): Morphological and molecular distinctness. Folia Malacol. 2016, 24, 25–30. [Google Scholar] [CrossRef]
- Szarowska, M.; Hofman, S.; Osikowski, A.; Falniowski, A. Divergence preceding Island formation among Aegean insular populations of the freshwater snail genus Pseudorientalia (Caenogastropoda: Truncatelloidea). Zool. Sci. 2014, 31, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Falniowski, A.; Szarowska, M.; Glöer, P.; Pešić, V. Molecules vs. morphology in the taxonomy of the Radomaniola/Grossuana group of Balkan Rissooidea (Mollusca: Caenogastropoda). J. Conchol. 2012, 41, 19–36. [Google Scholar]
- Szarowska, M.; Falniowski, A. Species distinctness of Sadleriana robici (Clessin, 1890) (Gastropoda: Rissooidea). Folia Malacol. 2013, 21, 127–133. [Google Scholar] [CrossRef]
- Falniowski, A. Species Distinction and Speciation in Hydrobioid Gastropods (Mollusca: Caenogastropoda: Truncatelloidea). Arch. Zool. Stud. 2018, 1, 003. [Google Scholar] [CrossRef]
- Pecina, L.; Vďačný, P. Morphological versus molecular delimitation of ciliate species: A case study of the family Clevelandellidae (Protista, Ciliophora, Armophorea). Eur. J. Taxon. 2020, 697, 1–46. [Google Scholar] [CrossRef]
- Duminil, J.; Di Michele, M. Plant species delimitation: A comparison of morphological and molecular markers. Plant Biosyst. 2009, 143, 528–542. [Google Scholar] [CrossRef]
- Tödter, L.; Worsaae, K.; Schmidt-Rhaesa, A. Comparative molecular and morphological species delineation of Halammohydra Remane, 1927 (Hydrozoa) with the description of four new species. Org. Divers. Evol. 2023, 23, 455–476. [Google Scholar] [CrossRef]
- Lefébure, T.; Douady, C.J.; Gouy, M.; Gibert, J. Relationship between morphological taxonomy and molecular divergence within Crustacea: Proposal of a molecular threshold to help species delimitation. Mol. Phylogenet. Evol. 2006, 40, 435–447. [Google Scholar] [CrossRef]
- Szarowska, M. Molecular phylogeny, systematics and morphological character evolution in the Balkan Rissooidea (Caenogastropoda). Folia Malacol. 2006, 14, 99–168. [Google Scholar] [CrossRef]
- Radoman, P. New Classification of Fresh and Brackish Water Prosobranchia from the Balkans and Asia Minor; Posebna Izdanja; Prirodnjacki Musej u Beogradu: Belgrade, Serbia, 1973; Volume 32, pp. 1–30. [Google Scholar]
- Radoman, P. Hydrobioidea, a Superfamily of Prosobranchia (Gastropoda). II. Origin, Zoogeography, Evolution in the Balkans and Asia Minor; Serbian Academy of Sciences and Arts, Faculty of Science–Department of Biology Monographs: Belgrade, Serbia, 1985; Volume 1, pp. 1–173. [Google Scholar]
- Lyubas, A.A.; Tomilova, A.A.; Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Eliseeva, T.A.; Aksenova, O.V.; Bovykina, G.V.; Kryuk, D.V.; et al. Phylogeography and genetic diversity of Duck Mussel Anodonta anatina (Bivalvia: Unionidae) in Eurasia. Diversity 2023, 15, 260. [Google Scholar] [CrossRef]
- Neubauer, T.A.; Wesselingh, F.P. The Early Pleistocene freshwater mollusks of the Denizli Basin (Turkey): A new long-lived lake fauna at the crossroads of Pontocaspian and Aegean-Anatolian realms. Zitteliana 2023, 97, 53–88. [Google Scholar] [CrossRef]
- Ghanavi, H.R.; Rahimi, P.; Tavana, M.; Tavabe, K.R.; Jouladeh- Roudbar, A.; Doadrio, I. The evolutionary journey of freshwater crabs of the genus Potamon (Decapoda: Brachyura: Potamidae). Mol. Phylogenet. Evol. 2023, 180, 107690. [Google Scholar] [CrossRef] [PubMed]
- Kosswig, C. Zoogeography of the Near East. Syst. Zool. 1955, 4, 49–73+96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).