Serum Lipid Reference Intervals of High-Density, Low-Density and Non-High-Density Lipoprotein Cholesterols and Their Association with Atherosclerosis and Other Factors in Psittaciformes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals and Sample Collection
2.2. Categorization of Variables
2.3. Data Subsets
2.4. Data Analysis
3. Results
3.1. Reference Ranges of HDL-C, LDL-C, and Non-HDL-C
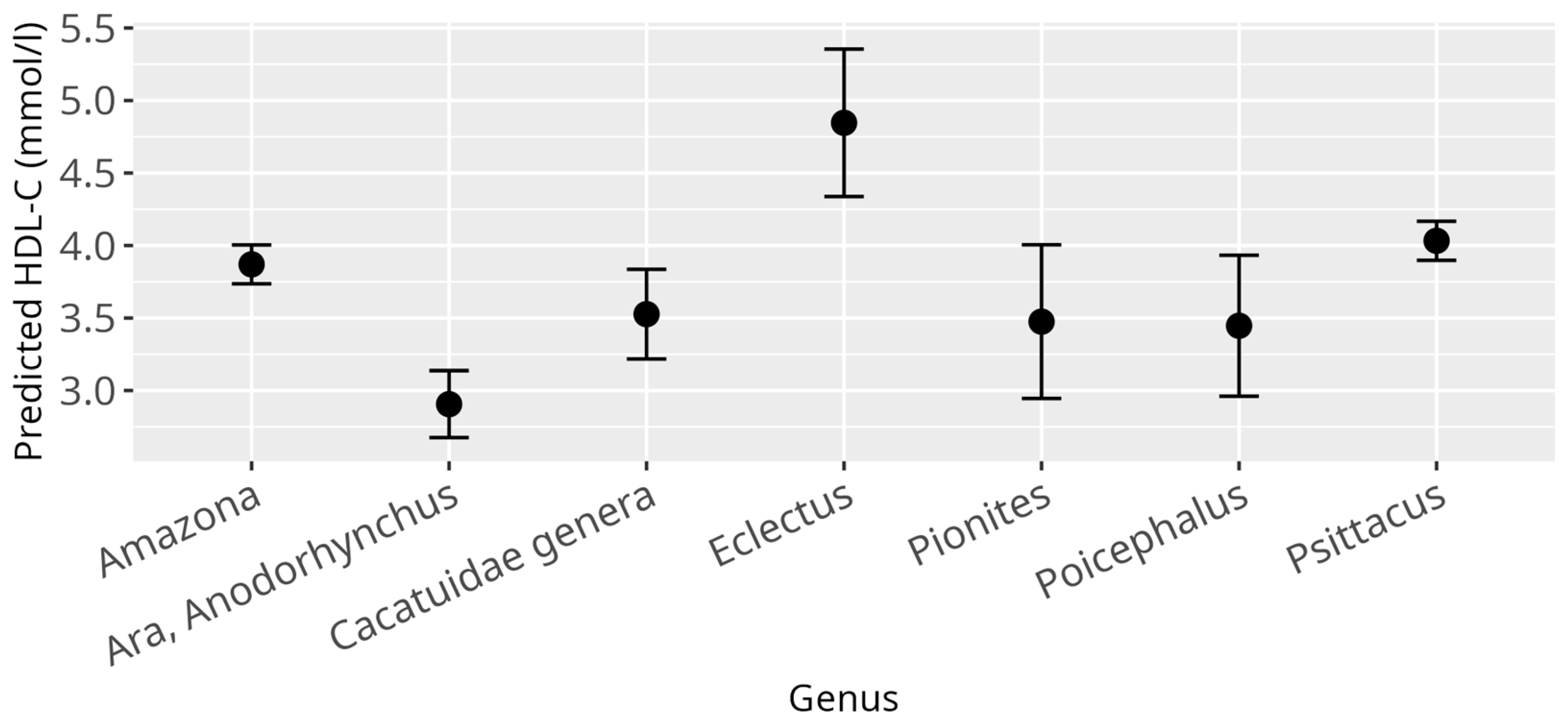
3.2. HDL-C Model
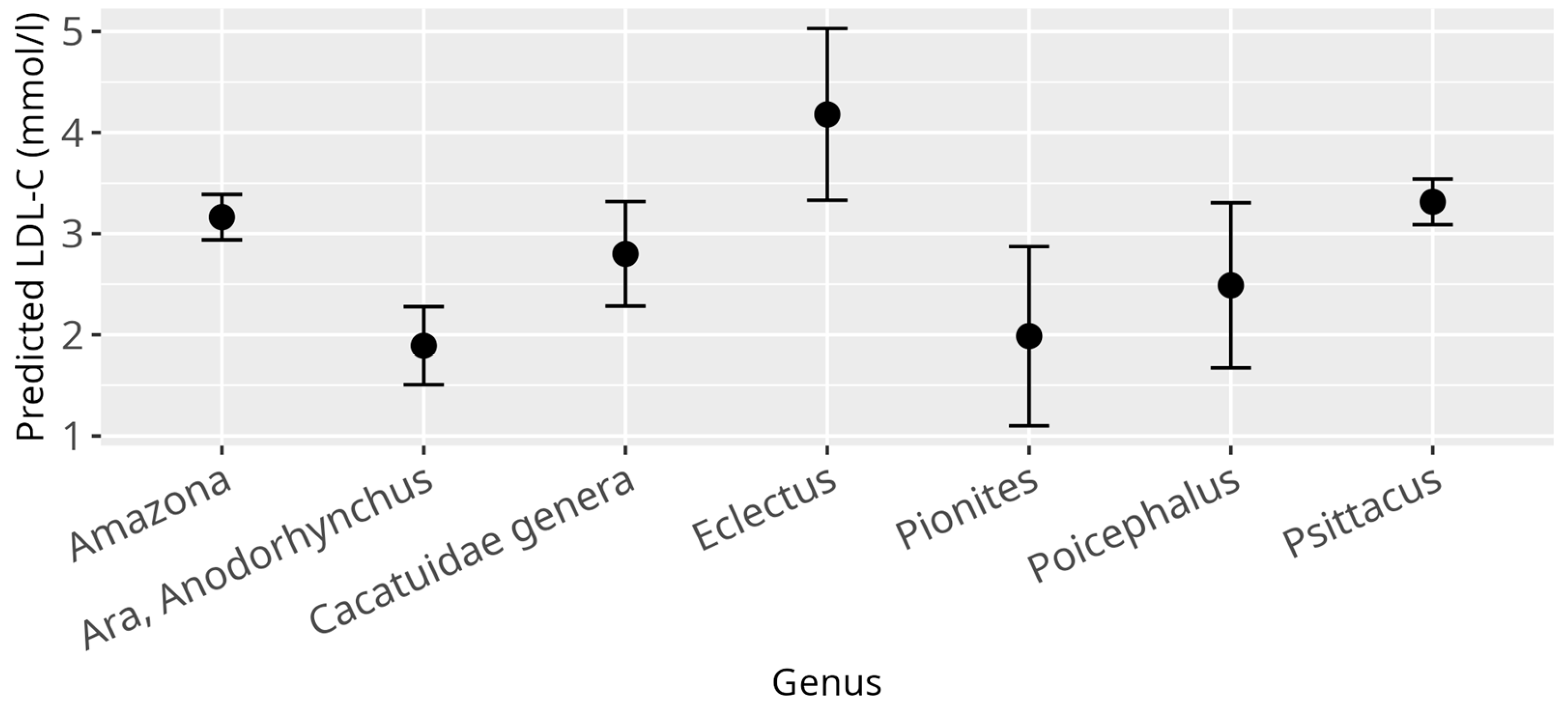
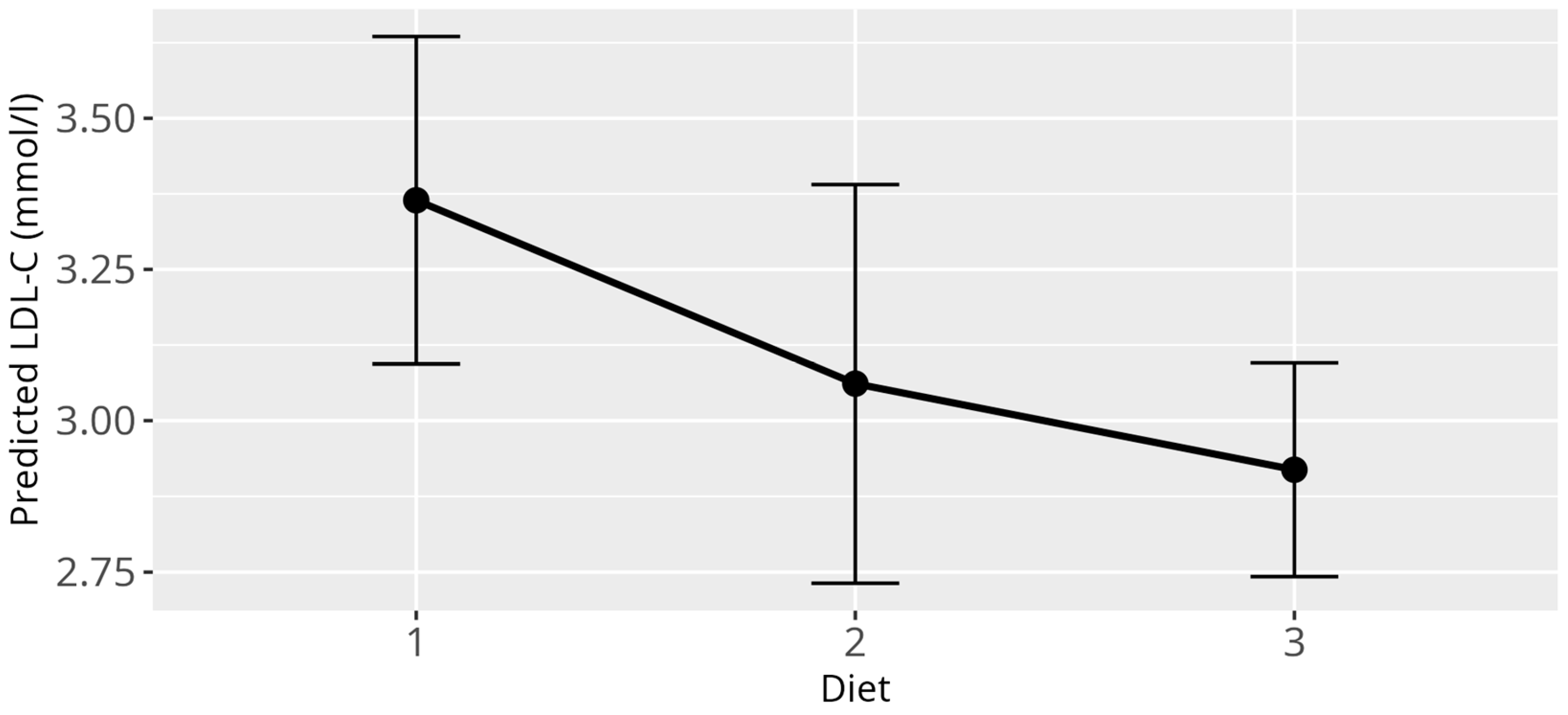
3.3. LDL-C Model
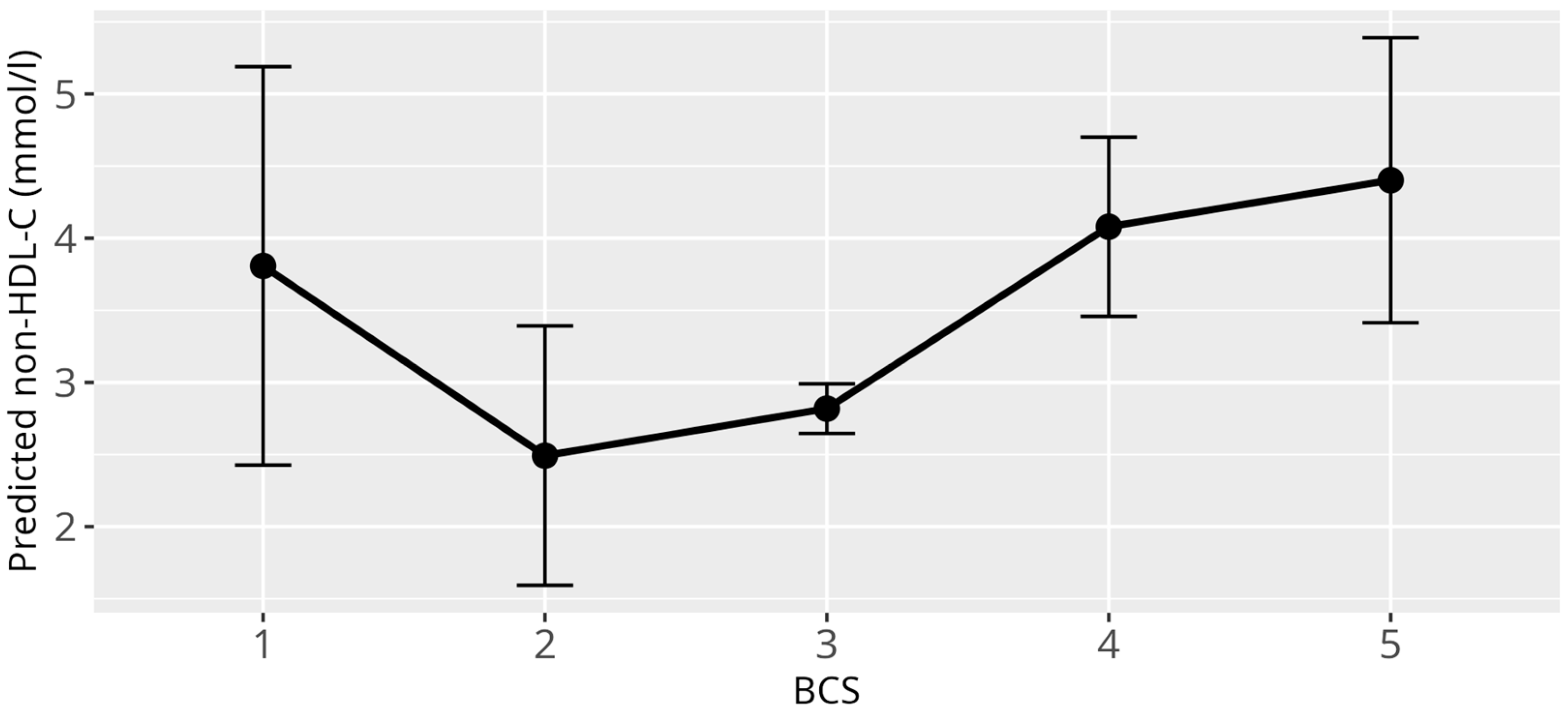
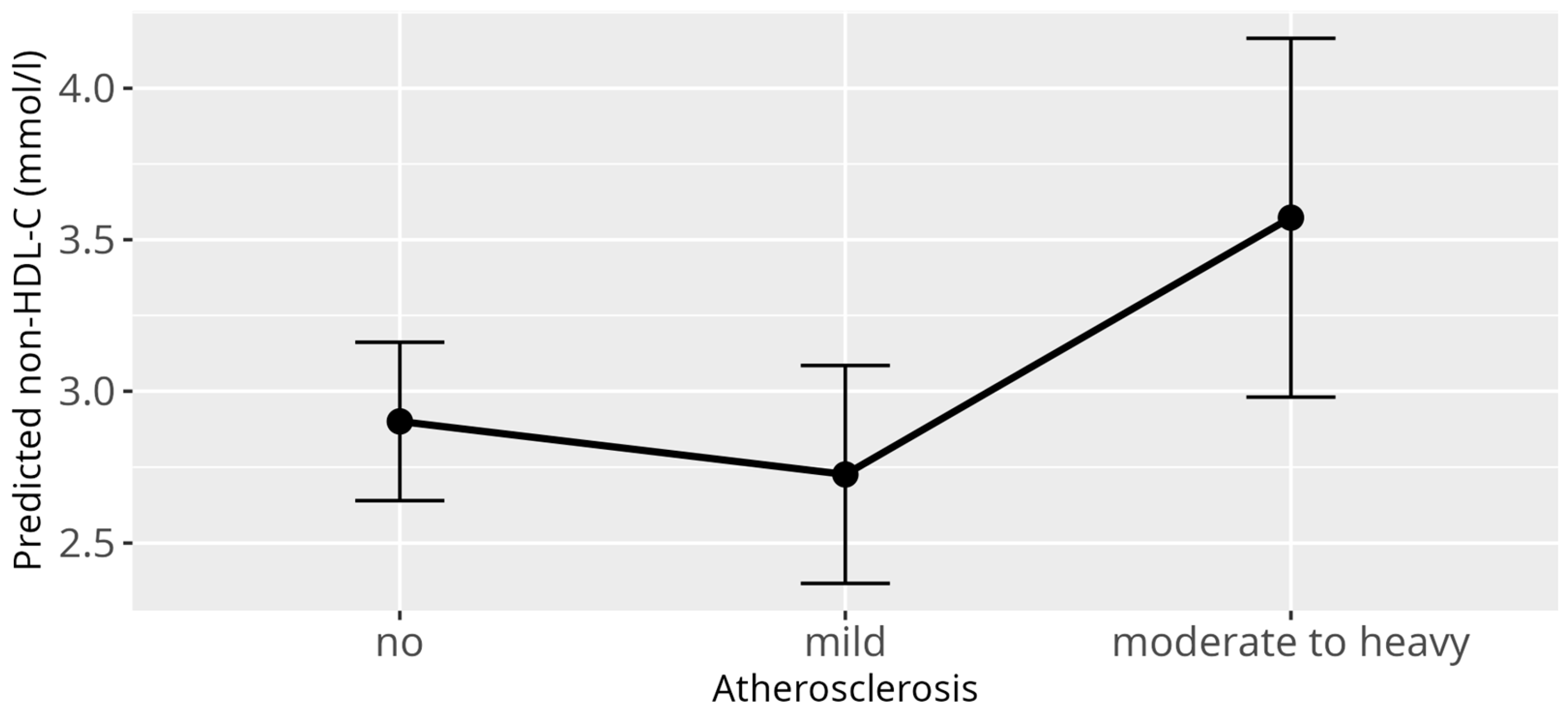
3.4. Non-HDL-C Model
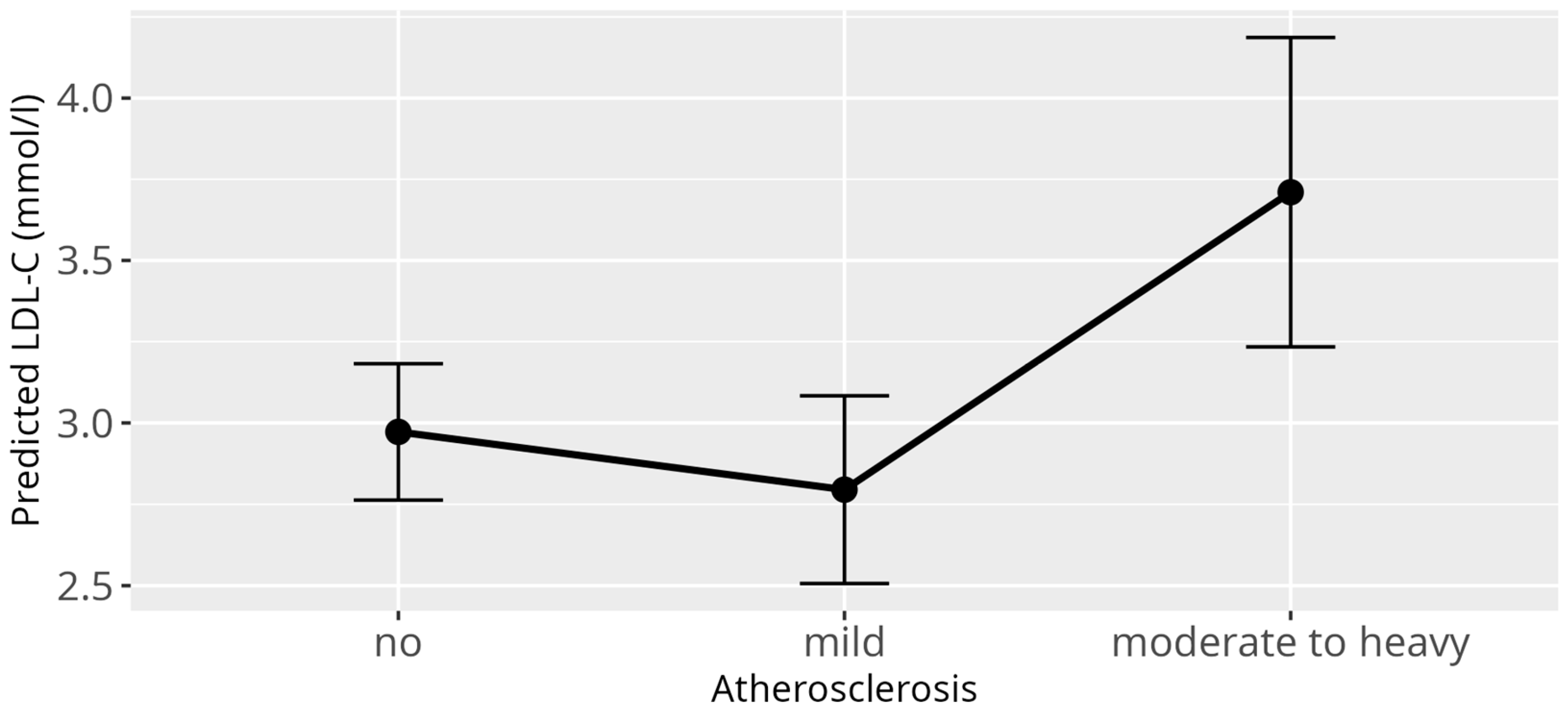
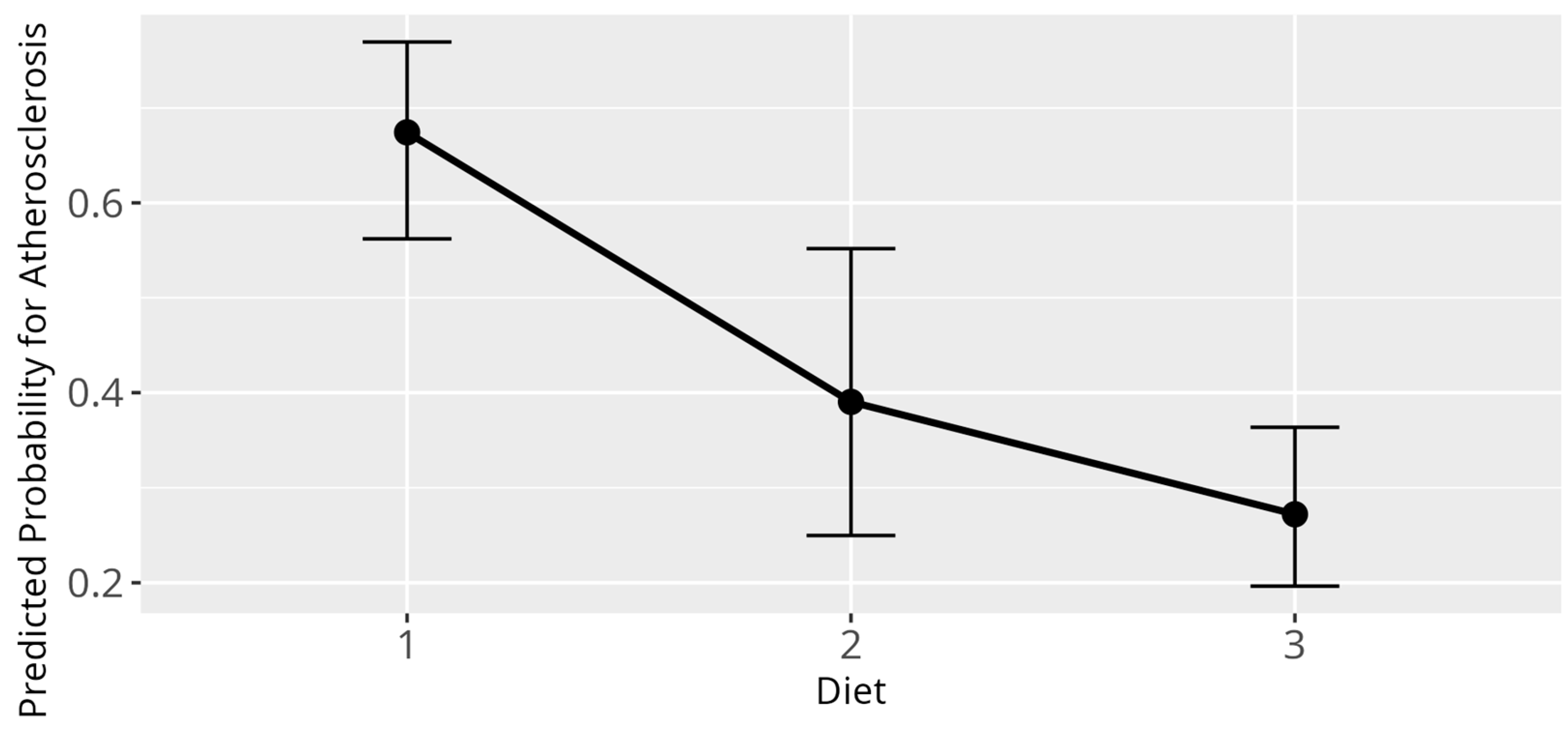
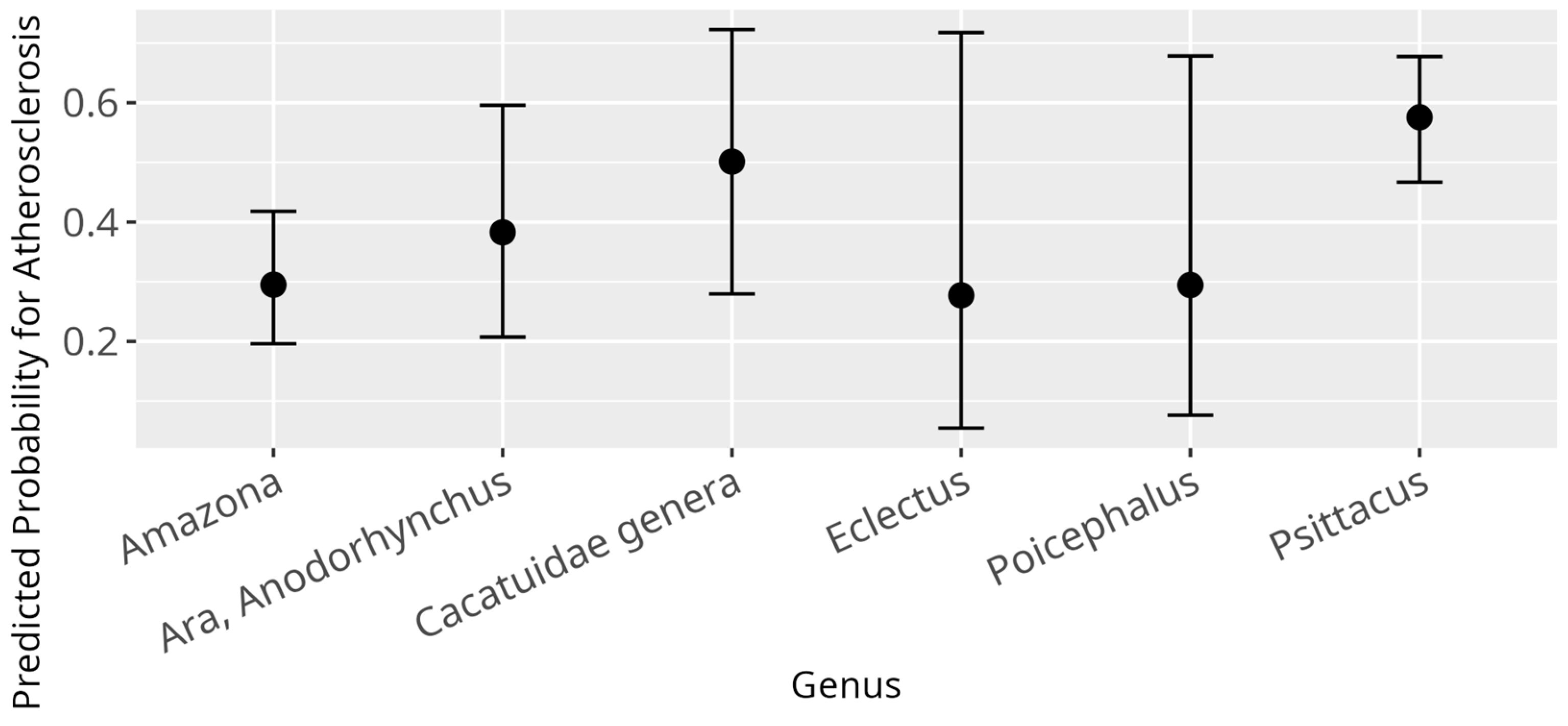
3.5. Atherosclerosis Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, C.; Klaphake, E.; Wellehan, J.F.X. Avian medicine: An overview. In Current Therapy in Avian Medicine and Surgery; Speer, B.L., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Silva, T. Psittaculture: A Manual for the Care and Breeding of Parrots; Mgr. Jan Sojka-Nová Exota: Olomouc, Czech Republic, 2018. [Google Scholar]
- Pires, S.F. The illegal parrot trade: A literature review. Global Crime 2012, 13, 176–190. [Google Scholar] [CrossRef]
- Oldest Parrot Ever. Available online: https://www.guinnessworldrecords.com/world-records/442525-oldest-parrot-ever (accessed on 25 October 2024).
- Reavill, D.R.; Dorrestein, G.M. Pathology of aging psittacines. Vet. Clin. North. Am. Exot. Anim. Pract. 2010, 13, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Beaufrere, H. Avian Atherosclerosis: Parrots and Beyond. J. Exot. Pet Med. 2013, 22, 336–347. [Google Scholar] [CrossRef]
- Fitzgerald, B.C.; Beaufrère, H. Cardiology. In Current Therapy in Avian Medicine and Surgery; Speer, B.L., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 252–328. [Google Scholar] [CrossRef]
- Johnson, J.H.P.D.; Kondik, V.H.; Phalen, D.N. Atherosclerosis in Psittacine Birds; Association of Avian Veterinarians: New Orleans, LA, USA, 1992; pp. 87–93. [Google Scholar]
- Beaufrère, H.; Ammersbach, M.; Reavill, D.R.; Garner, M.M.; Heatley, J.J.; Wakamatsu, N.; Nevarez, J.G.; Tully, T.N. Prevalence of and risk factors associated with atherosclerosis in psittacine birds. J. Am. Vet. Med. Assoc. 2013, 242, 1696–1704. [Google Scholar] [CrossRef]
- Beaufrère, H. Atherosclerosis: Comparative Pathogenesis, Lipoprotein Metabolism, and Avian and Exotic Companion Mammal Models. J. Exot. Pet Med. 2013, 22, 320–335. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.E.; Schulz, U.; Konicek, C.; Pees, M. Evaluation of diagnostic criteria in grey parrots (Psittacus erithacus) with suspected atherosclerosis. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2022, 50, 411–422. [Google Scholar] [CrossRef]
- Bavelaar, F.; Beynen, A. Atherosclerosis in parrots. A review. Vet. Q. 2004, 26, 50–60. [Google Scholar] [CrossRef]
- Pees, M.; Krautwald-Junghanns, M.E.; Straub, J. Evaluating and treating the cardiovascular system. Clin. Avian Med. 2006, 1, 384–387. [Google Scholar]
- Beaufrère, H.; Pariaut, R.; Rodriguez, D.; Tully, T.N. Avian vascular imaging: A review. J. Avian Med. Surg. 2010, 24, 174–184. [Google Scholar] [CrossRef]
- Phalen, D.N.; Hays, H.B.; Filippich, L.J.; Silverman, S.; Walker, M. Heart failure in a macaw with atherosclerosis of the aorta and brachiocephalic arteries. J. Am. Vet. Med. Assoc. 1996, 209, 1435–1440. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.E.; Pees, M.; Reese, S.; Tully, T. Diagnostic Imaging of Exotic Pets: Birds—Small Mammals—Reptiles; Schlütersche Verlagsgesellschaft mbH & Company KG: Hannover, Germany, 2010. [Google Scholar]
- Beaufrère, H.; Rodriguez, D.; Pariaut, R.; Gaschen Dvm, L.; Schnellbacher, R.; Nevarez, J.G.; Tully, T.N. Estimation of intrathoracic arterial diameter by means of computed tomographic angiography in Hispaniolan Amazon parrots. Am. J. Vet. Res. 2011, 72, 210–218. [Google Scholar] [CrossRef]
- Megan, L.; Brust, K.; Spriet, M.; Ruivo, P.; Gómez-Ponce, M.; Beaufrère, H. 18F-sodium fluoride positron emission tomography is a sensitive imaging technique to detect atherosclerosis in Amazon parrots (Amazona spp.). Am. J. Vet. Res. 2024, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/europe/news-room/fact-sheets/item/cardiovascular-diseases/ (accessed on 2 August 2025).
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiangdong, L.; Yuanwu, L.; Hua, Z.; Liming, R.; Qiuyan, L.; Ning, L. Animal models for the atherosclerosis research: A review. Protein Cell 2011, 2, 189–201. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Saleheen, D.; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Mitchell, M.; Sanchez-Migallon Guzman, D.; Knych, H.; Beaufrere, H. The pharmacokinetics of single-dose oral atorvastatin and its metabolites support therapeutic use in cockatiels (Nymphicus hollandicus). Am. J. Vet. Res. 2024, 85, 1–7. [Google Scholar] [CrossRef]
- Mikoni, N.; Sanchez-Migallon Guzman, D.; Knych, H.; Beaufrere, H. Pharmacokinetics of single-dose oral atorvastatin and its metabolites support therapeutic use in orange-winged amazon parrots (Amazona amazonica). Am. J. Vet. Res. 2023, 85, 1–7. [Google Scholar] [CrossRef]
- Beaufrere, H.; Barboza, T.; Burnett, A.; Stark, K.; Wood, R. Effects of Atorvastatin and Rosuvastatin on Blood Lipids in Quaker Parrots (Myiopsitta monachus). J. Avian Med. Surg. 2023, 37, 199–208. [Google Scholar] [CrossRef]
- Robertson, J.; Sanchez-Migallon Guzman, D.; Graham, J.; Stanhope, K.; Douglas, J.; Havel, P.; Beaufrere, H.; Knych, H.; Tully, T.; Paul-Murphy, J. Evaluation of Orally Administered Atorvastatin on Plasma Lipid and Biochemistry Profiles in Hypercholesterolemic Hispaniolan Amazon Parrots (Amazona ventralis). J. Avian Med. Surg. 2020, 34, 32. [Google Scholar] [CrossRef]
- Buyse, J.; Decuypere, E. Chapter 19—Adipose Tissue and Lipid Metabolism. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 443–453. [Google Scholar] [CrossRef]
- Beaufrère, H.; Reavill, D.; Heatley, J.; Susta, L. Lipid-Related Lesions in Quaker Parrots (Myiopsitta monachus). Vet. Pathol. 2019, 56, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, H.; Wood, D. Comparison of Lipoprotein Analysis Using Gel-Permeation High-Performance Liquid Chromatography and a Biochemistry Analyzer in Normolipidemic and Dyslipidemic Quaker Parrots (Myiopsitta monachus). J. Avian Med. Surg. 2023, 36, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, H.; Gardhouse, S.; Ammersbach, M. Lipoprotein characterization in Quaker parrots (Myiopsitta monachus) using gel-permeation high-performance liquid chromatography. Vet. Clin. Pathol. 2020, 49, 417–427. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Scharnagl, H.; Kleber, M.; Silbernagel, G.; Nauck, M.; Müller-Wieland, D.; von Eckardstein, A. Laboratory diagnostics of lipid metabolism disorders. Dtsch. Med. Wochenschr. 2023, 148, e120–e146. [Google Scholar] [CrossRef]
- Beaufrère, H.; Nevarez, J.G.; Wakamatsu, N.; Clubb, S.; Cray, C.; Tully, T.N. Experimental diet-induced atherosclerosis in Quaker parrots (Myiopsitta monachus). Vet. Pathol. 2013, 50, 1116–1126. [Google Scholar] [CrossRef]
- Beaufrère, H.; Cray, C.; Ammersbach, M.; Tully, T.N., Jr. Association of plasma lipid levels with atherosclerosis prevalence in psittaciformes. J. Avian Med. Surg. 2014, 28, 225–231. [Google Scholar] [CrossRef]
- Ravich, M.; Cray, C.; Hess, L.; Arheart, K.L. Lipid Panel Reference Intervals for Amazon Parrots (Amazona species). J. Avian Med. Surg. 2014, 28, 209–215. [Google Scholar] [CrossRef]
- König, H.E.; Korbel, R.; Liebich, H.G.; Klupiec, C. Avian Anatomy: Textbook and Colour Atlas, 2nd ed.; 5m Publishing: Sheffield, UK, 2016; pp. 289–303. [Google Scholar]
- Forshaw, J.M.; Knight, F. Parrots of the World; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Doneley, B.; Harrison, G.J.; Lightfoot, T.L. Maximizing Information from the Physical Examination. In Clinical Avian Medicine; Harrison, G.J., Lightfoot, T.L., Eds.; Spix Publishing: Palm Beach, FL, USA, 2006; Volume 1, p. 153. [Google Scholar]
- Fitzgerald, B. Atherosclerosis in birds—What you need to know. In Proceedings of the NAVC Conference, Orlando, FL, USA, 16–20 January 2016; pp. 1342–1344. [Google Scholar]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
- Finnegan, D. Referenceintervals: Reference Intervals, version 1.3.1; Comprehensive R Archive Network (CRAN): Vienna, Austria, 2024. [Google Scholar]
- Horn, P.S.; Pesce, A.J.; Copeland, B.E. A robust approach to reference interval estimation and evaluation. Clin. Chem. 1998, 44, 622–631. [Google Scholar] [CrossRef]
- Boyd, J. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines, CLSI Document C28-A3; CLSI: Wayne, PA, USA, 2010; Volume 28. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications: London, UK, 2011. [Google Scholar]
- Donovan, T.A.; Garner, M.M.; Phalen, D.; Reavill, D.; Monette, S.; Le Roux, A.B.; Hanson, M.; Chen, S.; Brown, C.; Echeverri, C.; et al. Disseminated coelomic xanthogranulomatosis in eclectus parrots (Eclectus roratus) and budgerigars (Melopsittacus undulatus). Vet. Pathol. 2022, 59, 143–151. [Google Scholar] [CrossRef]
- Guzman, D.S.-M.; Beaufrère, H.; Welle, K.R.; Heatley, J.; Visser, M.; Harms, C.A. Chapter 5—Birds. In Carpenter’s Exotic Animal Formulary, 6th ed.; Carpenter, J., Harms, C., Eds.; W.B. Saunders: New Delhi, India, 2023; pp. 222–443. [Google Scholar] [CrossRef]
- Fitzgerald, B.C. Introduction to Avian Cardiology. In Proceedings of the Association of Avian Veterinarians, Kansas City, MO, USA, 28 June 28–1 July 2024; pp. 165–176. [Google Scholar]
- Véniant, M.M.; Sullivan, M.A.; Kim, S.K.; Ambroziak, P.; Chu, A.; Wilson, M.D.; Hellerstein, M.K.; Rudel, L.L.; Walzem, R.L.; Young, S.G. Defining the atherogenicity of large and small lipoproteins containing apolipoprotein B100. J. Clin. Invest. 2000, 106, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Jacobs, F.; Feng, Y.; Thomassen, L.C.; Martens, J.A.; Lievens, J.; Snoeys, J.; De Geest, B. The relative atherogenicity of VLDL and LDL is dependent on the topographic site. J. Lipid Res. 2010, 51, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Stene, M.C.; Sethi, A.A.; Remaley, A.T.; Schnohr, P.; Grande, P.; Tybjaerg-Hansen, A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008, 299, 2524–2532. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Poernama, F.; Subramanian, R.; Cook, M.E.; Attie, A.D. High density lipoprotein deficiency syndrome in chickens is not associated with an increased susceptibility to atherosclerosis. Arterioscler. Thromb. 1992, 12, 601–607. [Google Scholar] [CrossRef]
- Allen, P.C.; Wong, H.Y.C. Effect of Atherogenic Diet on Chicken Plasma Lipids and Lipoproteins. Poult. Sci. 1993, 72, 1673–1678. [Google Scholar] [CrossRef]
- Schilliger, L.; Lemberger, K.; Chai, N.; Bourgeois, A.; Charpentier, M. Atherosclerosis associated with pericardial effusion in a central bearded dragon (Pogona vitticeps). J. Vet. Diagn. Invest. 2010, 22, 789–792. [Google Scholar] [CrossRef]
- Dangerfield, W.G.; Finlayson, R.; Myatt, G.; Mead, M.G. Serum lipoproteins and atherosclerosis in animals. Atherosclerosis 1976, 25, 95–106. [Google Scholar] [CrossRef]
- Ullrey, D.E.; Allen, M.E.; Baer, D.J. Formulated diets versus seed mixtures for psittacines. J. Nutr. 1991, 121, S193–S205. [Google Scholar] [CrossRef] [PubMed]
- Brightsmith, D.J. Nutritional levels of diets fed to captive Amazon parrots: Does mixing seed, produce, and pellets provide a healthy diet? J. Avian Med. Surg. 2012, 26, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Orosz, S.E. Clinical avian nutrition. Vet. Clin. North. Am. Exot. Anim. Pract. 2014, 17, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, R.; Hirchinson, V. Experimental atheroma in budgerigars. Nature 1961, 192, 369–370. [Google Scholar] [CrossRef]
- Beaufrère, H.; Holder, K.A.; Bauer, R.; Schnellbacher, R.; Pariaut, R.; Tully, T.N., Jr.; Wakamatsu, N. Intermittent claudication-like syndrome secondary to atherosclerosis in a yellow-naped Amazon parrot (Amazona ochrocephala auropalliata). J. Avian Med. Surg. 2011, 25, 266–276. [Google Scholar] [CrossRef]
- Grosset, C.; Guzman, D.S.; Keating, M.K.; Gaffney, P.M.; Lowenstine, L.; Zwingenberger, A.; Young, A.C.; Vernau, K.M.; Sokoloff, A.M.; Hawkins, M.G. Central vestibular disease in a blue and gold macaw (Ara ararauna) with cerebral infarction and hemorrhage. J. Avian Med. Surg. 2014, 28, 132–142. [Google Scholar] [CrossRef]
- Chalana, R.K.; Guraya, S.S. Seasonal fluctuation of cholesterol in ovarian compartments of birds. Ann. Biol. Anim. Biochim. Biophys. 1977, 17, 1005–1011. [Google Scholar] [CrossRef]
- Beaufrère, H. Blood Lipid Diagnostics in Psittacine Birds. Vet. Clin. North. Am. Exot. Anim. Pract. 2022, 25, 697–712. [Google Scholar] [CrossRef]
- Gustavsen, K.A.; Stanhope, K.L.; Lin, A.S.; Graham, J.L.; Havel, P.J.; Paul-Murphy, J.R. Effects of exercise on the plasma lipid profile in hispaniolan amazon parrots (Amazona ventralis) with naturally occurring hypercholesterolemia. J. Zoo. Wildl. Med. 2016, 47, 760–769. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein metabolism in poultry. World’s Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Tichý, L.; Freiberger, T.; Blaha, V.; Satny, M.; Hubacek, J.A. Genetics of Familial Hypercholesterolemia: New Insights. Front. Genet. 2020, 11, 574474. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M. Significance of cholesterol assays in the investigation of hepatic lipidosis and atherosclerosis in psittacine birds. Exotic DVM 2005, 7, 28. [Google Scholar]
- Berneis, K.K.; Krauss, R.M. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 2002, 43, 1363–1379. [Google Scholar] [CrossRef]
- Divers, S. Avian Diagnostic Endoscopy. Vet. Clin. North Am. Exot. Anim. Pract. 2010, 13, 187–202. [Google Scholar] [CrossRef]
- Lierz, M. Diagnostic value of endoscopy and biopsy. In Clinical Avian Medicine; Harrison, G.J., Lightfoot, T., Eds.; Spix Publishing: Palm Beach, FL, USA, 2006; Volume 2, pp. 665–681. [Google Scholar]


| Genus | Sample Size | RI Method 1 | RI 2 | Median | CI 3 Lower RI | CI 3 Upper RI | Min 4 | Max 4 |
|---|---|---|---|---|---|---|---|---|
| Amazona | 144 | n | [2.53, 6.36] | 3.70 | [2.32, 2.65] | [6.01, 6.78] | 2.41 | 6.99 |
| Psittacus | 122 | n | [2.93, 5.28] | 3.89 | [2.81, 2.97] | [4.93, 5.50] | 2.90 | 5.65 |
| Ara and Anodorhynchus | 64 | r | [1.53, 3.97] | 2.81 | [1.30, 1.72] | [3.78, 4.18] | 1.53 | 3.76 |
| Cacatuidae genera | 24 | r | [1.39, 5.67] | 3.36 | [0.67, 1.96] | [5.05, 6.50] | 1.89 | 5.96 |
| Genus | Sample Size | RI Method 1 | RI 1 | Median | CI 1 Lower RI | CI 1 Upper RI | Min 1 | Max 1 |
|---|---|---|---|---|---|---|---|---|
| Amazona | 144 | n | [1.19, 7.99] | 2.86 | [1.06, 1.28] | [6.86, 9.52] | 1.09 | 10.54 |
| Psittacus | 122 | n | [1.54, 5.70] | 3.37 | [1.22, 1.66] | [5.43, 6.24] | 1.42 | 5.96 |
| Ara and Anodorhynchus | 58 | r | [0.46, 3.29] | 2.00 | [0.19, 0.67] | [3.08, 3.55] | 0.67 | 3.21 |
| Cacatuidae genera | 24 | r | [1.03, 5.26] | 3.12 | [0.44, 1.52] | [4.68, 5.88] | 1.42 | 5.34 |
| Genus | Sample Size | RI Method 1 | RI 1 | Median | CI 1 Lower RI | CI 1 Upper RI | Min 1 | Max 1 |
|---|---|---|---|---|---|---|---|---|
| Amazona | 143 | n | [0.93, 9.52] | 2.48 | [0.77, 1.17] | [6.40, 12.21] | 0.52 | 15.61 |
| Psittacus | 122 | n | [1.51, 6.27] | 2.87 | [1.21, 1.57] | [3.64, 8.01] | 1.45 | 9.09 |
| Ara and Anodorhynchus | 62 | r | [−0.02, 2.86] | 1.52 | [−0.35, 0.28] | [2.55, 3.19] | 0.34 | 4.07 |
| Cacatuidae genera | 24 | r | [0.26, 3.96] | 2.11 | [−0.21, 0.75] | [3.32, 4.57] | 1.09 | 3.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janeczek, M.; Korbel, R.; Janeczek, F.; Alber, H.; Küchenhoff, H.; Rinder, M. Serum Lipid Reference Intervals of High-Density, Low-Density and Non-High-Density Lipoprotein Cholesterols and Their Association with Atherosclerosis and Other Factors in Psittaciformes. Animals 2025, 15, 2493. https://doi.org/10.3390/ani15172493
Janeczek M, Korbel R, Janeczek F, Alber H, Küchenhoff H, Rinder M. Serum Lipid Reference Intervals of High-Density, Low-Density and Non-High-Density Lipoprotein Cholesterols and Their Association with Atherosclerosis and Other Factors in Psittaciformes. Animals. 2025; 15(17):2493. https://doi.org/10.3390/ani15172493
Chicago/Turabian StyleJaneczek, Matthias, Rüdiger Korbel, Friedrich Janeczek, Helen Alber, Helmut Küchenhoff, and Monika Rinder. 2025. "Serum Lipid Reference Intervals of High-Density, Low-Density and Non-High-Density Lipoprotein Cholesterols and Their Association with Atherosclerosis and Other Factors in Psittaciformes" Animals 15, no. 17: 2493. https://doi.org/10.3390/ani15172493
APA StyleJaneczek, M., Korbel, R., Janeczek, F., Alber, H., Küchenhoff, H., & Rinder, M. (2025). Serum Lipid Reference Intervals of High-Density, Low-Density and Non-High-Density Lipoprotein Cholesterols and Their Association with Atherosclerosis and Other Factors in Psittaciformes. Animals, 15(17), 2493. https://doi.org/10.3390/ani15172493





