Allium mongolicum Regel Enhances Serum Immunity, Antioxidant, and Biochemical Indicators of Meat Sheep Achieved by Rumen Microbiota Regulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
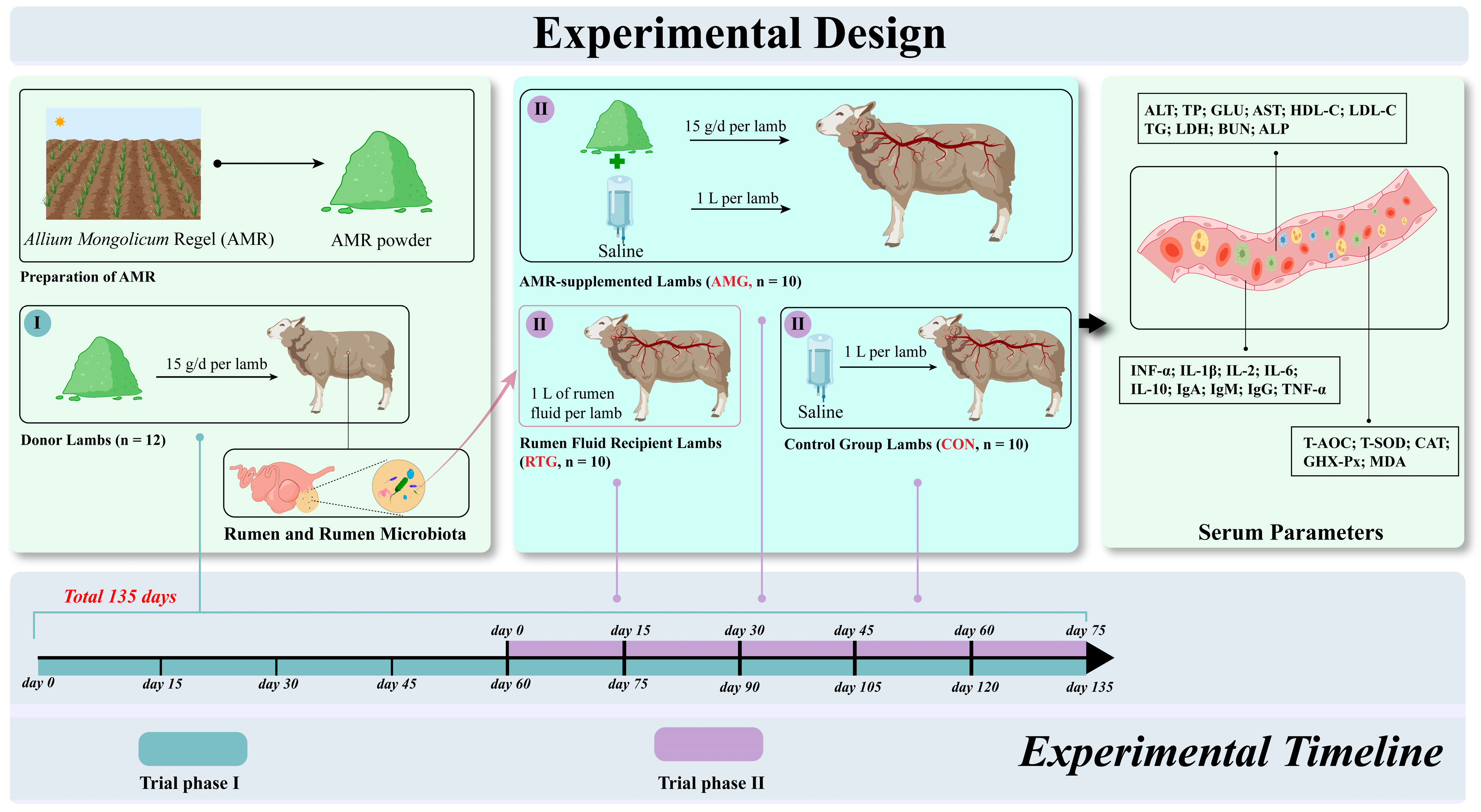
2.2. Preparation of AMR Powder
2.3. Experimental Design and Animal Management
2.3.1. Animal Rearing
2.3.2. Transplantation Procedure
2.4. Assessment of Growth Performance
2.5. Chemical Analysis of Feed Samples
2.6. Sample Acquisition and Processing
2.7. Serum Indicator Measurements
2.8. Microbial DNA Extraction and 16S rRNA Amplicon Sequencing
2.9. Data Analysis
3. Results
3.1. Growth Performance
3.2. Serum Immune Indicators
3.3. Serum Antioxidant Indicators
3.4. Serum Biochemical Indicators
3.5. Venn Map
3.6. Microbial Diversity Analysis
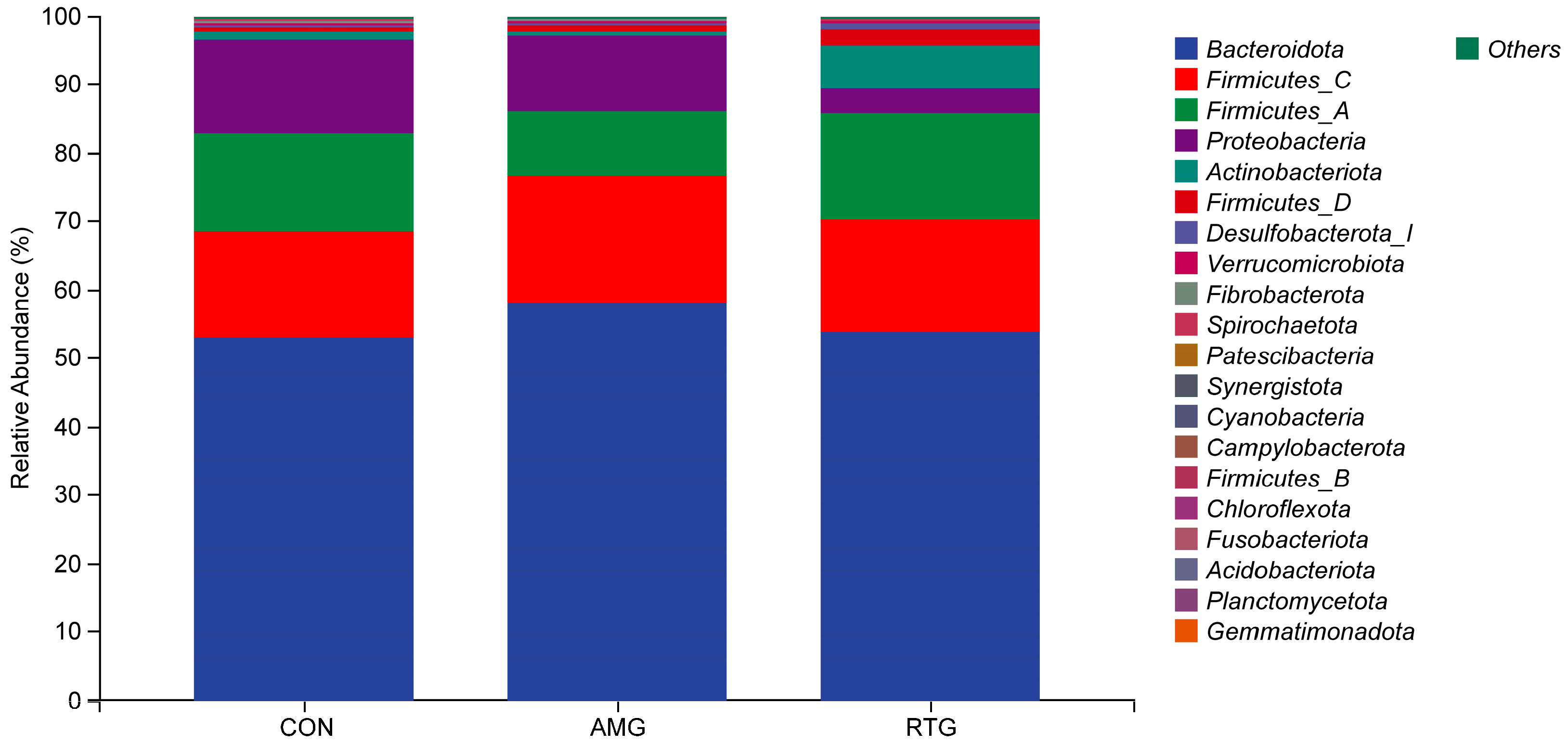
3.6.1. Multi-Sample Species Classification
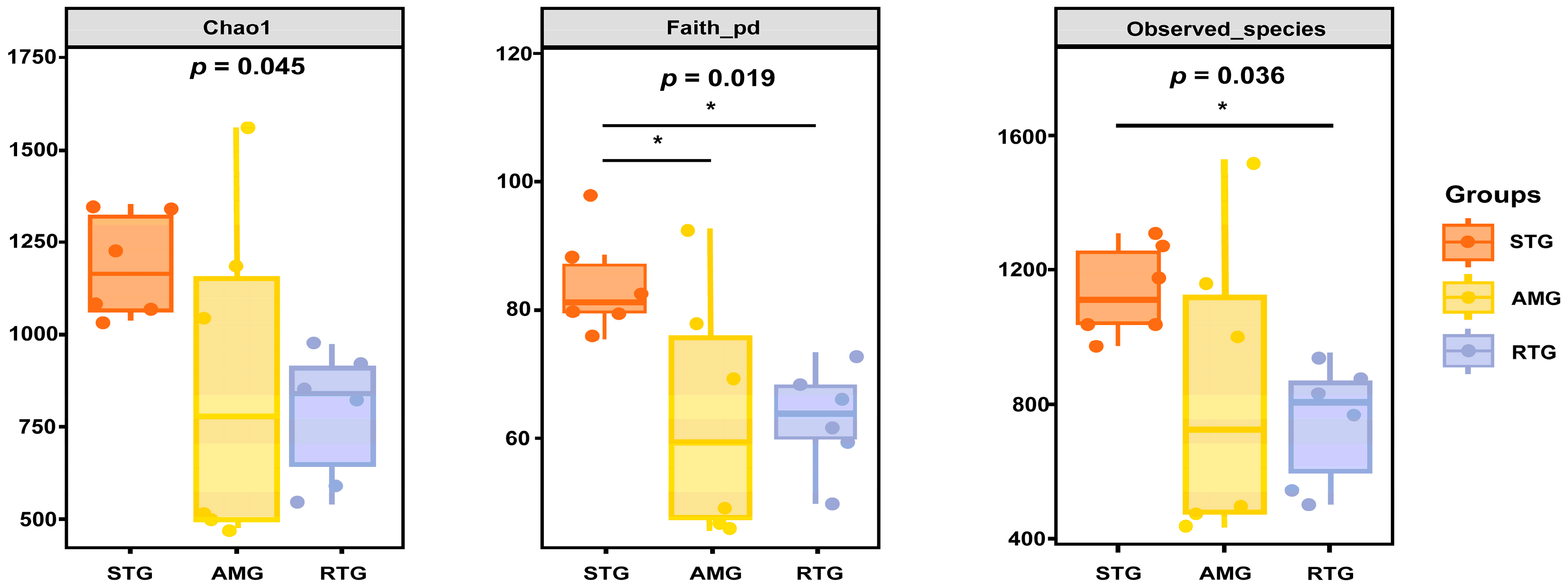
3.6.2. Alpha Diversity
3.6.3. Beta Diversity
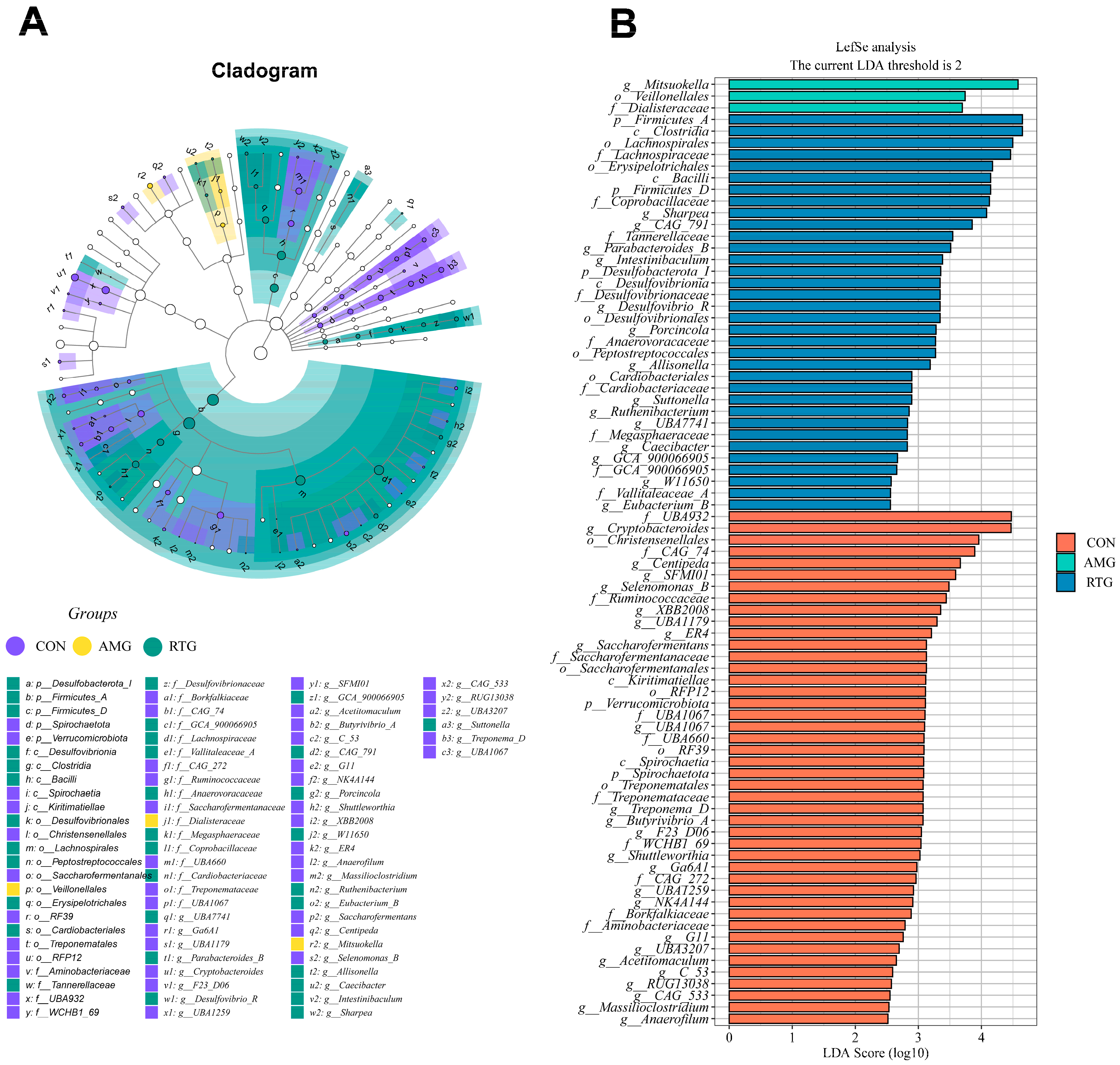
3.6.4. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
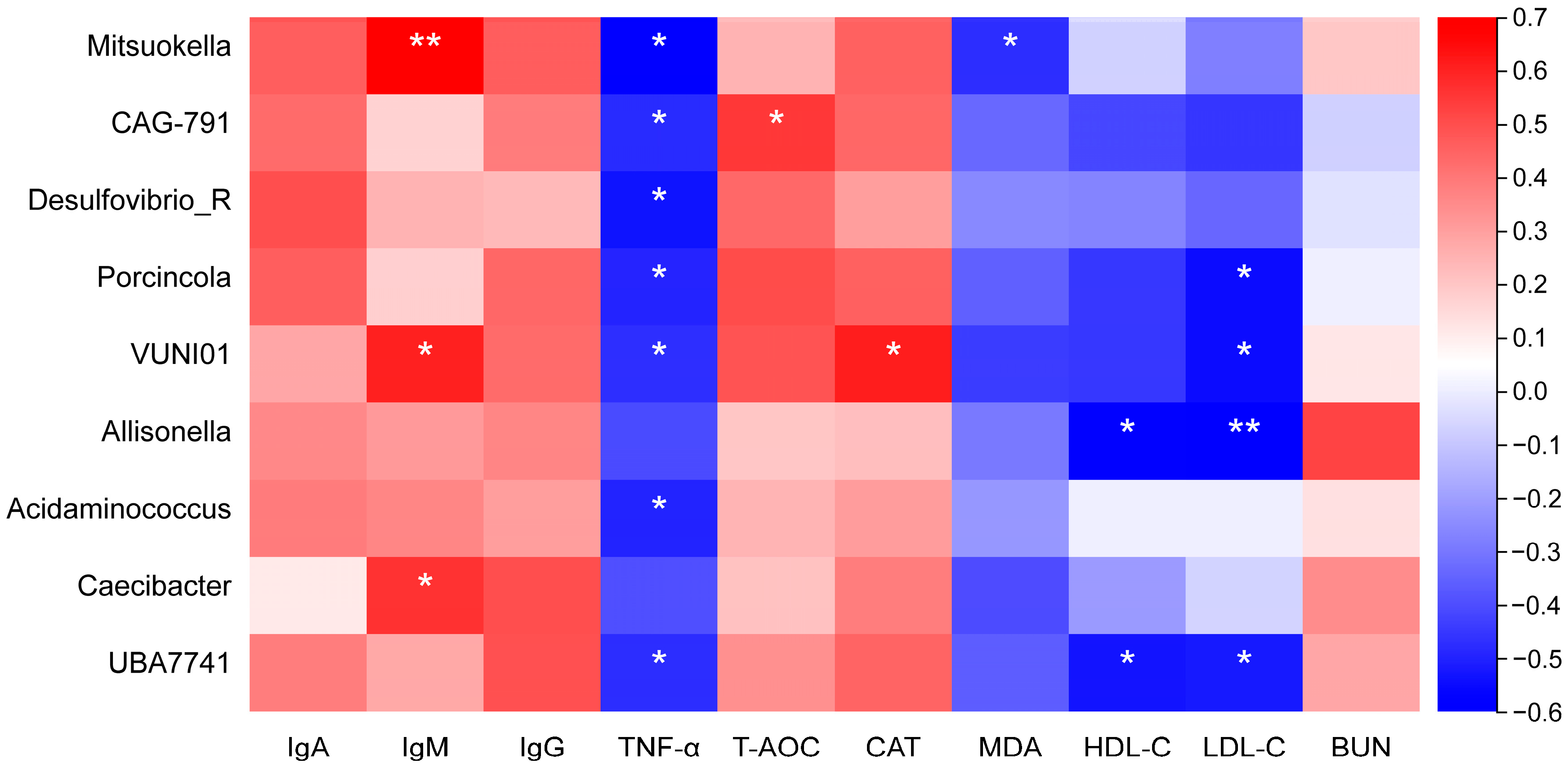
3.7. Correlation Analysis Between Ruminal Bacteria and Serum Biochemical Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Status
Acknowledgments
Conflicts of Interest
References
- Frankič, T.; Levart, A.; Salobir, J. The effect of vitamin E and plant extract mixture composed of carvacrol, cinnamaldehyde and capsaicin on oxidative stress induced by high PUFA load in young pigs. Animal 2010, 4, 572–578. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Tran, C.; Horyanto, D.; Stanley, D.; Cock, I.E.; Chen, X.J.; Feng, Y.J. Antimicrobial Properties of Bacillus Probiotics as Animal Growth Promoters. Antibiotics 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, M.M.; EI-Nomeary, Y.A.A.E.; Salman, F.M.; Shakweer, W.M.E. Improving the productive performance of growing lambs using prebiotic and probiotic as growth promoters. Trop. Anim. Health Prod. 2023, 55, 375. [Google Scholar] [CrossRef]
- Soni, R.; Keharia, H.; Bose, A.; Pandit, N.; Doshi, J.; Rao, S.V.R.; Paul, S.S.; Raju, M.V.L.N. Genome assisted probiotic characterization and application of Bacillus velezensis ZBG17 as an alternative to antibiotic growth promoters in broiler chickens. Genomics 2021, 113, 4061–4074. [Google Scholar] [CrossRef]
- Zhu, Q.D.; Sun, P.; Zhang, B.K.; Kong, L.L.; Xiao, C.P.; Song, Z.G. Progress on Gut Health Maintenance and Antibiotic Alternatives in Broiler Chicken Production. Front. Nutr. 2021, 8, 692839. [Google Scholar] [CrossRef]
- Alem, W.T. Effect of herbal extracts in animal nutrition as feed additives. Heliyon 2024, 10, e24973. [Google Scholar] [CrossRef]
- Piao, M.Y.; Tu, Y.; Zhang, N.F.; Diao, Q.Y.; Bi, Y.L. Advances in the application of phytogenic extracts as antioxidants and their potential mechanisms in ruminants. Antioxidants 2023, 12, 879. [Google Scholar] [CrossRef]
- Liu, W.J.; Yu, A.H.; Xie, Y.D.; Sun, C.X.; Gao, H.X.; He, J.J.; Ao, C.J.; Tang, D.F. Drying enhances the antioxidant activity of Allium mongolicum Regel through the phenylpropane and AA-MA pathway as shown by metabolomics. Food Chem. X 2024, 22, 101436. [Google Scholar] [CrossRef]
- Jiang, B.W.; Zhou, Y.X.; Wang, T.; Li, F. Nutritive value and ruminal degradation of seven Chinese herbs as forage for Tan sheep. Bioengineered 2020, 11, 1159–1169. [Google Scholar] [CrossRef]
- Zhang, X.H.; Sun, H.W.; Song, S.Z.; Li, Y.Y.; Zhang, X.L.; Zhang, W.T. Preparation and characterization of polyvinyl alcohol/pullulan/ZnO-Nps composite film and its effect on the postharvest quality of Allium mongolicum Regel. Int. J. Biol. Macromol. 2024, 279, 135380. [Google Scholar] [CrossRef]
- Liu, W.J.; Tang, D.F.; Ao, C.J. Adding of Allium mongolicum regel extracts to lamb feedlot diets influences 4-alkyl-branched fatty acids deposition and the meat quality during storage. Meat Sci. 2022, 193, 108951. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Zhang, Y.M.; Khas, E.; Bai, C.; Cao, Q.N.; Ao, C.J. Transcriptome analysis reveals candidate genes of the synthesis of branched-chain fatty acids related to mutton flavor in the lamb liver using Allium mongolicum Regel extract. J. Anim. Sci. 2022, 100, skac256. [Google Scholar] [CrossRef]
- Muqier; Chen, L.X.; Ao, C.J.; Erdene, K.; Bai, C.; Cao, Q.N.; Zheng, Y.K.; Hasi, J.; Bao, Y.T.; Sa, R.L. Effects of Allium mongolicum Regel Flavonoids on Immune Function of Mutton Sheep. Anim. Nutr. 2023, 35, 3943–3954. [Google Scholar] [CrossRef]
- Muqier; Qi, S.; Wang, T.; Chen, R.; Wang, C.; Ao, C. Effects of flavonoids from Allium mongolicum Regel on growth performance and growth-related hormones in meat sheep. Anim. Nutr. 2017, 3, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Erdene, K.; Chen, S.; Qi, S.; Bao, Z.; Zhao, Y.; Wang, C.; Zhao, G.; Ao, C. Correlation of the rumen fluid microbiome and the average daily gain with a dietary supplementation of Allium mongolicum Regel extracts in sheep. J. Anim. Sci. 2019, 97, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, C.; Erdene, K.; Umair, A.M.; Cao, Q.; Ao, C.; Jiang, L. Potential modulating effects of Allium mongolicum regel ethanol extract on rumen fermentation and biohydrogenation bacteria of dairy cows in vitro. Front. Microbiol. 2023, 14, 1272691. [Google Scholar] [CrossRef]
- Wang, B.; Luo, H.L. Effects of mulberry leaf silage on antioxidant and immunomodulatory activity and rumen bacterial community of lambs. BMC Microbiol. 2021, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Su, D.Y.; Song, L.J.; Dong, Q.; Zhang, A.; Lu, Z.; Wang, Y.N.; Feng, M.; Li, X.M.; Li, F.; Sun, X.S.; et al. Effects of herbal formula on growth performance, apparent digestibility, antioxidant capacity, and rumen microbiome in fattening lambs under heat stress. Environ. Sci. Pollut. Res. Int. 2024, 31, 51364–51380. [Google Scholar] [CrossRef]
- Liu, W.J.; Ao, C.J. Effect of dietary supplementation with Allium mongolicum Regel extracts on growth performance, carcass characteristics, and the fat color and flavor-related branched-chain fatty acids concentration in ram lambs. Anim. Biosci. 2020, 34, 134. [Google Scholar] [CrossRef]
- Liu, W.J.; Ding, H.; Li, S.Y.; Zhang, Z.Z.; Ao, C.J.; Li, Y. Effects of Allium mongolicum Regel Powder or Probiotic Complex Preparation on Fatty Acid and Volatile Flavor Compound Composition in Longissimus Dorsi Muscle of Dorper × Thin-Tailed Han Crossbred Mutton Lambs. Anim. Nutr. 2019, 31, 4349–4362. [Google Scholar]
- Liu, W.J.; Gao, H.X.; He, J.J.; Yu, A.H.; Sun, C.X.; Xie, Y.D.; Yao, H.B.; Wang, H.; Duan, Y.Y.; Hu, J.S.; et al. Effects of dietary Allium mongolicum Regel powder supplementation on the growth performance, meat quality, antioxidant capacity and muscle fibre characteristics of fattening Angus calves under heat stress conditions. Food Chem. 2024, 453, 139539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Li, H.W.; Zhu, W.Y.; Mao, S.Y. Dynamic changes in rumen fermentation and bacterial community following rumen fluid transplantation in a sheep model of rumen acidosis: Implications for rumen health in ruminants. FASEB J. 2019, 33, 8453–8467. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 18th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Hu, J.M.; Zhang, S.; Li, M.M.; Zhao, G.Y. Impact of dietary supplementation with β-alanine on the rumen microbial crude protein supply, nutrient digestibility and nitrogen retention in beef steers elucidated through sequencing the rumen bacterial community. Anim. Nutr. 2024, 17, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.B.; Qiao, Q.Q.; Gao, Y.; Hou, J.X.; Hu, M.Y.; Du, Y.F.; Zhao, K.; Li, X. Gut microbiota and their role in health and metabolic disease of dairy cow. Front. Nutr. 2021, 8, 701511. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Guan, L.L.; Liu, J.X. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J. Dairy. Sci. 2019, 102, 5031–5041. [Google Scholar] [CrossRef]
- Ma, P.; Hong, Y.F.; Liu, C.X.; Sun, Y.Q.; Liu, M.Z.; Yang, Z.G.; Ma, P.Y.; Wu, H.X.; Xue, F.G. Rumen microbiota responses to the enzymatic hydrolyzed cottonseed peptide supplement under high-concentrate diet feeding process. Front. Vet. Sci. 2022, 9, 984634. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Xu, Z.H.; Shen, Z.M.; Tian, Y.C.; Shen, H. Dietary Energy Level Promotes Rumen Microbial Protein Synthesis by Improving the Energy Productivity of the Ruminal Microbiome. Front. Microbiol. 2019, 10, 847. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Yu, C.H.; Zhou, Q.; Li, H.; Zhang, R.; Tang, J.Y.; Zhang, Z.M.; Luo, Z.; Jiang, X.M.; et al. Dietary supplementation with Bacillus subtilis PB6 alleviates diarrhea and improves growth performance and immune function in weaned piglets fed a high-protein diet. Front. Vet. Sci. 2025, 12, 1525354. [Google Scholar] [CrossRef]
- de Melo, H.S.A.; Ítavo, L.C.V.; de Castro, A.P.; Ítavo, C.C.B.F.; Caldas, R.d.A.; Mateus, R.G.; Niwa, M.V.G.; de Moraes, G.J.; Zornitta, C.d.S.; Gurgel, A.L.C.; et al. Effect of whole oilseeds in the diet on bacterial diversity in the solid fraction of the ruminal content of steers. Trop. Anim. Health Prod. 2023, 55, 32. [Google Scholar] [CrossRef]
- de Melo, H.S.A.; Ítavo, L.C.V.; de Castro, A.P.; Ítavo, C.C.B.F.; Caldas, R.d.A.; Mateus, R.G.; Niwa, M.V.G.; de Moraes, G.J.; Zornitta, C.d.S.; Gurgel, A.L.C.; et al. Bacterial species in the ruminal content of steers fed oilseeds in the diet. Trop. Anim. Health Prod. 2022, 54, 396. [Google Scholar] [CrossRef]
- Li, Z.P.; Mu, C.L.; Xu, Y.X.; Shen, J.S.; Zhu, W.Y. Changes in the solid-, liquid-, and epithelium-associated bacterial communities in the rumen of hu lambs in response to dietary urea supplementation. Front. Microbiol. 2020, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Redoy, M.R.A.; Shuvo, A.A.S.; Cheng, L.; AI-Mamun, M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal 2020, 14, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Makker, H.P.S.; Mele, M.; Priolo, A. Ruminal biohydrogenation as affected by tannins in vitro. Br. J. Nutr. 2009, 102, 82–92. [Google Scholar] [CrossRef]
- Chai, J.; Weiss, C.P.; Beck, P.A.; Zhao, W.; Li, Y.; Zhao, J.C. Diet and monensin influence the temporal dynamics of the rumen microbiome in stocker and finishing cattle. J. Anim. Sci. Biotechnol. 2024, 15, 12. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, L.Y.; Li, Y.D.; Wu, H.H.; Lu, L.P.; Zang, R.X.; Xu, H.W. Effects of tail vegetable fermented feed on the growth and rumen microbiota of lambs. Animals 2024, 14, 303. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet—Induced Metabolic Syndrome. Diabetes. 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.P.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z.P. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutritients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Li, Y.J.; Mao, K.; Zang, Y.T.; Lu, G.W.; Qiu, Q.H.; Ouyang, K.H.; Zhao, X.H.; Song, X.Z.; Xu, L.J.; Liang, H.; et al. Revealing the developmental characterization of rumen microbiome and its host in newly received cattle during receiving period contributes to formulating precise nutritional strategies. Microbiome 2023, 11, 238. [Google Scholar] [CrossRef]
- Wu, D.W.; He, X.M.; Lu, Y.; Gao, Z.D.; Chong, Y.Q.; Hong, J.Y.; Wu, J.; Deng, W.D.; Xi, D.M. Effects of different dietary combinations on blood biochemical indicators and rumen microbial ecology in wenshan cattle. Microorganisms 2024, 12, 2154. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Khas, E.; Ao, C.; Bai, C. Effects of Allium mongolicum Regel ethanol extract on three flavor-related rumen branched-chain fatty acids, rumen fermentation and rumen bacteria in lambs. Front. Microbiol. 2022, 13, 978057. [Google Scholar] [CrossRef]
- Guo, H.R.; Zhou, G.C.; Tian, G.J.; Liu, Y.Y.; Dong, N.; Li, L.F.; Zhang, S.J.; Chai, H.C.; Chen, Y.L.; Yang, Y.X. Changes in rumen microbiota affect metabolites, immune responses and antioxidant enzyme activities of sheep under cold stimulation. Animals 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.; Zhang, S.F.; Zhong, R.Q.; Su, D.; Xia, B.; Liu, L.; Chen, L.; Zhang, H.F. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl. Microbiol. Biotechnol. 2021, 105, 8441–8456. [Google Scholar] [CrossRef]
- Leffler, J.; Trend, S.; Ward, N.C.; Grau, G.E.; Hawke, S.; Byrne, S.N.; Kermode, A.G.; French, M.A.; Hart, P.H. Circulating Memory B Cells in Early Multiple Sclerosis Exhibit Increased IgA+ Cells, Globally Decreased BAFF-R Expression and an EBV-Related IgM+ Cell Signature. Front. Immunol. 2022, 13, 812317. [Google Scholar] [CrossRef]
- Koike, S.; Ueno, M.; Miura, H.; Saegusa, A.; Inouchi, K.; Inabu, Y.; Sugino, T.; Guan, L.L.; Oba, M.; Kobayashi, Y. Rumen microbiota and its relation to fermentation in lactose-fed calves. J. Dairy. Sci. 2021, 104, 10744–10752. [Google Scholar] [CrossRef]
- López-García, A.; Saborío-Montero, A.; Gutiérrez-Rivas, M.; Atxaerandio, R.; Goiri, I.; García-Rodríguez, A.; Jiménez-Montero, J.A.; González, C.; Tamames, J.; Puente-Sánchez, F.; et al. Fungal and ciliate protozoa are the main rumen microbes associated with methane emissions in dairy cattle. Gigascience 2022, 11, giab088. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 827, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.L.; Ji, W.W.; Yun, Y.; Zhang, Y.F.; Li, Z.F.; Wang, C. Influence of probiotic supplementation on the growth performance, plasma variables, and ruminal bacterial community of growth-retarded lamb. Front. Microbiol. 2023, 14, 1216534. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gloria, J.L.; Martínez-Olivares, C.E.; Rojas-Morales, P.; Hernández-Pando, R.; Carbó, R.; Rubio-Gayosso, I.; Arellano-Buendía, A.S.; Rada, K.M.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Anti-Inflammatory effect of allicin associated with fibrosis in pulmonary arterial hypertension. Int. J. Mol. Sci. 2021, 22, 8600. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, W.A. Potential effects of garlic (Allium sativum L.) on the performance, immunity, gut health, anti-oxidant status, blood parameters, and intestinal microbiota of poultry: An updated comprehensive review. Animals 2024, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- ElKatcha, M.I.; Soltan, M.; Essi, M.S. Effect of garlic extract supplementation on growth performance, nutrient digestibility and some blood serum biochemical changes of fattening lambs. Alex. J. Vet. Sci. 2016, 48, 124. [Google Scholar] [CrossRef]
- Xie, K.L.; Wang, Z.F.; Wang, Y.J.; Wang, C.M.; Chang, S.H.; Zhang, C.; Zhu, W.H.; Hou, F.J. Effects of Allium mongolicum Regel supplementation on the digestibility, methane production, and antioxidant capacity of Simmental calves in northwest China. Anim. Sci. J. 2020, 91, e13392. [Google Scholar] [CrossRef]
- Takegami, M.; Watanabe, M.; Higashiyama, A.; Tatsumi, Y.; Nakai, M.; Nakao, Y.M.; Nishimura, K.; Kokubo, Y.; Miyamoto, Y. Usefulness of LDL-C to HDL-C ratio in assessing risk of cardiovascular diseases: The suita study: Misa takegami. Eur. J. Public Health. 2015, 25, ckv175.108. [Google Scholar] [CrossRef]
- Schoch, L.; Sutelman, P.; Suades, R.; Casani, L.; Padro, T.; Badimon, L.; Vilahur, G. Hypercholesterolemia-Induced HDL dysfunction can be reversed: The impact of diet and statin treatment in a preclinical animal model. Int. J. Mol. Sci. 2022, 23, 8596. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef]
- Xue, H.L.; Chen, X.; Yu, C.; Deng, Y.Q.; Zhang, Y.; Chen, S.; Chen, X.C.; Chen, K.; Yang, Y.; Ling, W.H. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Ciri Res. 2022, 131, 404–420. [Google Scholar] [CrossRef]
- Lv, O.; Wang, L.F.; Li, J.K.; Ma, Q.Q.; Zhao, W. Effects of pomegranate peel polyphenols on lipid accumulation and cholesterol metabolic transformation in L-02 human hepatic cells via the PPARγ-ABCA1/CYP7A1 pathway. Food Funct. 2016, 7, 4976–4983. [Google Scholar] [CrossRef]
- Ohara, K.; Wakabayashi, H.; Taniguchi, Y.; Shido, K.; Yajima, H.; Yoshido, A. Quercetin-3-O-glucuronide induces ABCA1 expression by LXRα activation in murine macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 929–934. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Wang, Z.Z.; Ma, Y.; Qu, Y.H.; Lu, X.N.; Luo, H.L. Effects of dietary lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot bamei lamb. Asian-Australas. J. Anim. Sci. 2015, 28, 958–965. [Google Scholar] [CrossRef]
- Sirichaiwetchakoon, K.; Eumkeb, G. Free radical scavenging and anti-isolated human LDL oxidation activities of Butea superba Roxb. extract. BMC Complement. Med. Ther. 2024, 24, 75. [Google Scholar] [CrossRef]
- Osorio-Olivares, M.E.; Vásquez-Martínez, Y.; Díaz, K.; Canelo, J.; Taborga, L.; Espinoza-Catalán, L. Antibacterial and Antioxidant Activity of Synthetic Polyoxygenated Flavonoids. Int. J. Mol. Sci. 2024, 25, 5999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Zhang, Y.C. Multiple free radical scavenging reactions of aurones. Phytochemistry 2021, 190, 112853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.J.; Li, Z.W.; Moalin, M.; Hartog, G.J.M.D.; Zhang, M. Computational Chemistry Strategies to Investigate the Antioxidant Activity of Flavonoids-An Overview. Molecules 2024, 29, 2627. [Google Scholar] [CrossRef] [PubMed]



| Item | Content |
|---|---|
| CP | 32.55 |
| Ether extract | 6.25 |
| NDF | 17.55 |
| ADF | 15.87 |
| Ca | 0.97 |
| P | 0.58 |
| Item | Content |
|---|---|
| Ingredients (%) | |
| Corn silage | 19.67 |
| Alfalfa meal | 16.35 |
| Corn stalk | 10.01 |
| Wheat bran | 7.30 |
| Corn | 25.20 |
| Soybean meal | 14.53 |
| Extruded soybeans | 1.83 |
| Premixes 1 | 2.48 |
| NaCl | 1.50 |
| Limestone | 1.13 |
| Total | 100.00 |
| Nutrients levels | |
| Metabolic energy (MJ/kg) | 11.82 |
| CP (%) | 14.50 |
| Ether extract (%) | 2.50 |
| NDF (%) | 34.60 |
| ADF (%) | 16.43 |
| OM (%) | 15.00 |
| Calcium (%) | 2.00 |
| Phosphorus (%) | 0.80 |
| Item 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AMG | RTG | |||
| Initial body weight, kg | 22.47 | 22.53 | 24.18 | 0.65 | 0.128 |
| Final body weight, kg | 44.78 b | 48.33 a | 49.87 a | 0.69 | 0.005 |
| Dry matter intake, kg/d | 1.61 | 1.56 | 1.58 | 0.05 | 0.702 |
| ADG, kg/d | 0.34 b | 0.42 a | 0.45 a | 0.01 | <0.001 |
| F/G | 4.91 | 4.66 | 5.41 | 0.17 | 0.163 |
| Item 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AMG | RTG | |||
| Day 0 | |||||
| IFN-γ (pg/mL) | 54.43 | 56.69 | 57.03 | 1.52 | 0.465 |
| IL-1β (pg/mL) | 138.66 | 122.33 | 122.63 | 7.21 | 0.259 |
| IL-2 (pg/mL) | 148.29 | 138.98 | 140.47 | 4.67 | 0.384 |
| IL-6 (pg/mL) | 180.71 | 164.01 | 162.76 | 7.59 | 0.249 |
| IL-10 (pg/mL) | 223.28 | 242.04 | 247.44 | 7.41 | 0.132 |
| IgA (mg/dL) | 30.21 | 31.70 | 30.86 | 0.55 | 0.238 |
| IgM (mg/dL) | 13.41 | 13.74 | 13.86 | 0.20 | 0.317 |
| IgG (mg/dL) | 1319.11 | 1374.69 | 1365.56 | 18.37 | 0.217 |
| TNF-α (IU/mL) | 1093.81 | 1070.81 | 1086.98 | 23.73 | 0.788 |
| Day 30 | |||||
| IFN-γ (pg/mL) | 57.73 | 60.77 | 59.73 | 1.02 | 0.180 |
| IL-1β (pg/mL) | 127.83 | 111.81 | 112.33 | 4.84 | 0.096 |
| IL-2 (pg/mL) | 135.34 | 129.88 | 130.02 | 2.42 | 0.268 |
| IL-6 (pg/mL) | 165.51 | 155.91 | 157.84 | 4.08 | 0.286 |
| IL-10 (pg/mL) | 247.55 b | 260.92 a | 263.66 a | 3.62 | 0.041 |
| IgA (mg/dL) | 29.53 b | 34.09 a | 33.20 a | 0.49 | <0.001 |
| IgM (mg/dL) | 13.47 b | 14.73 a | 15.11 a | 0.24 | <0.001 |
| IgG (mg/dL) | 1358.16 b | 1423.79 a | 1451.72 a | 22.97 | 0.027 |
| TNF-α (IU/mL) | 1059.23 | 1048.57 | 1044.43 | 7.59 | 0.418 |
| Day 60 | |||||
| IFN-γ (pg/mL) | 57.46 | 55.33 | 56.94 | 0.83 | 0.247 |
| IL-1β (pg/mL) | 130.10 | 130.86 | 130.44 | 0.98 | 0.863 |
| IL-2 (pg/mL) | 136.98 | 137.23 | 131.98 | 1.57 | 0.096 |
| IL-6 (pg/mL) | 180.42 | 179.20 | 178.73 | 1.71 | 0.779 |
| IL-10 (pg/mL) | 241.94 | 245.78 | 246.44 | 1.55 | 0.165 |
| IgA (mg/dL) | 30.23 b | 33.62 a | 33.73 a | 0.46 | <0.001 |
| IgM (mg/dL) | 13.49 b | 14.86 a | 14.65 a | 0.25 | 0.003 |
| IgG (mg/dL) | 1334.35 b | 1503.18 a | 1501.36 a | 21.31 | <0.001 |
| TNF-α (IU/mL) | 1123.64 a | 954.89 b | 934.18 b | 13.82 | <0.001 |
| Item 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AMG | RTG | |||
| Day 0 | |||||
| T-AOC (U/mL) | 9.81 | 10.35 | 10.27 | 0.35 | 0.521 |
| T-SOD (pg/mL) | 279.41 | 295.18 | 295.14 | 7.21 | 0.276 |
| CAT (ng/L) | 261.10 | 289.42 | 287.82 | 8.96 | 0.121 |
| GSH-Px (pmol/mL) | 73.35 | 80.55 | 79.49 | 3.00 | 0.258 |
| MDA (nmol/mL) | 13.74 | 12.51 | 13.11 | 0.44 | 0.220 |
| Day 30 | |||||
| T-AOC (U/mL) | 10.39 | 10.65 | 10.87 | 0.18 | 0.254 |
| T-SOD (pg/mL) | 313.53 | 320.80 | 318.66 | 5.49 | 0.691 |
| CAT (ng/L) | 286.86 | 302.34 | 302.38 | 5.46 | 0.146 |
| GSH-Px (pmol/mL) | 81.99 | 85.71 | 85.49 | 1.33 | 0.165 |
| MDA (nmol/mL) | 13.78 a | 11.23 b | 10.90 b | 0.53 | <0.001 |
| Day 60 | |||||
| T-AOC (U/mL) | 9.90 b | 10.70 a | 11.04 a | 0.13 | <0.001 |
| T-SOD (pg/mL) | 290.68 | 292.44 | 294.35 | 4.08 | 0.819 |
| CAT (ng/L) | 265.35 b | 282.10 a | 285.02 a | 2.72 | <0.001 |
| GSH-Px (pmol/mL) | 76.39 | 77.89 | 79.91 | 1.02 | 0.082 |
| MDA (nmol/mL) | 14.21 a | 11.18 b | 10.69 b | 0.24 | <0.001 |
| Item 1 | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | AMG | RTG | |||
| Day 0 | |||||
| GLU (mmol/L) | 3.83 | 3.60 | 3.86 | 0.24 | 0.712 |
| TP (g/L) | 53.11 | 53.06 | 50.85 | 1.35 | 0.449 |
| ALB (g/L) | 26.05 | 26.36 | 25.03 | 1.11 | 0.691 |
| ALT (U/L) | 12.49 | 12.59 | 11.16 | 1.55 | 0.784 |
| AST (U/L) | 100.63 | 104.96 | 110.53 | 5.54 | 0.490 |
| ALP (U/L) | 318.51 | 354.08 | 316.40 | 29.89 | 0.627 |
| HDL-C (mmol/L) | 1.38 | 1.25 | 1.05 | 0.11 | 0.128 |
| LDL-C (mmol/L) | 0.64 | 0.43 | 0.46 | 0.09 | 0.215 |
| TG (mmol/L) | 0.48 | 0.38 | 0.36 | 0.04 | 0.162 |
| LDH (U/L) | 392.69 | 405.46 | 418.13 | 15.12 | 0.525 |
| BUN (mmol/L) | 4.80 | 5.69 | 5.97 | 0.49 | 0.246 |
| Day 30 | |||||
| GLU (mmol/L) | 3.83 | 3.60 | 3.89 | 0.18 | 0.482 |
| TP (g/L) | 45.68 | 44.94 | 46.62 | 2.67 | 0.907 |
| ALB (g/L) | 24.19 | 24.31 | 25.84 | 1.14 | 0.551 |
| ALT (U/L) | 10.25 | 10.25 | 10.83 | 1.22 | 0.928 |
| AST (U/L) | 91.56 | 88.16 | 86.89 | 5.92 | 0.851 |
| ALP (U/L) | 396.43 | 401.48 | 408.87 | 27.81 | 0.951 |
| HDL-C (mmol/L) | 1.15 | 1.02 | 1.18 | 0.08 | 0.337 |
| LDL-C (mmol/L) | 0.38 | 0.39 | 0.37 | 0.03 | 0.909 |
| TG (mmol/L) | 0.32 | 0.27 | 0.28 | 0.02 | 0.290 |
| LDH (U/L) | 466.21 | 452.09 | 467.83 | 29.05 | 0.916 |
| BUN (mmol/L) | 4.45 | 5.49 | 4.46 | 0.41 | 0.160 |
| Day 60 | |||||
| GLU (mmol/L) | 4.08 | 4.89 | 3.99 | 0.34 | 0.152 |
| TP (g/L) | 58.32 | 69.93 | 58.16 | 3.90 | 0.528 |
| ALB (g/L) | 29.31 | 30.79 | 28.95 | 1.59 | 0.700 |
| ALT (U/L) | 9.96 | 10.90 | 9.16 | 1.69 | 0.776 |
| AST (U/L) | 93.68 | 92.80 | 80.32 | 6.52 | 0.337 |
| ALP (U/L) | 443.78 | 400.97 | 417.82 | 41.21 | 0.769 |
| HDL-C (mmol/L) | 1.16 b | 1.50 a | 0.99 b | 0.11 | 0.013 |
| LDL-C (mmol/L) | 0.68 a | 0.47 b | 0.33 b | 0.05 | 0.010 |
| TG (mmol/L) | 0.37 | 0.37 | 0.36 | 0.02 | 0.900 |
| LDH (U/L) | 525.02 | 589.69 | 571.21 | 44.42 | 0.597 |
| BUN (mmol/L) | 6.41 a | 4.73 b | 3.91 b | 0.33 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Bai, C.; Erdene, K.; Zheng, Y.; Cao, Q.; Han, G.; Ao, C. Allium mongolicum Regel Enhances Serum Immunity, Antioxidant, and Biochemical Indicators of Meat Sheep Achieved by Rumen Microbiota Regulation. Animals 2025, 15, 2491. https://doi.org/10.3390/ani15172491
Wang X, Bai C, Erdene K, Zheng Y, Cao Q, Han G, Ao C. Allium mongolicum Regel Enhances Serum Immunity, Antioxidant, and Biochemical Indicators of Meat Sheep Achieved by Rumen Microbiota Regulation. Animals. 2025; 15(17):2491. https://doi.org/10.3390/ani15172491
Chicago/Turabian StyleWang, Xiaoyuan, Chen Bai, Khas Erdene, Yankai Zheng, Qina Cao, Guoli Han, and Changjin Ao. 2025. "Allium mongolicum Regel Enhances Serum Immunity, Antioxidant, and Biochemical Indicators of Meat Sheep Achieved by Rumen Microbiota Regulation" Animals 15, no. 17: 2491. https://doi.org/10.3390/ani15172491
APA StyleWang, X., Bai, C., Erdene, K., Zheng, Y., Cao, Q., Han, G., & Ao, C. (2025). Allium mongolicum Regel Enhances Serum Immunity, Antioxidant, and Biochemical Indicators of Meat Sheep Achieved by Rumen Microbiota Regulation. Animals, 15(17), 2491. https://doi.org/10.3390/ani15172491





