Identification of Patterns of Trace Mineral Deficiencies in Dairy and Beef Cattle Herds in Spain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Selection
2.2. Trace Mineral Analysis and Quality Assurance
2.3. Statistical Analysis
3. Results and Discussion
3.1. Trace Mineral Status in Cattle According to Production System
3.2. Farm-Level Trace Mineral Deficiency Patterns in Different Production Systems and Clinical Reason for Submission
3.3. Multivariate Analysis of Trace Mineral Profiles and Deficiency Patterns
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palomares, R.A. Trace Minerals Supplementation with Great Impact on Beef Cattle Immunity and Health. Animals 2022, 12, 2839. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N. Mineral Nutrition of Livestock, 5th ed.; CABI: Wallingford, UK, 2022. [Google Scholar]
- Corbett, R.B. Trace Mineral Nutrition in Confinement Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2023, 39, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; López-Alonso, M. Trace Minerals Imbalance in Cattle. In Encyclopedia of Livestock Medicine for Large Animal and Poultry Production; Simões, J., Ed.; Springer Nature: Cham, Switzerland, 2025; pp. 1–11. ISBN 978-3-031-52133-1. [Google Scholar]
- Spears, J.W.; Brandao, V.L.N.; Heldt, J. INVITED REVIEW: Assessing Trace Mineral Status in Ruminants, and Factors That Affect Measurements of Trace Mineral Status. Appl. Anim. Sci. 2022, 38, 252–267. [Google Scholar] [CrossRef]
- Arthington, J.D.; Ranches, J. Trace Mineral Nutrition of Grazing Beef Cattle. Animals 2021, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Sager, B.; Van Saun, R.J. Trace Mineral Supplementation for Beef Cows: Dry Range Environment. Vet. Clin. N. Am. Food Anim. Pract. 2023, 39, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Orjales, I.; Herrero-Latorre, C.; Miranda, M.; Rey-Crespo, F.; Rodríguez-Bermúdez, R.; López-Alonso, M. Evaluation of Trace Element Status of Organic Dairy Cattle. Animal 2018, 12, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Juszczak–Czasnojć, M.; Tomza–Marciniak, A. Ratio of Selenium Concentrations between Soil, Forage Plants and Blood Serum of Beef Cattle Studied in Organic and Conventional Farms. Arch. Anim. Nutr. 2021, 75, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Guyot, H.; Saegerman, C.; Lebreton, P.; Sandersen, C.; Rollin, F. Epidemiology of Trace Elements Deficiencies in Belgian Beef and Dairy Cattle Herds. J. Trace Elem. Med. Biol. 2009, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Enjalbert, F.; Lebreton, P.; Salat, O. Effects of Copper, Zinc and Selenium Status on Performance and Health in Commercial Dairy and Beef Herds: Retrospective Study. J. Anim. Physiol. Anim. Nutr. 2006, 90, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.; Pecoraro, H.L.; Webb, B.T.; Choi, B.J.; Idamawatta, C.; Mostrom, M.S.; Steichen, Q.P.; Hoppe, K. Copper and Manganese Levels Are Associated with Infectious Abortions, Stillbirths, and Early Neonatal Deaths in Upper Midwest Beef Cattle Herds. J. Am. Vet. Med. Assoc. 2025, 263, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.; Srivastava, R.; Mani, V.; Arulkumar, S.; Tyagi, N.; Mondal, G. The Importance of Zinc, Copper and Manganese and Their Impact on Growth, Immunity and Fertility of Male Cattle: A Review. BioMetals 2025, 38, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Swecker, W.S. Trace Mineral Feeding and Assessment. Vet. Clin. N. Am. Food Anim. Pract. 2023, 39, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Penedo, I.; Shore, R.F.; Miranda, M.; Benedito, J.L.; López-Alonso, M. Factors Affecting Trace Element Status in Calves in NW Spain. Livest. Sci. 2009, 123, 198–208. [Google Scholar] [CrossRef]
- Luna, D.; Miranda, M.; Minervino, A.H.H.; Piñeiro, V.; Herrero-Latorre, C.; López-Alonso, M. Validation of a Simple Sample Preparation Method for Multielement Analysis of Bovine Serum. PLoS ONE 2019, 14, e0211859. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420093681. [Google Scholar]
- López-Alonso, M.; Rey-Crespo, F.; Herrero-Latorre, C.; Miranda, M. Identifying Sources of Metal Exposure in Organic and Conventional Dairy Farming. Chemosphere 2017, 185, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M. Copper Supplementation, a Challenge in Cattle. Animals 2020, 10, 1890. [Google Scholar] [CrossRef]
- Hansen, S.L.; Spears, J.W. Bioaccessibility of Iron from Soil Is Increased by Silage Fermentation. J. Dairy Sci. 2009, 92, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Libera, K.; Konieczny, K.; Witkowska, K.; Żurek, K.; Szumacher-Strabel, M.; Cieslak, A.; Smulski, S. The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review. Animals 2021, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Langova, L.; Novotna, I.; Nemcova, P.; Machacek, M.; Havlicek, Z.; Zemanova, M.; Chrast, V. Impact of Nutrients on the Hoof Health in Cattle. Animals 2020, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
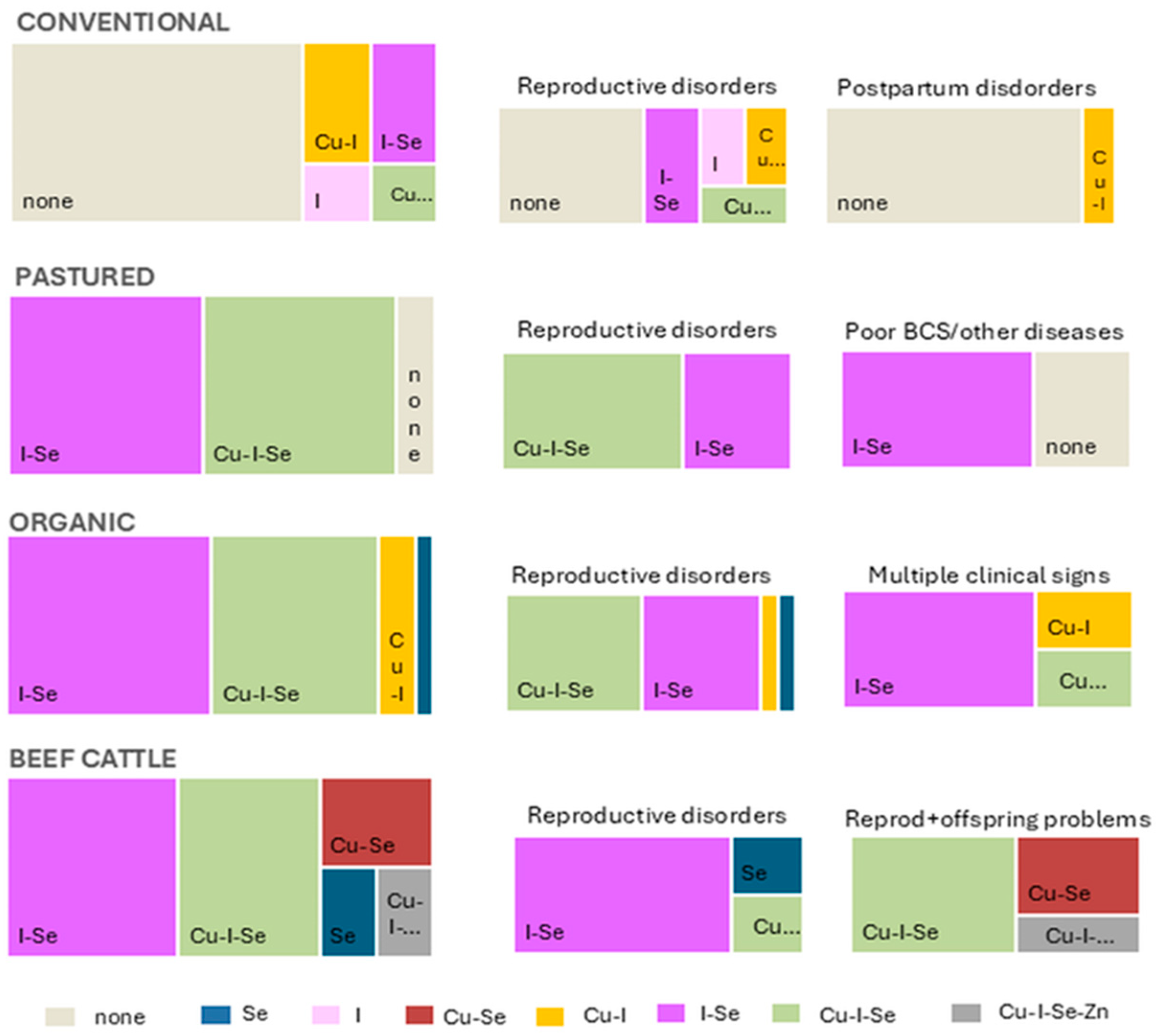
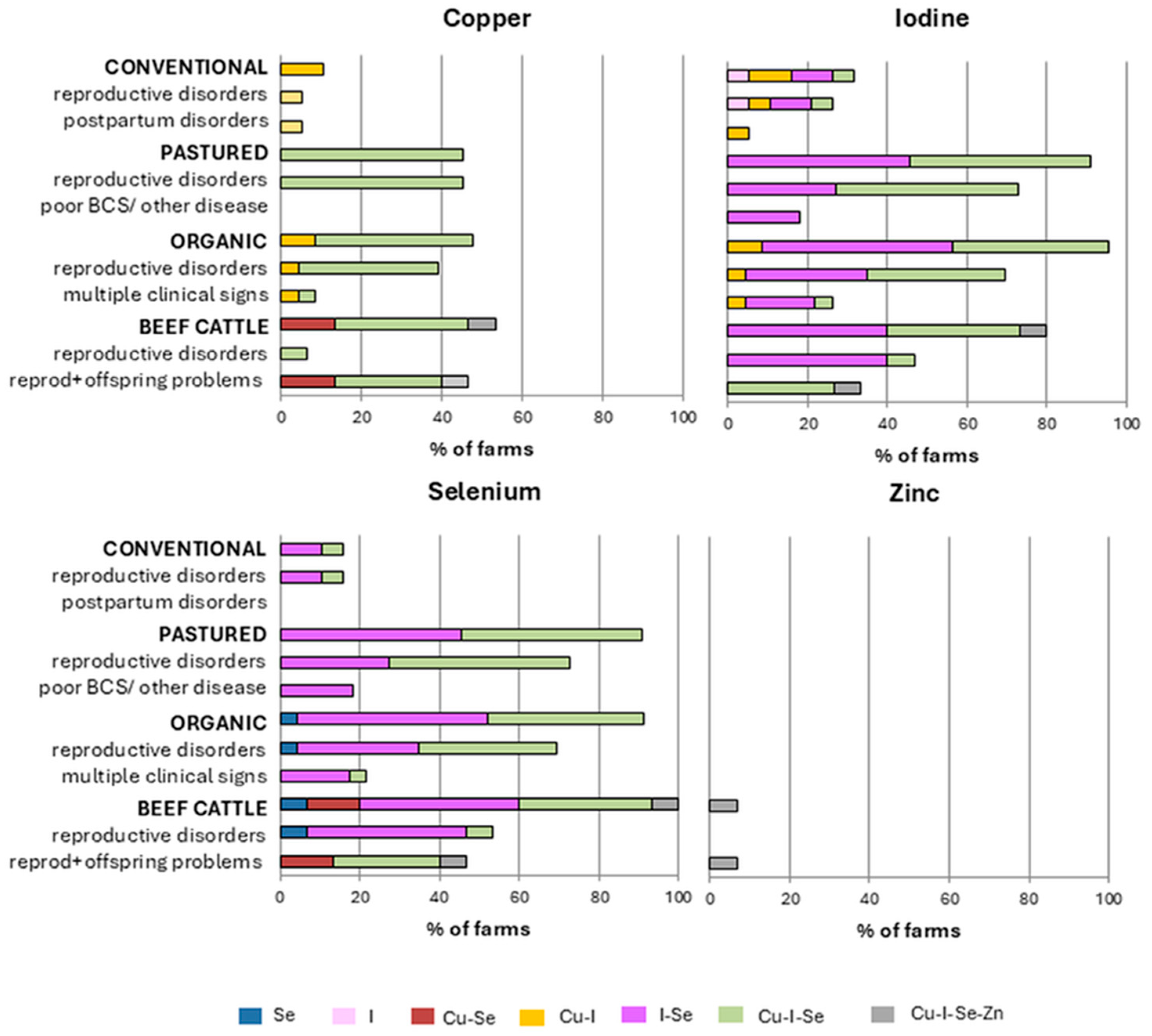
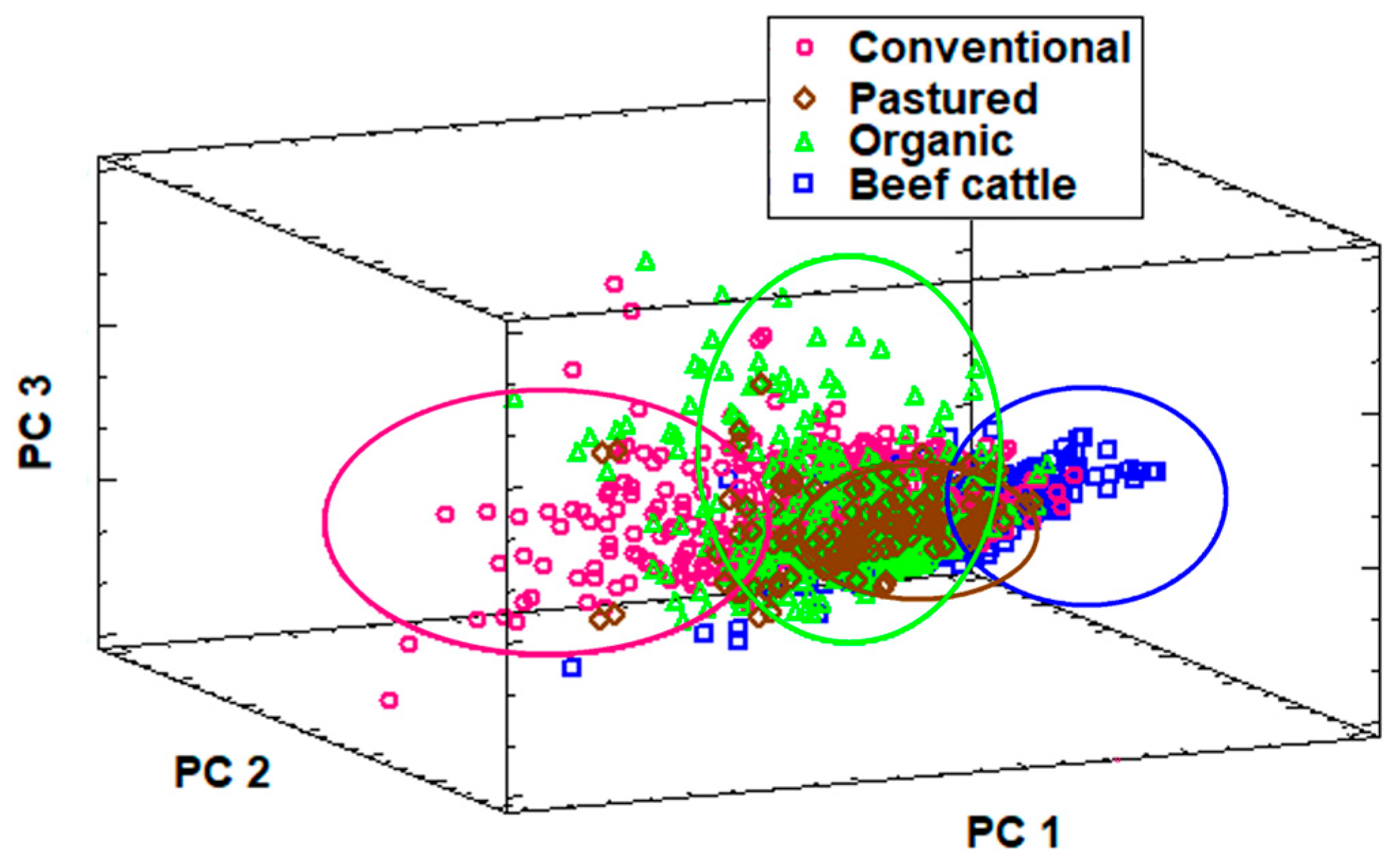
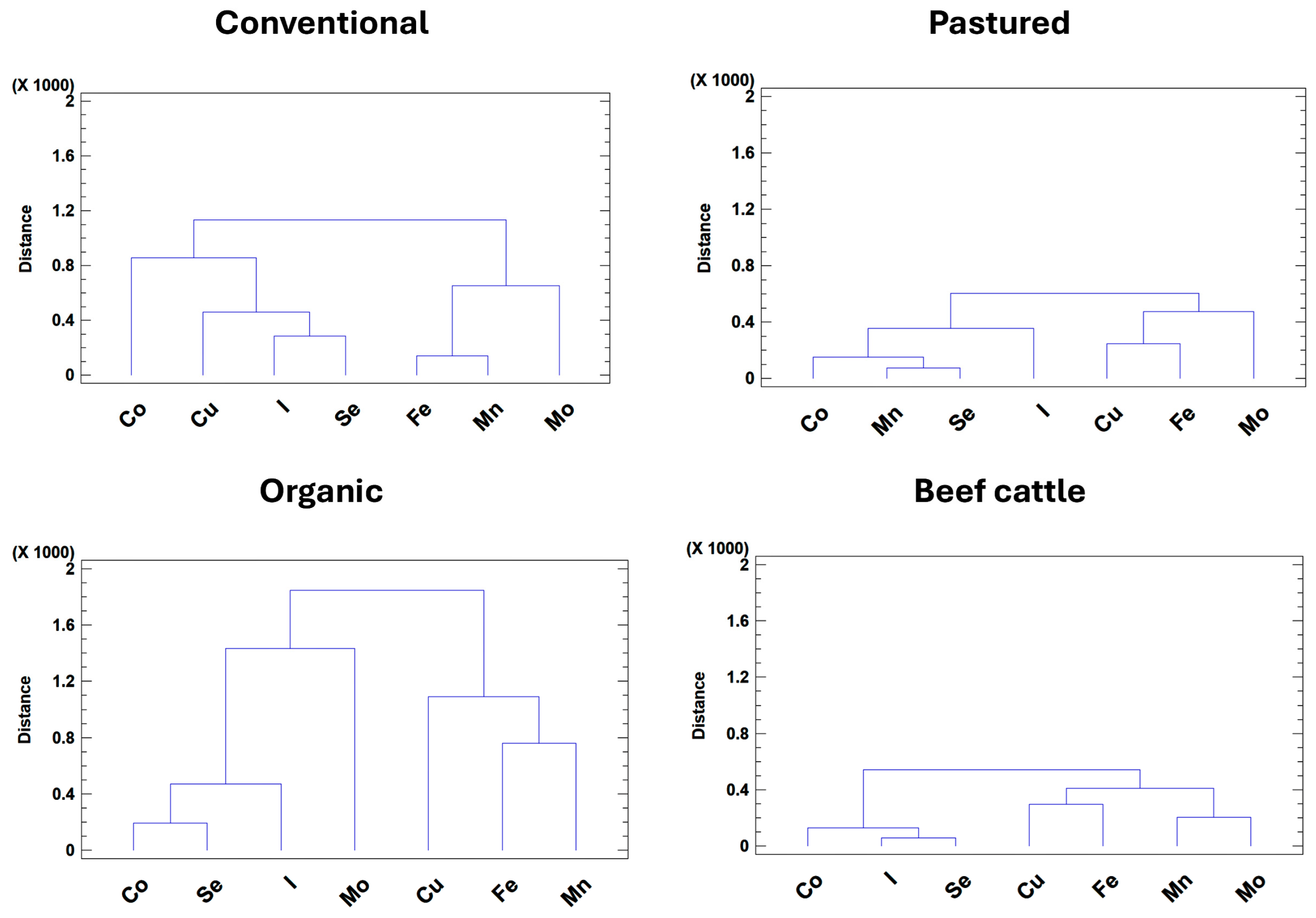
| Production System | No. Farms | Total no. Animals Sampled | Main Clinical Reason for Submission |
|---|---|---|---|
| Conventional dairy | 46 | 466 | Reproductive disorders (n = 24) Postpartum disorders (n = 22) |
| Pasture-based dairy | 11 | 120 | Reproductive disorders (n = 8) Poor BCS 1/other diseases (n = 3) |
| Organic dairy | 25 | 464 | Reproductive disorders (n = 19) Multiple clinical signs (n = 6) |
| Beef cattle | 35 | 223 | Reproductive disorders (n = 18) Reproductive and offspring problems (n = 17) |
| Total | 117 | 1273 |
| Type of Farm | ||||||
|---|---|---|---|---|---|---|
| Conventional Dairy | Pasture-Based Dairy | Organic Dairy | Beef Cattle | p-Value | ||
| Co (µg/L) | Mean ± SD | 0.776 ± 0.748 b | 1.031 ± 0.489 a | 1.147 ± 0.424 a | 0.270 ± 0.328 c | <0.001 |
| Median | 0.748 | 1.051 | 1.024 | 0.246 | ||
| (min–max) | (0.029–3.390) | (0.376–1.984) | (0.401–3.426) | (0.024–0.922) | ||
| Cu (mg/L) | Mean ± SD | 0.721 ± 0.128 a | 0.712 ± 0.194 a | 0.642 ± 0.154 b | 0.592 ± 0.180 b | <0.001 |
| Median | 0.720 | 0.675 | 0.636 | 0.583 | ||
| (min–max) | (0.392–1.410) | (0.381–1.367) | (0.134–1.162) | (0.291–0.927) | <0.001 | |
| Fe (mg/L) | Mean ± SD | 2.20 ± 0.63 ab | 1.81 ± 0.76 c | 1.98 ± 0.77 cb | 2.27 ± 0.83 a | |
| Median | 2.17 | 1.61 | 1.82 | 2.13 | ||
| (min–max) | (0.56–4.44) | (0.66–5.04) | (0.17–5.04) | (1.32–3.90) | ||
| I (µg/L) | Mean ± SD | 98.0 ± 62.9 a | 65.9 ± 45.0 b | 62.0 ± 63.9 b | 62.6 ± 29.5 b | <0.001 |
| Median | 85.3 | 62.4 | 52.7 | 47.7 | ||
| (min–max) | (14.9–490) | (19.8–196) | (2.4–468) | (15.7–498) | ||
| Mn (µg/L) | Mean ± SD | 3.97 ± 1.48 a | 3.18 ± 1.52 b | 3.72 ± 2.04 a | 3.14 ± 1.08 b | <0.001 |
| Median | 3.61 | 3.08 | 3.44 | 2.95 | ||
| (min–max) | (1.66–22.59) | (1.44–6.57) | (1.15–10.3) | (0.89–9.96) | ||
| Mo (µg/L) | Mean ± SD | 21.1 ± 9.33 b | 18.0 ± 30.5 b | 29.7 ± 22.8 a | 8.9 ± 14.5 c | <0.001 |
| Median | 14.4 | 12.1 | 21.1 | 5.3 | ||
| (min–max) | (1.7–170) | (2.4–76.1) | (1.5–353) | (0.8–34.0) | ||
| Se (µg/L) | Mean ± SD | 79.2 ± 16.6 a | 47.6 ± 28.1 b | 47.1 ± 29.0 b | 34.3 ± 21.5 c | <0.001 |
| Median | 84.2 | 48.0 | 42.4 | 33.7 | ||
| (min–max) | (19.8–141) | (11.2–99.1) | (7.2–133) | (6.6–80.7) | ||
| Zn (mg/L) | Mean ± SD | 0.875 ± 0.210 | 0.859 ± 0.228 | 0.866 ± 0.202 | 0.883 ± 0.246 | 0.399 |
| Median | 0.862 | 0.805 | 0.841 | 0.843 | ||
| (min–max) | (0.360–1.672) | (0.306–1.798) | (0.293–1.829) | (0.236–1.614) |
| 0 Elements | 1 Element | 2 Elements | ≥3 Elements | |
|---|---|---|---|---|
| Conventional dairy | 32 (68.4%) | 7 (15.8%) | 5 (10.5%) | 2 (5.3%) |
| Pasture-based dairy | 1 (9.1%) | 0 (0%) | 5 (45.5%) | 5 (45.5%) |
| Organic dairy | 0 (0%) | 1 (4.3%) | 14 (56.5%) | 10 (39.1%) |
| Beef | 0 (0%) | 2 (6.7%) | 19 (53.3%) | 14 (40.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Villa, C.; Rigueira, L.; López-Alonso, M.; Larrán, B.; Orjales, I.; Herrero-Latorre, C.; Pereira, V.; Miranda, M. Identification of Patterns of Trace Mineral Deficiencies in Dairy and Beef Cattle Herds in Spain. Animals 2025, 15, 2480. https://doi.org/10.3390/ani15172480
Fernández-Villa C, Rigueira L, López-Alonso M, Larrán B, Orjales I, Herrero-Latorre C, Pereira V, Miranda M. Identification of Patterns of Trace Mineral Deficiencies in Dairy and Beef Cattle Herds in Spain. Animals. 2025; 15(17):2480. https://doi.org/10.3390/ani15172480
Chicago/Turabian StyleFernández-Villa, Candela, Lucas Rigueira, Marta López-Alonso, Belén Larrán, Inmaculada Orjales, Carlos Herrero-Latorre, Víctor Pereira, and Marta Miranda. 2025. "Identification of Patterns of Trace Mineral Deficiencies in Dairy and Beef Cattle Herds in Spain" Animals 15, no. 17: 2480. https://doi.org/10.3390/ani15172480
APA StyleFernández-Villa, C., Rigueira, L., López-Alonso, M., Larrán, B., Orjales, I., Herrero-Latorre, C., Pereira, V., & Miranda, M. (2025). Identification of Patterns of Trace Mineral Deficiencies in Dairy and Beef Cattle Herds in Spain. Animals, 15(17), 2480. https://doi.org/10.3390/ani15172480








