Motility Performance of Thawed Spermatozoa of Bulls from the Tropics Throughout the Year
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Experimental Design
2.3. Animals and Care
2.4. Semen Collection and Cryopreservation
2.5. Motility
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morrell, J.M. Heat stress and bull fertility. Theriogenology 2020, 153, 62–67. [Google Scholar] [CrossRef]
- Capela, L.; Leites, I.; Romão, R.; Lopes-da-Costa, L.; Pereira, R.M.L.N. Impact of heat stress on bovine sperm quality and competence. Animals 2022, 12, 975. [Google Scholar] [CrossRef]
- Johnson, W.H. The significance to bull fertility of morphologically abnormal sperm. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 255–270. [Google Scholar] [CrossRef]
- Fisch, H.; Andrews, H.F.; Fisch, K.S.; Golden, R.; Liberson, G.; Olsson, C.A. The relationship of long term global temperature change and human fertility. Med. Hypotheses 2003, 61, 21–28. [Google Scholar] [CrossRef]
- Hansen, P.J. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82–83, 349–360. [Google Scholar] [CrossRef]
- Koivisto, M.B.; Costa, M.T.A.; Perri, S.H.V.; Vicente, W.R.R. The effect of season on semen characteristics and freezability in Bos indicus and Bos taurus bulls in the Southeastern region of Brazil. Reprod. Domest. Anim. 2009, 44, 587–592. [Google Scholar] [CrossRef]
- Landaeta-Hernández, A.J.; Gil-Araujo, M.A.; Ungerfeld, R.; Rae, D.O.; Urdaneta-Moyer, A.; Parra-Núñez, A.; Kaske, M.; Bollwein, H.; Chenoweth, P.J. Effect of season and genotype on values for bull semen variables under tropical conditions. Anim. Reprod. Sci. 2020, 221, 106592. [Google Scholar] [CrossRef]
- Freitas, A.; dos Santos, G.F.F.; Fernandes, A.R.; Mendonça, G.G.; de Paz, C.C.P.; Filho, A.E.V.; Faro, L.E. Effect of thermal stress on basic seminal characteristics of Gyr bulls. Int. J. Biometeorol. 2020, 64, 1649–1656. [Google Scholar] [CrossRef]
- Cabrera, P.; Pantoja, C. Sperm viability and acrosome integrity in national frozen bull semen. Rev. Investig. Vet. Perú 2012, 23, 192–200. [Google Scholar]
- Pathak, P.K.; Dhami, A.J.; Chaudhari, D.V. Correlations of motion characteristics and kinematic attributes of fresh and frozen-thawed spermatozoa of Gir bulls. Indian J. Vet. Sci. Biotechnol. 2019, 15, 9–13. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Chacón, L.; Navarro, O.; Ladino, C.; Martins, J.; Pérez, J.; Ardila, A. Sexual behavior and seminal characteristics of Brahman bulls in the Colombian tropical flooded savanna: Effects of reproductive management systems and climatic periods. Trop. Anim. Health Prod. 2022, 54, 81. [Google Scholar] [CrossRef]
- Menegassi, S.R.O.; Pereira, G.R.; Bremm, C.; Koetz, C.; Lopes, F.G.; Fiorentini, E.C.; McManus, C.; Dias, E.A.; da Rocha, M.K.; Lopes, R.B.; et al. Effects of ambient air temperature, humidity, and wind speed on seminal traits in Braford and Nellore bulls at the Brazilian Pantanal. Int. J. Biometeorol. 2016, 60, 1787–1794. [Google Scholar] [CrossRef]
- Kastelic, J.P. Male involvement in fertility and factors affecting semen quality in bulls. Anim. Front. 2013, 3, 20–25. [Google Scholar] [CrossRef]
- Ahirwar, M.K.; Kataktalware, M.; Prasad, K.; Pal, R.P.; Barman, D.; Thul, M.; Rawat, N. Effect of non-genetic factors on semen quality in bulls: A review. J. Entomol. Zool. Stud. 2018, 6, 38–45. [Google Scholar]
- Boe-Hansen, G.B.; Rêgo, J.P.A.; Satake, N.; Venus, B.; Sadowski, P.; Nouwens, A.; Li, Y.; McGowan, M. Effects of increased scrotal temperature on semen quality and seminal plasma proteins in Brahman bulls. Mol. Reprod. Dev. 2020, 87, 574–597. [Google Scholar] [CrossRef]
- Rekwot, P.I.; Voh, A.A., Jr.; Oyedipe, E.O.; Opaluwa, G.I.; Sekoni, V.O.; Dawuda, P.M. Influence of season on characteristics of the ejaculate from bulls in an artificial insemination centre in Nigeria. Anim. Reprod. Sci. 1987, 14, 187–194. [Google Scholar] [CrossRef]
- Chacón, J.; Perez, E.; Rodriguez-Martinez, H. Seasonal variations in testicular consistency, scrotal circumference and spermiogramme parameters of extensively reared Brahman (Bos indicus) bulls in the tropics. Theriogenology 2002, 58, 41–50. [Google Scholar] [CrossRef]
- Wildeus, S.; Hammond, A.C. Testicular, semen and blood parameters in adapted and non-adapted Bos taurus bulls in the semi-arid tropics. Theriogenology 1993, 40, 345–355. [Google Scholar] [CrossRef]
- Cunha, M.S.; Bonato, D.V.; Taira, A.R.; Teixeira, P.P.M. Degeneração testicular em machos: Dos animais ao homem. Investigação 2015, 14, 54–61. Available online: https://scispace.com/pdf/degeneracao-testicular-em-machos-dos-animais-ao-homem-4t165ivtxn.pdf (accessed on 6 July 2025).
- Thom, E.C. The discomfort index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Residiwati, G.; Tuska, H.S.; Budiono-Kawai, G.K.; Seifi-Jamadi, A.; Santoro, D.; Leemans, B.; Boccart, C.; Pascottini, O.B.; Opsomer, G.; Van Soom, A. Practical methods to assess the effects of heat stress on the quality of frozen-thawed Belgian Blue semen in field conditions. Anim. Reprod. Sci. 2020, 221, 106572. [Google Scholar] [CrossRef]
- Leite, R.F.; Losano, J.D.A.; Angrimani, D.S.R.; Sousa, R.G.B.; Alves, A.M.; Cavallin, M.D.; Kawai, G.K.V.; Cortada, C.N.M.; Zuge, R.M.; Nichi, M. Reproductive parameters of Bos taurus and Bos indicus bulls during different seasons in tropical conditions: Focus on an alternative approach to testicular assessments using ultrasonography. Anim. Reprod. Sci. 2020, 225, 106668. [Google Scholar] [CrossRef] [PubMed]
- Rizzoto, G.; Ferreira, J.C.P.; Codognoto, V.M.; Oliveira, K.C.; Mogollón García, H.M.; Pupulim, A.G.R.; Teixeira-Neto, F.J.; Castilho, A.; Nunes, S.G.; Thundathil, J.C.; et al. Testicular hyperthermia reduces testosterone concentrations and alters gene expression in testes of Nelore bulls. Theriogenology 2020, 152, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sidibé, M.; Franco, L.A.; Fredriksson, G.; Madej, A.; Malgrem, L. Effects on testosterone, LH, and cortisol concentrations and on testicular ultrasonographic appearance of induced testicular degeneration in bulls. Acta Vet. Scand. 1992, 33, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A.; Ason-Berg, A.; Damber, J.E.; Hammar, M.; Selstam, G. Steroid biosynthesis and Leydig cell morphology in adult unilateral cryptorchid rats. Acta Endocrinol. 1984, 107, 556–562. [Google Scholar] [CrossRef]
- Stellflug, J.N. Comparison of cortisol, luteinizing hormone, and testosterone responses to a defined stressor in sexually inactive rams and sexually active female-oriented and male-oriented rams. J. Anim. Sci. 2006, 84, 1520–1525. [Google Scholar] [CrossRef]
- Rasooli, A.; Jalali, M.T.; Nouri, M.; Mohammadian, B.; Barati, F. Effects of chronic heat stress on testicular structures, serum testosterone and cortisol concentrations in developing lambs. Anim. Reprod. Sci. 2010, 117, 55–59. [Google Scholar] [CrossRef]
- Pathak, P.K.; Dhami, A.J.; Chaudhari, D.V.; Patel, J.A. Motion and kinematics parameters of frozen-thawed bovine spermatozoa assessed by Biovis CASA. Indian J. Dairy Sci. 2019, 72, 302–306. [Google Scholar] [CrossRef]
- Airani, K.; Renukaradhya, G.J.; Sahadev, A.; Naveenkumar, S.; Satisha, K.B.; Ningaraju, K. Seminal attributes and motion kinematics of Gir bulls in their non-breeding tract. Pharma Innov. 2023, 12, 379–384. [Google Scholar]
- Pathak, P.K.; Dhami, A.J.; Chaudhari, D.V.; Hadiya, K.K. Comparative evaluation of motility and kinematics of fresh versus frozen-thawed spermatozoa of cattle and buffalo bull by CASA. Indian J. Anim. Res. 2020, 54, 1188–1194. [Google Scholar] [CrossRef]
- Ferraz, M.A.M.M.; Morato, R.; Yeste, M.; Arcarons, N.; Pena, A.I.; Tamargo, C.; Hidalgo, C.O.; Muiño, R.; Mogas, T. Evaluation of sperm subpopulation structure in relation to in vitro sperm–oocyte interaction of frozen-thawed semen from Holstein bulls. Theriogenology 2014, 81, 1067–1072. [Google Scholar] [CrossRef]
- Anchieta, M.C.; Vale-Filho, V.R.; Colosimo, E.; Sampaio, I.B.M.; Andrade, V.J. Semen discarded and freezing ability of Zebu and European bulls from a Brazilian artificial insemination centre. Arq. Bras. De Med. Veterinária E Zootec. 2005, 57, 196–204. [Google Scholar] [CrossRef]
- Teixeira, H.C.A.; Nascimento, N.V.; McManus, C.; Egito, A.A.; Mariante, A.S.; Ramos, A.F. Seasonal influence on semen traits and freezability from locally adapted Curraleiro bulls. Anim. Reprod. Sci. 2011, 125, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nichi, M.; Bols, P.E.J.; Zuge, R.M.; Barnabe, V.H.; Goovaerts, I.G.F.; Barnabe, R.C.; Cortada, C.N.M. Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology 2006, 66, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.P.; Sinha, A.K.; Singh, B.K.; Prasad, R.L. The effect of glutathione on the motility, enzyme leakage and fertility of frozen goat semen. Anim. Reprod. Sci. 1996, 41, 237–243. [Google Scholar] [CrossRef]
- Wildeus, S.; Entwistle, K.W. Spermiogram and sperm reserves in hybrid Bos indicus—Bos taurus bulls after scrotal insulation. J. Reprod. Fertil. 1983, 69, 711–716. [Google Scholar] [CrossRef]
- Waites, G.M.H.; Setchell, B.P. Effects of local heating on blood flow and metabolism in the testis of the conscious ram. J. Reprod. Fertil. 1964, 8, 339–349. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Kim, B.; Park, K.; Rhee, K. Heat stress response of male germ cells. Cell. Mol. Life Sci. 2013, 70, 2623–2636. [Google Scholar] [CrossRef]
- Menegassi, S.R.O.; Pereira, G.R.; Dias, E.A.; Koetz, C., Jr.; Lopes, F.G.; Bremm, C.; McManus, C.P.; Lopes, R.B.; Rocha, M.K.; Carvalho, H.R.; et al. The uses of infrared thermography to evaluate the effects of climatic variables in bull’s reproduction. Int. J. Biometeorol. 2016, 60, 151–157. [Google Scholar] [CrossRef]
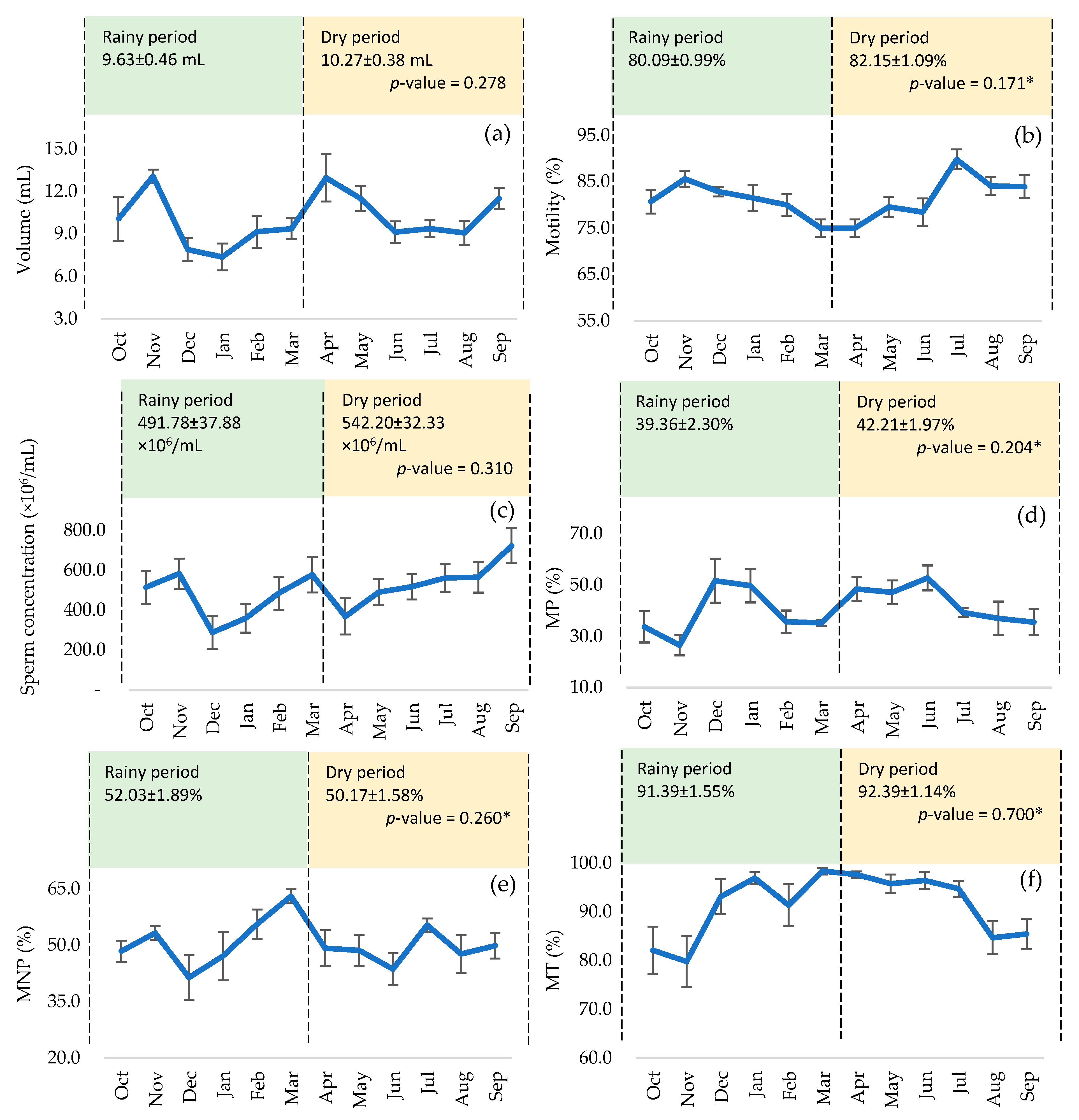
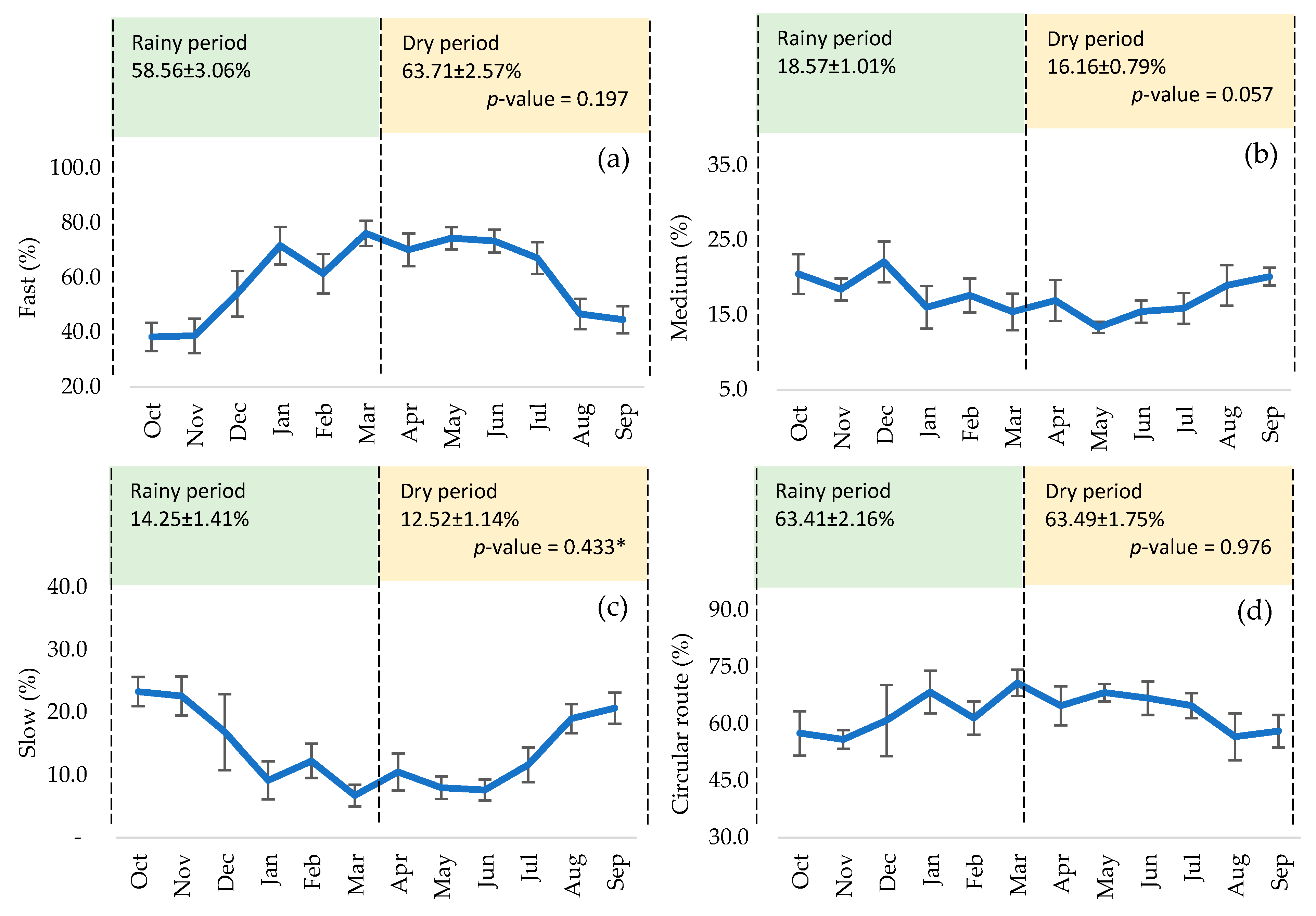
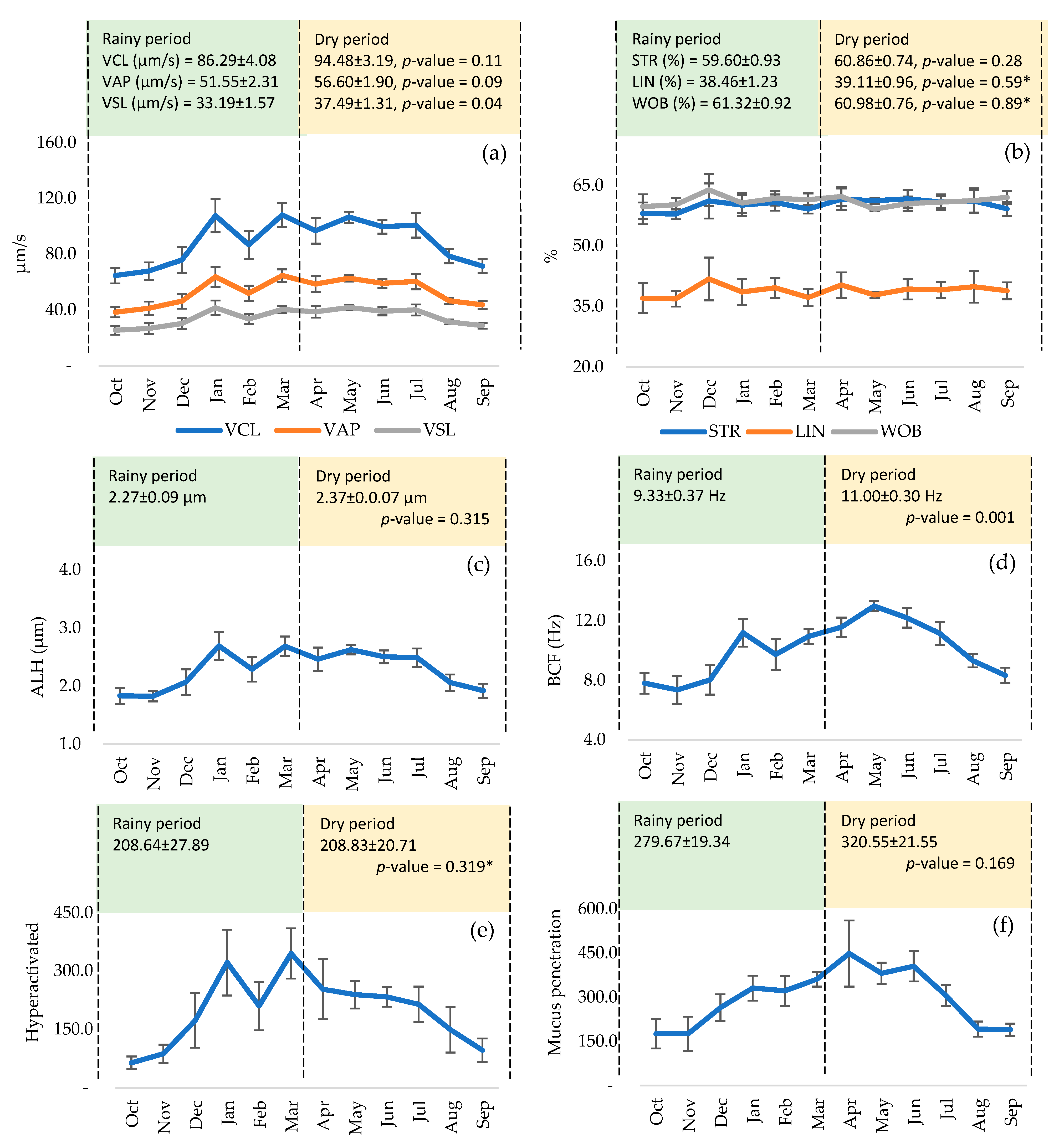
| ID | n | Volume (mL) | MM | MI (%) | Concent. (×106/mL) | MP (%) | MNP (%) | MT (%) | Fast (%) | Medium (%) | Slow (%) | Circular Route (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA-GY | 43 | 8.9 ± 0.4 b | 2.9 ± 0.1 bc | 83.4 ± 1.2 | 468.0 ± 40.9 b | 39.5 ± 2.5 bc | 52.9 ± 2.1 a | 92.4 ± 1.6 ab | 62.4 ± 3.4 a | 16.5 ± 1.1 | 13.5 ± 1.7 ab | 63.0 ± 2.5 b |
| NA-GY | 21 | 9.2 ± 0.7 b | 2.8 ± 0.1 c | 78.3 ± 1.9 | 278.4 ± 41.4 c | 54.9 ± 3.6 a | 41.9 ± 3.5 b | 96.8 ± 0.7 a | 67.6 ± 3.1 a | 19.1 ± 1.4 | 10.0 ± 1.4 b | 71.6 ± 2.6 a |
| PA-GY | 28 | 9.7 ± 0.6 b | 3.1 ± 0.1 ab | 79.5 ± 1.4 | 472.7 ± 48.2 b | 44.8 ± 3.4 b | 49.1 ± 2.9 a | 93.8 ± 2.0 a | 68.8 ± 4.3 a | 14.8 ±1.3 | 10.2 ± 1.9 b | 64.6 ± 3.0 ab |
| SO-BR | 37 | 12.0 ± 0.6 a | 3.2 ± 0.1 a | 81.6 ± 1.6 | 751.7 ± 29.2 a | 31.8 ± 1.8 c | 55.5 ± 1.3 a | 87.3 ± 2.0 b | 51.1 ± 3.9 b | 18.9 ± 1.3 | 17.3 ± 1.6 a | 58.5 ± 2.4 b |
| p-value | <0.001 | 0.001 ** | 0.084 | <0.001 | <0.001 | 0.002 | 0.001 ** | 0.005 | 0.072 | 0.013 | 0.018 | |
| Total | 129 | 10.0 ± 0.3 | 3.0 ± 0.1 | 81.2 ± 0.8 | 519.5 ± 24.6 | 40.9 ± 1.5 | 51.0 ± 1.2 | 91.9 ± 0.9 | 61.4 ± 2.0 | 17.2 ± 0.6 | 13.3 ± 0.9 | 63.5 ± 1.4 |
| ID | VCL (µm/s) | VAP (µm/s) | VSL (µm/s) | STR (%) | LIN (%) | WOB (%) | ALH (µm) | BCF (Hz) | Hyperactivated | Mucus Penetration |
|---|---|---|---|---|---|---|---|---|---|---|
| CA-GY | 95.7 ± 5.0 a | 58.0 ± 2.9 a | 38.5 ± 2.0 a | 60.7 ± 1.1 | 39.5 ± 1.4 | 61.3 ± 1.0 | 2.4 ± 0.1 ab | 10.1 ± 0.4 ab | 228.7 ± 33.2 | 298.4 ± 21.5 bc |
| NA-GY | 87.4 ± 4.3 ab | 52.1 ± 3.1 ab | 33.1 ± 2.4 ab | 57.5 ± 1.5 | 36.1 ± 1.7 | 59.5 ± 1.3 | 2.3 ± 0.1 ab | 10.4 ± 0.5 ab | 204.5 ± 37.5 | 394.1 ± 45.0 a |
| PA-GY | 100.6 ± 5.7 a | 59.3 ± 3.2 a | 38.8 ± 2.1 a | 61.4 ± 1.4 | 39.1 ± 1.6 | 60.5 ± 1.1 | 2.6 ± 0.1 a | 11.6 ± 0.5 a | 256.3 ± 40.2 | 345.8 ± 34.5 ab |
| SO-BR | 79.6 ± 4.1 b | 47.6 ± 2.3 b | 31.1 ± 1.5 b | 60.5 ± 0.9 | 39.4 ± 1.5 | 62.4 ± 1.2 | 2.1 ± 0.1 b | 9.3 ± 0.5 b | 152.0 ± 22.9 | 221.4 ± 20.1 c |
| p-value | 0.015 | 0.013 | 0.008 | 0.181 | 0.485 | 0.429 | 0.017 | 0.016 | 0.183 ** | 0.001 |
| Total | 90.8 ± 2.6 | 54.3 ± 1.5 | 35.6 ± 1.0 | 60.3 ± 0.6 | 38.8 ± 0.8 | 61.1 ± 0.6 | 2.3 ± 0.1 | 10.3 ± 0.3 | 208.7 ± 16.9 | 302.2 ± 14.8 |
| Variables | Winter (21 June–20 September) | Spring (21 September–20 December) | Summer (21 December–20 March) | Autumn (21 March–20 June) | p-Value |
|---|---|---|---|---|---|
| n | 37 | 25 | 33 | 34 | |
| Volume (mL) | 9.60 ± 0.45 ab | 10.83 ± 0.67 a | 8.72 ± 0.58 b | 11.01 ± 0.61 a | 0.017 |
| MM | 3.09 ± 0.08 | 2.96 ± 0.10 | 2.97 ± 0.09 | 3.10 ± 0.09 | 0.754 ** |
| MI (%) | 85.62 ± 1.50 a | 82.80 ± 1.00 a | 78.03 ± 1.47 b | 78.38 ± 1.33 b | <0.001 |
| Concent. (×106/mL) | 596.70 ± 42.66 | 508.72 ± 58.98 | 478.94 ± 50.03 | 482.88 ± 47.59 | 0.249 |
| MP (%) | 37.69 ± 2.75 | 38.84 ± 4.05 | 39.76 ± 2.70 | 47.14 ± 2.63 | 0.093 |
| MNP (%) | 50.65 ± 2.06 | 47.21 ± 2.38 | 55.68 ± 2.65 | 49.65 ± 2.46 | 0.110 |
| MT (%) | 88.34 ± 1.78 b | 86.04 ± 2.62 b | 95.44 ± 1.55 a | 96.79 ± 0.95 a | <0.01 |
| Fast (%) | 53.32 ± 3.59 b | 45.52 ± 3.82 b | 68.45 ± 3.73 a | 75.02 ± 2.57 a | <0.001 |
| Medium (%) | 18.09 ± 1.16 a | 20.57 ± 1.21 a | 17.06 ± 1.48 ab | 14.05 ± 0.94 b | 0.005 |
| Slow (%) | 16.91 ± 1.61 a | 19.96 ± 2.16 a | 9.93 ± 1.48 b | 7.73 ± 1.16 b | <0.001 ** |
| Circular route (%) | 59.40 ± 2.67 b | 58.96 ± 3.36 b | 66.78 ± 2.72 ab | 67.94 ± 1.99 a | 0.027 |
| VCL (µm/s) | 84.16 ± 4.30 b | 71.05 ± 3.86 b | 97.84 ± 5.82 a | 105.71 ± 3.96 a | <0.001 |
| VAP (µm/s) | 50.77 ± 2.59 b | 42.78 ± 2.41 c | 58.20 ± 3.19 ab | 62.93 ± 2.40 a | <0.001 |
| VSL (µm/s) | 33.98 ± 1.83 b | 27.77 ± 1.84 c | 37.29 ± 2.14 ab | 41.32 ± 1.68 a | <0.001 |
| STR (%) | 60.75 ± 1.13 | 58.95 ± 1.59 | 60.09 ± 1.11 | 60.99 ± 0.95 | 0.662 |
| LIN (%) | 39.65 ± 1.55 | 38.56 ± 1.99 | 38.38 ± 1.58 | 38.52 ± 1.10 | 0.922 |
| WOB (%) | 61.61 ± 1.23 | 61.36 ± 1.55 | 61.28 ± 1.14 | 60.29 ± 0.87 | 0.978 ** |
| ALH (µm) | 2.15 ± 0.09 b | 1.95 ± 0.09 b | 2.50 ± 0.12 a | 2.62 ± 0.08 a | <0.001 |
| BCF (Hz) | 9.76 ± 0.41 b | 7.87 ± 0.44 c | 10.44 ± 0.48 b | 12.35 ± 0.31 a | <0.001 |
| Hyperactivated | 152.62 ± 25.24 b | 119.08 ± 26.44 b | 276.48 ± 41.27 a | 270.00 ± 30.41 a | <0.001 ** |
| Mucus penetration | 238.11 ± 19.41 c | 207.84 ± 26.74 c | 334.09 ± 23.44 b | 410.26 ± 33.81 a | <0.001 ** |
| Max. temperature (°C) | 34.37 ± 0.16 c | 35.13 ± 0.19 d | 33.51 ± 0.18 b | 31.75 ± 0.15 a | <0.001 ** |
| Humidity (%) | 71.16 ± 0.40 a | 71.58 ± 0.47 a | 73.52 ± 0.54 b | 77.01 ± 0.47 c | <0.001 ** |
| THI | 88.08 ± 0.19 c | 89.30 ± 0.24 d | 87.23 ± 0.20 b | 85.13 ± 0.17 a | <0.001 ** |
| Accumulated rainfall (mm) | 296.5 | 511.9 | 720.1 | 624.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poclín-Rojas, A.Y.; Arbaiza Barnechea, M.D.; Segura Portocarrero, G.T.; Ampuero-Trigoso, G.; Bernilla Carrillo, D.; Depaz-Hizo, B.A.; Vásquez-Tarrillo, R.W.; Diaz-Quevedo, C.; Quispe-Ccasa, H.A. Motility Performance of Thawed Spermatozoa of Bulls from the Tropics Throughout the Year. Animals 2025, 15, 2451. https://doi.org/10.3390/ani15162451
Poclín-Rojas AY, Arbaiza Barnechea MD, Segura Portocarrero GT, Ampuero-Trigoso G, Bernilla Carrillo D, Depaz-Hizo BA, Vásquez-Tarrillo RW, Diaz-Quevedo C, Quispe-Ccasa HA. Motility Performance of Thawed Spermatozoa of Bulls from the Tropics Throughout the Year. Animals. 2025; 15(16):2451. https://doi.org/10.3390/ani15162451
Chicago/Turabian StylePoclín-Rojas, Annie Y., Martin Daniel Arbaiza Barnechea, Gleni T. Segura Portocarrero, Gustavo Ampuero-Trigoso, Diana Bernilla Carrillo, Benjamín A. Depaz-Hizo, Ronald W. Vásquez-Tarrillo, Clavel Diaz-Quevedo, and Hurley A. Quispe-Ccasa. 2025. "Motility Performance of Thawed Spermatozoa of Bulls from the Tropics Throughout the Year" Animals 15, no. 16: 2451. https://doi.org/10.3390/ani15162451
APA StylePoclín-Rojas, A. Y., Arbaiza Barnechea, M. D., Segura Portocarrero, G. T., Ampuero-Trigoso, G., Bernilla Carrillo, D., Depaz-Hizo, B. A., Vásquez-Tarrillo, R. W., Diaz-Quevedo, C., & Quispe-Ccasa, H. A. (2025). Motility Performance of Thawed Spermatozoa of Bulls from the Tropics Throughout the Year. Animals, 15(16), 2451. https://doi.org/10.3390/ani15162451







