Characterizing Spatio-Temporal Variation in Macroinvertebrate Communities and Ecological Health Assessment in the Poyang Lake Basin During the Early Stage of a Fishing Ban
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Data Analysis
2.3. Biological Integrity Evaluation System for Macroinvertebrates
3. Results
3.1. Environmental Factors
3.2. Species Composition and Dominant Species
3.3. Spatio-Temporal Variation in Density and Biomass
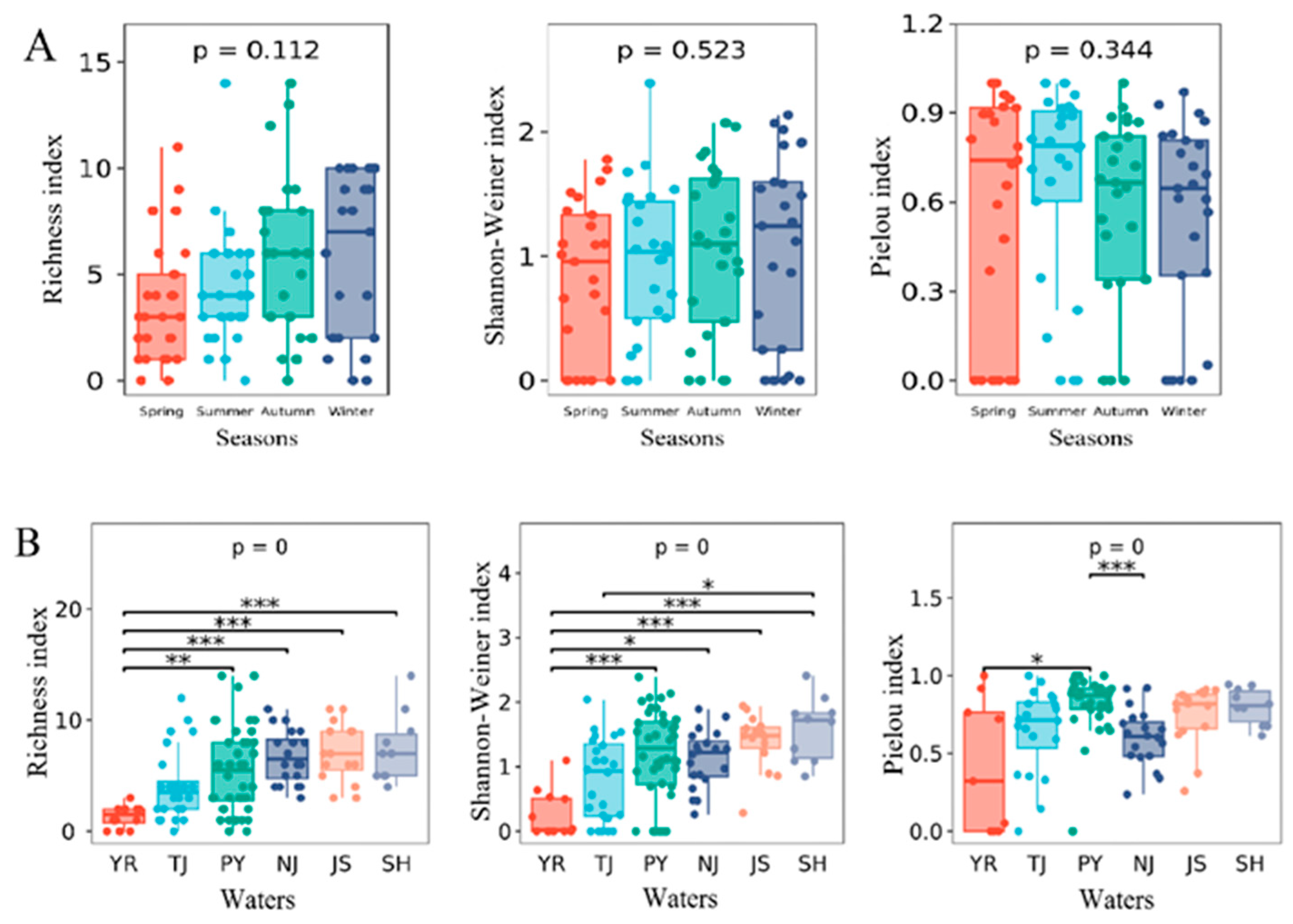
3.4. Diversity and Community Structure
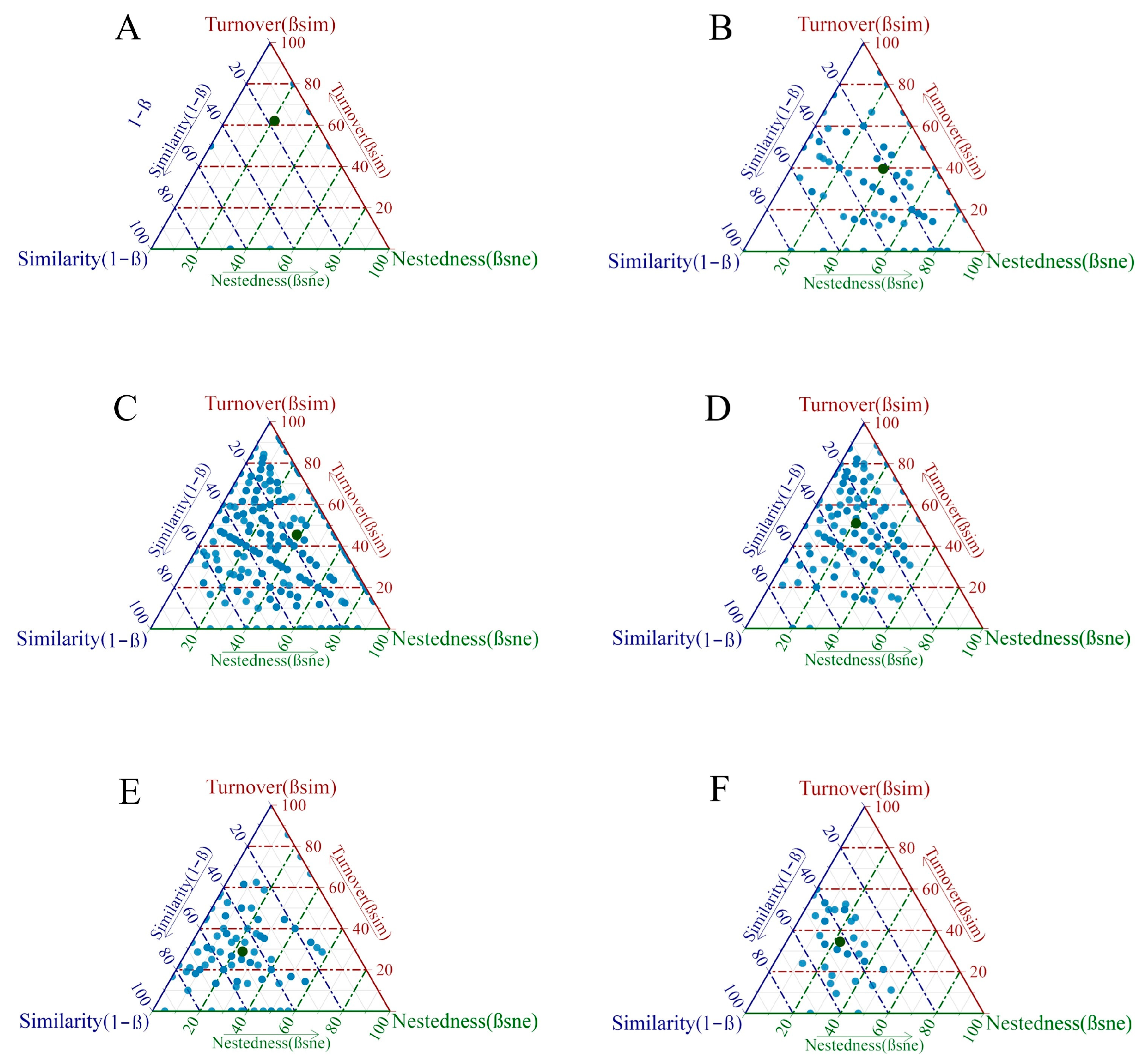
3.5. Evaluation of Community ABC Curves
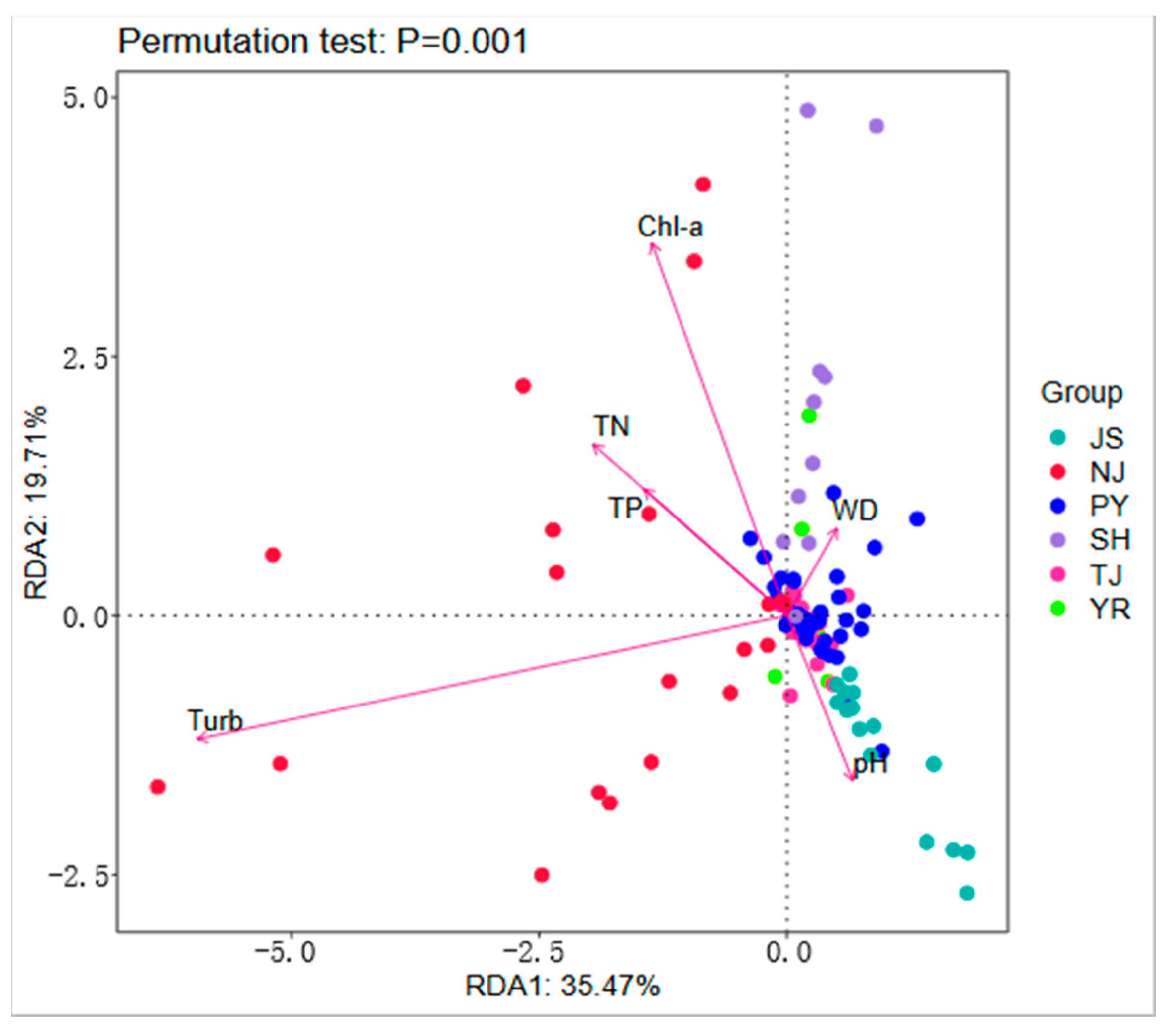
3.6. Redundancy Analysis of Environmental Factors and the Community Structure of Macroinvertebrates
3.7. Screening and Establishment of Biological Integrity Indicators
3.8. Scoring and Evaluation
4. Discussion
4.1. Characteristics of the Macroinvertebrate Communities
4.2. Macroinvertebrate Diversity and Community Structure
4.3. Effects of Environmental Factors on Macroinvertebrates
4.4. Health Evaluation of the Poyang Lake Basin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter Attribute | Parameter Number | Biological Parameter | Parameter Description | Response to Disturbance |
|---|---|---|---|---|
| Species composition and abundance | M1 | Total taxa | Number of benthic macroinvertebrate taxa in the sample | Decrease |
| M2 | EPT taxa | Number of Ephemeroptera + Plecoptera + Trichoptera taxa in the sample | Decrease | |
| M3 | Crustacea + Mollusca taxa | Number of crustacean and mollusk taxa in the sample | Decrease | |
| M4 | Ephemeroptera taxa | Number of Ephemeroptera taxa in the sample | Decrease | |
| M5 | Coleoptera taxa | Number of Coleoptera taxa in the sample | Decrease | |
| M6 | Trichoptera taxa | Number of Trichoptera taxa in the sample | Decrease | |
| M7 | Diptera taxa | Number of Diptera taxa in the sample | Decrease | |
| M8 | Chironomidae taxa | Number of Chironomidae taxa in the sample | Decrease | |
| M9 | EPT (%) | (Ephemeroptera + Plecoptera + Trichoptera individuals)/Total individuals in the sample | Decrease | |
| M10 | Crustacea + Mollusca (%) | (Crustacean + Mollusca individuals)/Total individuals in the sample | Decrease | |
| M11 | Ephemeroptera (%) | Ephemeroptera individuals/Total individuals in the sample | Decrease | |
| M12 | Coleoptera (%) | Coleoptera individuals/Total individuals in the sample | Decrease | |
| M13 | Trichoptera (%) | Trichoptera individuals/Total individuals in the sample | Decrease | |
| M14 | Diptera (%) | Diptera individuals/Total individuals in the sample | Increase | |
| M15 | Chironomidae (%) | Chironomidae individuals/Total individuals in the sample | Increase | |
| M16 | Oligochaeta (%) | Oligochaete individuals/Total individuals in the sample | Increase | |
| Diversity | M17 | Shannon–Wiener index | Calculated by formula | Decrease |
| M18 | Pielou index | Calculated by formula | Decrease | |
| M19 | Richness index | Calculated by formula | Decrease | |
| M20 | Simpson index | Calculated by formula | Decrease | |
| Sensitivity and tolerance | M21 | Sensitive taxa (TV ≤ 3) | Number of taxa with Tolerance Value (TV) ≤ 3 | Decrease |
| M22 | Tolerant taxa (TV ≥ 7) | Number of taxa with Tolerance Value (TV) ≥ 7 | Increase | |
| M23 | Sensitive taxa (%) | (Individuals with TV ≤ 3)/Total individuals in the sample | Decrease | |
| M24 | Tolerant Taxa (%) | (Individuals with TV ≥ 7)/Total individuals in the sample | Increase | |
| M25 | Dominant Species (%) | (Individuals of most dominant species)/Total individuals in the sample | Increase | |
| M26 | Top 3 Dominant Species (%) | (Individuals of top 3 dominant species)/Total individuals in the sample | Increase | |
| Feeding Functional Groups | M27 | Shredders (%) | Shredder individuals/Total individuals in the sample | Decrease |
| M28 | Collectors (%) | Collector individuals/Total individuals in the sample | Increase | |
| M29 | Filterers (%) | Filterers individuals/Total individuals in the sample | Increase | |
| M30 | Scrapers (%) | Scraper individuals/Total individuals in the sample | Decrease | |
| M31 | Predators (%) | Predator individuals/Total individuals in the sample | Decrease |
| Sample | Richness Index | Shannon–Weiner | Pielou Index |
|---|---|---|---|
| CYR1 | 0 | 0 | 0 |
| CYR2 | 1 | 0 | 0 |
| CYR3 | 3 | 1.09861228866811 | 1 |
| DYR1 | 2 | 0.529706199057654 | 0.76420450650862 |
| DYR2 | 0 | 0 | 0 |
| DYR3 | 2 | 0.0358990284409962 | 0.051791350304557 |
| QYR1 | 2 | 0.223718076065834 | 0.322756958897398 |
| QYR2 | 2 | 0.636514168294813 | 0.918295834054489 |
| QYR3 | 1 | 0 | 0 |
| XYR1 | 0 | 0 | 0 |
| XYR2 | 1 | 0 | 0 |
| XYR3 | 2 | 0.500402423538188 | 0.721928094887362 |
| CTJ4 | 4 | 1.09059947377948 | 0.786701226208884 |
| CTJ5 | 3 | 1.09861228866811 | 1 |
| CTJ6 | 1 | 0 | 0 |
| CTJ7 | 2 | 0.410116318288409 | 0.591672778582327 |
| CTJ8 | 4 | 1.33217904021012 | 0.960964047443681 |
| CTJ9 | 4 | 1.242453325 | 0.896240625180289 |
| DTJ4 | 2 | 0.25095480435762 | 0.362051251733998 |
| DTJ5 | 2 | 0.244930026794635 | 0.353359335021421 |
| DTJ6 | 9 | 1.58157310741415 | 0.719804941073228 |
| DTJ7 | 1 | 0 | 0 |
| DTJ8 | 4 | 0.914285581467099 | 0.659517637169433 |
| DTJ9 | 10 | 1.40451606968384 | 0.609973578808134 |
| QTJ4 | 3 | 0.362501021739676 | 0.329962649679761 |
| QTJ5 | 1 | 0 | 0 |
| QTJ6 | 0 | 0 | 0 |
| QTJ7 | 9 | 1.48786138269121 | 0.677154897154393 |
| QTJ8 | 3 | 0.955699891112534 | 0.869915529773626 |
| QTJ9 | 12 | 2.04045519011617 | 0.821139574917332 |
| XTJ4 | 4 | 0.199099914156536 | 0.143620229397526 |
| XTJ5 | 6 | 1.53672246943722 | 0.857661140252986 |
| XTJ6 | 1 | 0 | 0 |
| XTJ7 | 4 | 1.03501663348432 | 0.746606682182711 |
| XTJ8 | 2 | 0.562335144618808 | 0.811278124459133 |
| XTJ9 | 8 | 1.47733677100331 | 0.71044881108313 |
| CPY10 | 6 | 1.60520710745546 | 0.895883144486481 |
| CPY11 | 3 | 0.955699891112534 | 0.869915529773626 |
| CPY12 | 2 | 0.562335144618808 | 0.811278124459133 |
| CPY13 | 6 | 1.69574253416963 | 0.946411928215015 |
| CPY14 | 3 | 1.01140426470735 | 0.920619835714305 |
| CPY15 | 5 | 1.47507631105469 | 0.916516443199602 |
| CPY16 | 2 | 0.693147180559945 | 1 |
| CPY17 | 1 | 0 | 0 |
| CPY18 | 1 | 0 | 0 |
| CPY19 | 1 | 0 | 0 |
| CPY20 | 0 | 0 | 0 |
| DPY10 | 10 | 1.90720474094698 | 0.828288494852995 |
| DPY11 | 10 | 2.13425532309787 | 0.926895309794048 |
| DPY12 | 10 | 1.48803119085618 | 0.646243735088764 |
| DPY13 | 8 | 2.01615371726138 | 0.969564989854281 |
| DPY14 | 4 | 1.11874333598575 | 0.80700273142711 |
| DPY15 | 10 | 2.06751244168107 | 0.897909244688407 |
| DPY16 | 0 | 0 | 0 |
| DPY17 | 7 | 1.54278207838702 | 0.792833152720848 |
| DPY18 | 1 | 0 | 0 |
| DPY19 | 1 | 0 | 0 |
| DPY20 | 9 | 1.91533317167766 | 0.871705692460301 |
| QPY20 | 8 | 1.70355993434049 | 0.819239156376716 |
| XPY20 | 7 | 1.73153540826228 | 0.889833176060516 |
| QPY10 | 9 | 1.62297601153479 | 0.73864821478667 |
| QPY11 | 5 | 1.16018624397852 | 0.720864243979353 |
| QPY12 | 13 | 1.66666497996901 | 0.649784751157216 |
| QPY13 | 14 | 2.070231994 | 0.784458893905427 |
| QPY14 | 8 | 1.84074872856928 | 0.885213020743189 |
| QPY15 | 6 | 1.58678470752805 | 0.885601407320416 |
| QPY16 | 3 | 1.09861228866811 | 1 |
| QPY17 | 8 | 1.80551494922852 | 0.868269154500956 |
| QPY18 | 1 | 0 | 0 |
| QPY19 | 6 | 0.926760564602932 | 0.51723491937353 |
| XPY10 | 4 | 1.08325501051851 | 0.781403315846587 |
| XPY11 | 6 | 1.4402347497046 | 0.803810318538513 |
| XPY12 | 6 | 1.41324169041344 | 0.788745205304989 |
| XPY13 | 14 | 2.39025733681791 | 0.905723915124794 |
| XPY14 | 2 | 0.693147180559945 | 1 |
| XPY15 | 3 | 0.735621939758795 | 0.669591945535779 |
| XPY16 | 6 | 1.67698777432242 | 0.935944697445866 |
| XPY17 | 3 | 1.05492016798614 | 0.960229717860761 |
| XPY18 | 3 | 1.09861228866811 | 1 |
| XPY19 | 3 | 0.974314752869349 | 0.886859507142915 |
| CNJ21 | 11 | 1.77605476212089 | 0.740672364747694 |
| CNJ22 | 8 | 1.36419303723545 | 0.656038176544945 |
| CNJ23 | 4 | 0.659872013784827 | 0.475997040954392 |
| CNJ24 | 8 | 1.51129077395926 | 0.726777234977422 |
| CNJ25 | 9 | 0.80978214954624 | 0.368547738769593 |
| DNJ21 | 8 | 1.27416326986915 | 0.612743010241028 |
| DNJ22 | 10 | 1.89236951566801 | 0.821845638376544 |
| DNJ23 | 6 | 0.866406431097394 | 0.483550636107797 |
| DNJ24 | 9 | 1.24296164577051 | 0.565696223586485 |
| DNJ25 | 10 | 1.59621685497326 | 0.693228172035848 |
| QNJ21 | 6 | 0.874455398810259 | 0.488042850521114 |
| QNJ22 | 4 | 0.470900127917266 | 0.339682639650109 |
| QNJ23 | 8 | 1.31057323357431 | 0.630252501599822 |
| QNJ24 | 7 | 1.05543195677292 | 0.542384733069668 |
| QNJ25 | 6 | 1.19203251272669 | 0.665286012547351 |
| XNJ21 | 5 | 0.970552590755782 | 0.603038230463906 |
| XNJ22 | 5 | 1.47507631105469 | 0.916516443199602 |
| XNJ23 | 4 | 1.27703425946614 | 0.921185496588554 |
| XNJ24 | 3 | 0.259717624933192 | 0.236405170060547 |
| XNJ25 | 4 | 0.478150579382401 | 0.344912734836587 |
| DJS26 | 7 | 1.59453248461001 | 0.819427600695805 |
| DJS27 | 6 | 1.51749809498804 | 0.846931812584097 |
| DJS28 | 9 | 1.9415071796013 | 0.883617996825371 |
| DJS29 | 3 | 0.283936266755864 | 0.258449927863169 |
| DJS30 | 7 | 1.7388948450374 | 0.893615178420025 |
| QJS30 | 3 | 0.858740913006287 | 0.781659664527667 |
| QJS26 | 5 | 1.46481638489081 | 0.91014159264798 |
| QJS27 | 6 | 1.44691898293633 | 0.807540860135486 |
| QJS28 | 6 | 1.62602069242075 | 0.90749942743224 |
| QJS29 | 4 | 1.21488965394912 | 0.876357639489852 |
| XJS26 | 11 | 1.54803838135041 | 0.645582148191082 |
| XJS27 | 9 | 1.36307341326878 | 0.620361444764691 |
| XJS28 | 11 | 0.893435780026477 | 0.372591659928428 |
| XJS29 | 9 | 1.47835813629106 | 0.672829783327534 |
| XJS30 | 10 | 1.89026255383218 | 0.820930596477666 |
| DSH31 | 5 | 1.08658415270008 | 0.675132693411418 |
| DSH32 | 5 | 1.27801195876342 | 0.79407347676467 |
| DSH33 | 8 | 1.7003183727299 | 0.817680294756605 |
| DSH34 | 4 | 0.851108979568139 | 0.613945352039511 |
| DSH35 | 9 | 1.73972154347212 | 0.791781396138056 |
| QSH31 | 11 | 2.06528328861355 | 0.861290028819042 |
| QSH32 | 7 | 1.82387331598544 | 0.937285473777337 |
| QSH33 | 14 | 2.40919349182936 | 0.912899263230706 |
| QSH34 | 7 | 1.83437197028162 | 0.94268071481726 |
| QSH35 | 5 | 1.08701095739633 | 0.675397882079428 |

References
- Chen, L.; Guo, F.; Shao, C.; Lou, F.; Li, B.; Li, G.; Qie, H.; Wu, Z.; Jiang, Y.; Liu, F. Characteristics and controlling factors of danxia landscapes in Jiangxi Province. Acta Geol. Sin. 2022, 96, 4023–4037. [Google Scholar]
- Liu, X.; Ni, D.; Zhong, X.; Zhang, Z. Structure of benthic food web and trophic relationship of macrofauna in the Yellow Sea. Period. Ocean Univ. China 2020, 50, 20–33. [Google Scholar]
- Serrana, J.M.; Li, B.; Watanabe, K. Cross-taxa assessment of species diversity and phylogenetic structure of benthic communities in a dam-impacted river undergoing habitat restoration. Sci. Total Environ. 2025, 958, 177886. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, Q.; Zeng, J.; Yin, Z.; Liu, J.; Liu, G.; Peng, M. Macroinvertebrate community structure, pollution tolerance, diversity and feeding functional groups in polluted urban rivers under different black and odorous levels. Ecol. Indic. 2023, 156, 111148. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Li, H.; Jiang, X.; Li, Z.; Meng, X.; Xie, Z. Construction of macroinvertebrate integrity-based health assessment framework for the Chishui River. Environ. Monit. China 2018, 34, 62–72. [Google Scholar]
- Zhan, Y.; Wang, S.; Chen, J.; Wang, Y.; Wang, Y. Evaluation of aquatic ecosystem health in the middle and upper reaches of the Heihe River based on macrobenthic integrity index. J. Desert Res. 2022, 43, 271–280. [Google Scholar]
- Zhang, Q.; Ye, X.; Werner, A.D.; Li, Y.; Yao, J.; Li, X.; Xu, C. An investigation of enhanced recessions in Poyang Lake: Comparison of Yangtze River and local catchment impacts. J. Hydrol. 2014, 517, 425–434. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Q.; Li, Y.; Chen, Y.; Chen, L. An analysis of period, tendency and response characteristics of annual precipitation in Poyang Lake basin. J. Xi’an Univ. Technol. 2014, 30, 225–230. [Google Scholar]
- Zhou, C.; Jian, S.; Jiang, Z.; Chen, J.; Ouyang, S.; Wu, X. Environmental DNA reveals spatial and temporal variation in fish communities before the 10-year fishing ban in the Poyang Lake Basin. Fish. Res. 2025, 281, 107192. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y. A preliminary study of oligochaetes in Poyang Lake, the largest freshwater lake of China, and its vicinity, with description of a new species of Limnodrilus. Hydrobiologia 2001, 463, 29–38. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Joseph, S. Effect of reduced anthropogenic activities on water quality in Lake Vembanad, India during COVID-19 lockdown. Sci. Total Environ. 2021, 755, 142579. [Google Scholar]
- Paital, B.; Pati, S.G.; Panda, F.; Jally, S.K.; Agrawal, P.K. Changes in physicochemical, heavy metals and air quality linked to spot Aplocheilus panchax along Mahanadi industrial belt of India under COVID-19-induced lockdowns. Environ. Geochem. Health 2023, 45, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhan, C.; Chen, T.; Wu, H.; Wu, X. Species diversity and resource assessment of macrozoobenthos in Poyang Lake. J. Nanchang Univ. Eng. Technol. 2009, 31, 9–13. [Google Scholar]
- Shun, W.; Li, K.; Qin, H.; Liu, X.; Lv, Q.; Nie, X.; Ouyang, S.; Wu, X.; Hu, B.; Wan, S. The seasonal dynamics of macrozoobenthos in the Shallow-Lakes of Poyang Lake Wetland. J. Nanchang Univ. Nat. Sci. 2020, 44, 172–179. [Google Scholar]
- Cai, Y.; Lu, Y.; Wu, Z.; Chen, Y.; Zhang, L.; Lu, Y. Community structure and decadal changes in macrozoobenthic assemblages in Lake Poyang, the largest freshwater lake in China. Knowl. Managt. Aquatic Ecosyst. 2014, 414, 21. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zhou, Y.; Xu, Y.; Lv, Q.; Ouyang, S.; Wu, X. Temporal and spatial changes in macrozoobenthos diversity in Poyang Lake Basin, China. Ecol. Evol. 2019, 9, 6353–6365. [Google Scholar] [CrossRef]
- Zou, L.; Zou, W.; Zhang, Q.; Li, Y.; Gong, Z.; Zhang, Y.; Lu, S.; Cai, Y. Characteristics and driving factors of spatiotemporal succession of macrozoobenthos in Poyang Lake. China Environ. Sci. 2021, 41, 2881–2892. [Google Scholar]
- Xiang, L.; Xie, Z.; Du, Z.; Xie, C.; Zhang, H.; Jiang, Y.; Wu, Y. Establishment of an indicator system for health assessment of wetland ecosystem in Poyang Lake based on PSR. J. Anhui Agric. Sci. 2015, 43, 105–107. [Google Scholar]
- You, Q.; Liu, L.; Fang, N.; Yang, W.; Zhang, H.; Li, J.; Wu, Y.; Qi, S. Assessing ecological health of Poyang Lake wetland, using benthic macroinvertebrate-based index of biotic integrity (B-IBI). Acta Ecol. Sin. 2019, 39, 6631–6641. [Google Scholar] [CrossRef]
- Lu, J.; Zhong, X.; Wu, H.; Wang, H. Application of GIS for evaluating ecosystem health of Poyang Lake using benthic index of biotic integrity. Chin. J. Environ. Eng. 2016, 10, 1553–1559. [Google Scholar]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Global Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- Wang, C.; Huang, D.; Zhang, Y.; Tian, Q.; Yin, K.; Xiong, L.; Chen, Y. Assessment of integrity of macrobenthos in Lake Dongting (1988–2021) and impacts from environmental stress. J. Lake Sci. 2023, 35, 1765–1773. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Griffith, G.E.; Frydenborg, R.; Mccarron, E.; White, J.S.; Bastian, M.L. A framework for biological criteria for Florida streams using benthic macroinvertebrates. J. N. Am. Benthol. Soc. 1996, 15, 185–211. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, M.; Liu, Z.; Chen, H.; Qi, S. A health assessment using a benthic-index of biotic integrity in Ganjiang River Basin. Acta Hydrobiol. Sin. 2011, 35, 963–971. [Google Scholar]
- Blocksom, K.A.; Kurtenbach, J.P.; Klemm, D.J.; Fulk, F.A.; Coemier, S.M. Development and evaluation of the Lake Macroinvertebrate Integrity Index (LMII) for New Jersey lakes and reservoirs. Environ. Monit. Assess. 2002, 77, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Shi, H.; Li, L.; Huang, D.; Tian, Q.; Chen, X. Macrobenthic community succession during last thirty years in Dongting Lake. Ecol. Environ. Sci. 2015, 24, 1348–1353. [Google Scholar]
- Zhou, C.; Chen, J.; Guo, T.; Ouyang, S.; Wu, X. Environmental DNA metabarcoding reveals the biological community structure in Poyang Lake, China. Conserv. Genet. Resour. 2022, 14, 437–448. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, B.; Chen, C.; Hu, H. The effect of environment factors on life activity of zoobenthos. J. Zhejiang Ocean Univ. Nat. Sci. 2005, 24, 253–280. [Google Scholar]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Benzina, I.; Bachir, A.S.; Santoul, F.; Céréghino, R. Macroinvertebrate functional trait responses to environmental gradients and anthropogenic disturbance in arid-land streams of North Africa. J. Arid Environ. 2021, 195, 104626. [Google Scholar] [CrossRef]
- Gao, Y.; Rong, L.; Cao, L.; Li, K.; Lin, C.; Zhang, Z.; Xiang, H.; Yang, H. Temporal changes in headwater streams macroinvertebrate assemblages during the snowmelt season in Northeast China. Front. Environ. Sci. 2022, 10, 960254. [Google Scholar] [CrossRef]
- Chen, H.; Qu, X.; Wang, F. Research progress of river dynamic influences on the distribution of macroinvertebrates. Res. Environ. Sci. 2019, 32, 758–765. [Google Scholar]
- Wang, Y.; Yan, L.; Zeng, Q.; Tang, J.; Zhang, F.; Hu, P. Hydrological conditions for the benthic macroinvertebrates of the Western Route of the South-to-North Water Transfers Project. South-North Water Divers. Water Sci. Technol. 2024, 22, 557–565. [Google Scholar]
- Stone, N.M.; Earll, R.; Hodgson, A.; Mather, J.G.; Parker, J.; Woodward, F.R. The distributions of three sympatric mussel species (Bivalvia: Unionidae) in budworth mere, Cheshire. J. Molluscan Stud. 1982, 48, 266–274. [Google Scholar] [CrossRef]
- Ren, H.; Yuan, X.; Liu, H.; Zhang, Y.; Zhou, S. The effects of environment factors on community structure of benthic invertebrate in rivers. Acta Ecol. Sin. 2015, 35, 3148–3156. [Google Scholar] [CrossRef]
- LaZerte, B.D.; Dillon, P.J. Relative importance of anthropogenic versus natural sources of acidity in lakes and streams of central Onrario Canada. Can. J. Fish. Aquat. Sci. 1984, 41, 1664–1677. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Q.; Tang, T.; Wu, N.; Fu, X.; Li, F.; Liu, R. Spatial distribution of macroinvertebrates in Xiangxi River. Chin. J. Appl. Ecol. 2008, 19, 2443–2448. [Google Scholar]
- Lei, Y.; Zhou, C.; Ouyang, S.; Wu, X. Differences in environmental DNA monitoring of freshwater mussels from different environmental sample types. Acta Hydrobiol. Sin. 2023, 47, 412–423. [Google Scholar]
- Luo, Q.; Chiu, M.; Tan, L.; Cai, Q. Hydrological season can have unexpectedly insignificant influences on the elevational patterns of functional diversity of riverine macroinvertebrates. Biology 2022, 11, 208. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Y.; Hao, X.; Li, J.; Chen, L.; Liu, Q.; Hu, Z. On macrozoobenthic community and its relationship to environmental factors in Dishui Lake. Oceanol. Limnol. Sin. 2020, 51, 528–535. [Google Scholar]




| Screening Criteria | Reference Sampling Point | Disturbed Sampling Point |
|---|---|---|
| Shannon–Wiener diversity index (H′) | H′ ≥ 3 | H′ < 3 |
| Human disturbance activities | Minimal or no disturbance | Strong disturbance |
| Vegetation coverage | High vegetation cover, predominantly non-agricultural | Severe vegetation degradation, dominated by agricultural vegetation |
| Human residents | No human residents | Presence of human residents |
| Parameter | YR | TJ | PY | NJ | JS | SH |
|---|---|---|---|---|---|---|
| WD (m) | 12.49 ± 10.19a | 10.33 ± 4.78a | 6.5 ± 4.5b | 4.23 ± 1.95b | 4.68 ± 1.4b | 11.53 ± 8.8a |
| V (m/s) | 0.38 ± 0.2ab | 0.32 ± 0.18a | 0.21 ± 0.12b | 0.1 ± 0.02c | 0.15 ± 0.03c | 0.11 ± 0.22c |
| Turb (NTU+) | 13.5 ± 6.99bc | 25.78 ± 25.12b | 9.94 ± 8.85c | 71.33 ± 50.90a | 8.35 ± 6.11bc | 10.27 ± 7.14c |
| T (°C) | 19.4 ± 5.63a | 19.69 ± 8.40a | 20.58 ± 8.11a | 20.63 ± 0.61a | 19.16 ± 9.61a | 17.6 ± 8.61a |
| pH | 7.86 ± 0.19a | 7.55 ± 0.33b | 7.31 ± 0.60b | 7.00 ± 0.43c | 7.41 ± 0.47b | 6.74 ± 0.62c |
| Sal (mg/L) | 0.14 ± 0.07a | 0.06 ± 0.02bc | 0.04 ± 0.02c | 0.06 ± 0.11b | 0.05 ± 0.04bc | 0.03 ± 0.01c |
| DO (mg/L) | 9.29 ± 0.28b | 9.79 ± 0.45b | 9.8 ± 2.00b | 9.72 ± 1.60b | 10.15 ± 0.48b | 11.94 ± 1.61a |
| Chl-a (μg/L) | 2.49 ± 3.57c | 1.56 ± 0.91cd | 2.06 ± 1.31cd | 6.51 ± 4.16b | 0.85 ± 0.42d | 15.18 ± 4.44a |
| TN (mg/L) | 2.58 ± 0.41b | 2.21 ± 0.41a | 2.2 ± 0.55a | 2.45 ± 1.11a | 1.26 ± 0.2a | 2.51 ± 1.35a |
| TP (mg/L) | 0.14 ± 0.05bc | 0.13 ± 0.07c | 0.13 ± 0.04c | 0.08 ± 0.09a | 0.07 ± 0.02d | 0.18 ± 0.08ab |
| Dominant Species | Degree of Dominance | |||||
|---|---|---|---|---|---|---|
| YR | TJ | PY | NJ | JS | SH | |
| Nephtys oligobranchia | — | 0.15 | 0.04 | — | 0.05 | — |
| Tubifex sinicus | — | — | — | — | 0.02 | — |
| Branchiura sowerbyi | — | — | — | — | 0.06 | — |
| Bellamya purificata | — | 0.07 | 0.07 | 0.5 | — | 0.05 |
| Parafossarulus eximius | — | 0.02 | 0.05 | — | — | |
| Corbicula fluminea | — | 0.03 | 0.02 | — | — | — |
| Gammarus sp. | 0.38 | 0.16 | — | — | — | — |
| Chironomus sinicus | — | — | — | — | 0.47 | — |
| Clinotanypus sp. | — | — | — | — | 0.05 | — |
| Tanypus punctipennis | — | — | — | — | 0.02 | 0.35 |
| Ceratopogonus sp. | — | — | — | — | — | 0.3 |
| Waters | Βsor | Βsim | βsne | βsim% | βsne% |
|---|---|---|---|---|---|
| YR | 0.829 | 0.620 | 0.208 | 74.86 | 25.14 |
| TJ | 0.778 | 0.395 | 0.383 | 50.78 | 49.22 |
| PY | 0.838 | 0.454 | 0.384 | 54.18 | 45.82 |
| NJ | 0.722 | 0.511 | 0.211 | 70.78 | 29.22 |
| JS | 0.524 | 0.289 | 0.235 | 55.10 | 44.90 |
| SH | 0.570 | 0.345 | 0.225 | 60.49 | 39.51 |
| M1 | M7 | M8 | M15 | M17 | M19 | M20 | M22 | M28 | |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 1 | ||||||||
| M7 | 0.665 ** | 1 | |||||||
| M8 | 0.656 ** | 0.983 ** | 1 | ||||||
| M15 | 0.469 ** | 0.834 ** | 0.811 ** | 1 | |||||
| M17 | 0.845 ** | 0.686 ** | 0.689 ** | 0.452 ** | 1 | ||||
| M19 | 0.874 ** | 0.730 ** | 0.736 ** | 0.486 ** | 0.967 ** | 1 | |||
| M20 | −0.552 ** | −0.384 ** | −0.390 ** | −0.154 | −0.762 ** | −0.670 ** | 1 | ||
| M22 | 0.692 ** | 0.500 ** | 0.492 ** | 0.318 * | 0.692 ** | 0.687 ** | −0.482 ** | 1 | |
| M28 | 0.516 ** | 0.822 ** | 0.796 ** | 0.977 ** | 0.495 ** | 0.524 ** | −0.198 | 0.363 ** | 1 |
| Healthy | Sub-Healthy | Moderate | Poor | Extremely Poor | |
|---|---|---|---|---|---|
| B-IBI | IBI > 2.07 | 1.55 < IBI ≤ 2.07 | 1.04 < IBI ≤ 1.55 | 0.52 < IBI ≤ 1.04 | IBI ≤ 0.52 |
| Health Condition | YR | TJ | PY | NJ | JS | SH | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Points | Proportion | Sample Points | Proportion | Sample Points | Proportion | Sample Points | Proportion | Sample Points | Proportion | Sample Points | Proportion | Sample Points | Proportion | |
| Healthy | 1 | 33.33% | 1 | 16.67% | 4 | 36.36% | 3 | 60.00% | 3 | 60.00% | 1 | 20.00% | 13 | 37.14% |
| Sub-healthy | — | — | — | — | 4 | 36.36% | 2 | 40.00% | 2 | 40.00% | 2 | 40.00% | 10 | 28.57% |
| Moderate | — | — | 5 | 83.33% | 1 | 9.09% | — | — | — | — | 2 | 40.00% | 8 | 22.86% |
| Poor | 1 | 33.33% | — | — | 1 | 9.09% | — | — | — | — | — | — | 2 | 5.71% |
| Extremely Poor | 1 | 33.33% | — | — | 1 | 9.09% | — | — | — | — | — | — | 2 | 5.71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhao, R.; Xia, W.; Zeng, F.; Deng, Y.; Wang, W.; Ouyang, S.; Wu, X. Characterizing Spatio-Temporal Variation in Macroinvertebrate Communities and Ecological Health Assessment in the Poyang Lake Basin During the Early Stage of a Fishing Ban. Animals 2025, 15, 2440. https://doi.org/10.3390/ani15162440
Zhou C, Zhao R, Xia W, Zeng F, Deng Y, Wang W, Ouyang S, Wu X. Characterizing Spatio-Temporal Variation in Macroinvertebrate Communities and Ecological Health Assessment in the Poyang Lake Basin During the Early Stage of a Fishing Ban. Animals. 2025; 15(16):2440. https://doi.org/10.3390/ani15162440
Chicago/Turabian StyleZhou, Chunhua, Ruobing Zhao, Wenxin Xia, Fangfa Zeng, Yanqing Deng, Wenhao Wang, Shan Ouyang, and Xiaoping Wu. 2025. "Characterizing Spatio-Temporal Variation in Macroinvertebrate Communities and Ecological Health Assessment in the Poyang Lake Basin During the Early Stage of a Fishing Ban" Animals 15, no. 16: 2440. https://doi.org/10.3390/ani15162440
APA StyleZhou, C., Zhao, R., Xia, W., Zeng, F., Deng, Y., Wang, W., Ouyang, S., & Wu, X. (2025). Characterizing Spatio-Temporal Variation in Macroinvertebrate Communities and Ecological Health Assessment in the Poyang Lake Basin During the Early Stage of a Fishing Ban. Animals, 15(16), 2440. https://doi.org/10.3390/ani15162440





