Pilot Study: Heart Rate and Heart Rate Variability Indices in Mules Evaluated by 24-Hour Electrocardiogram
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. aECG Recordings
2.3. Statistical Analysis
3. Results
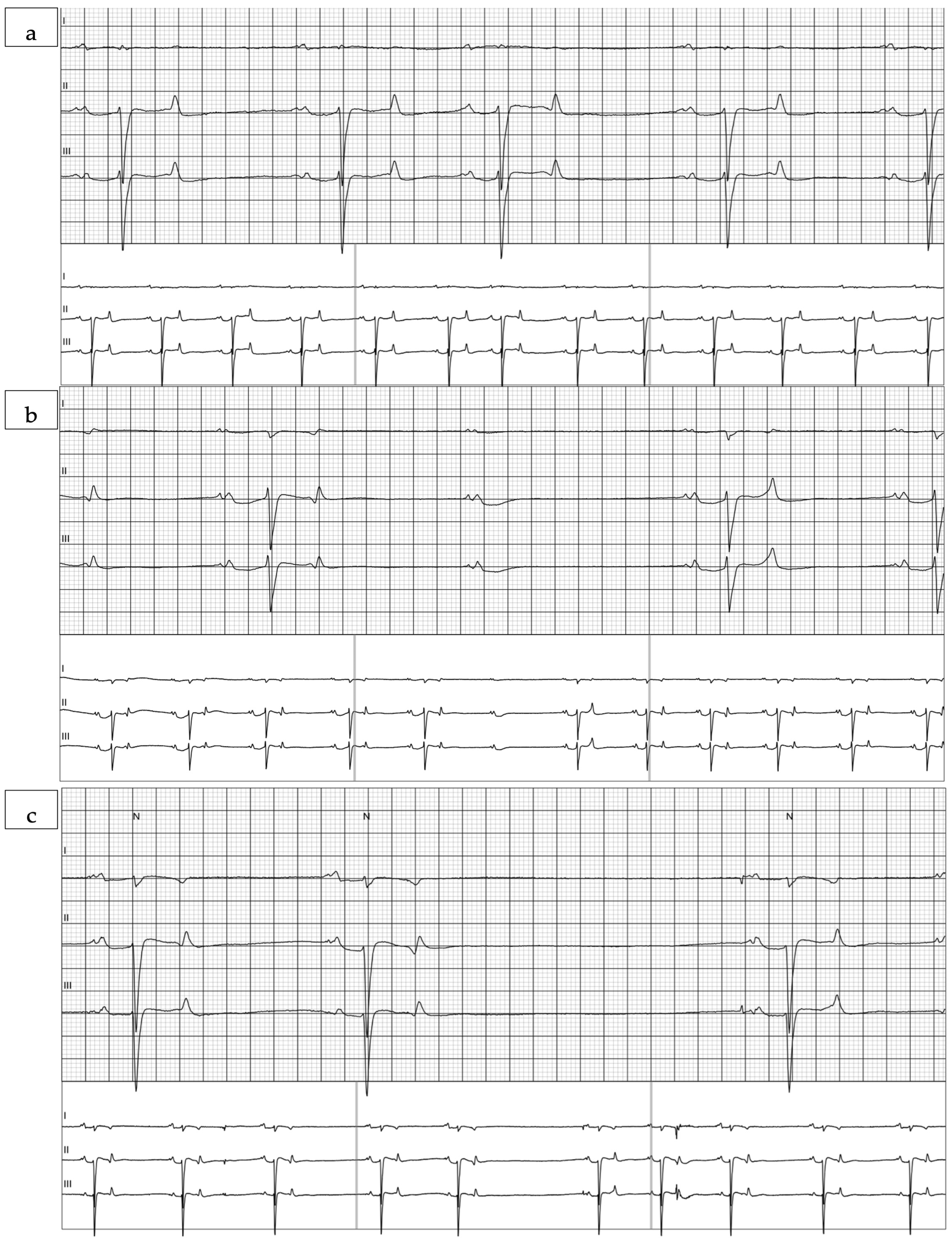
3.1. Auscultation Findings
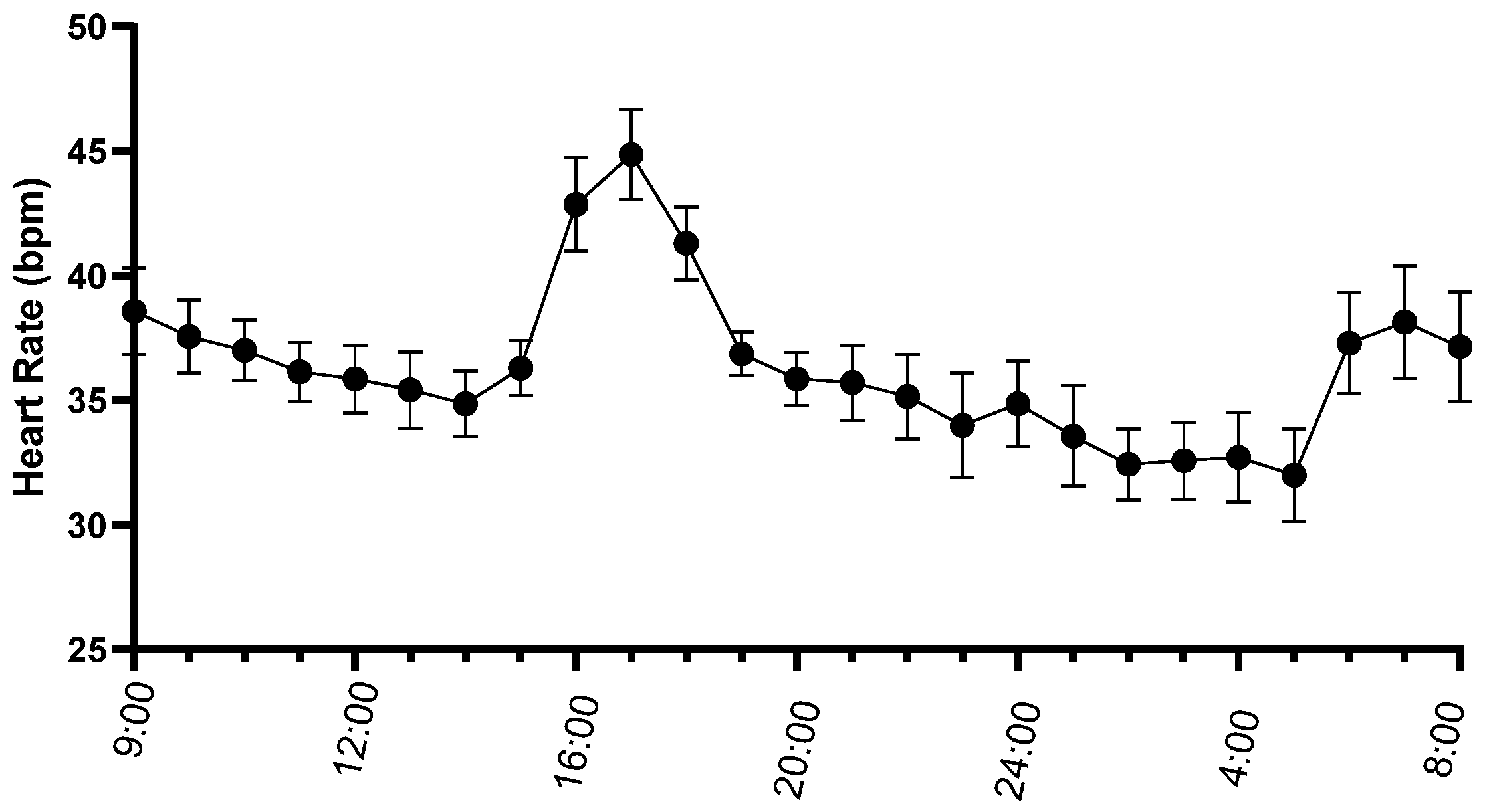
3.2. aECG Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| HRV | Heart rate variability |
| HR | Heart rate |
| aECG | Ambulatory electrocardiogram |
| 2DAVB | Second-degree atrioventricular block |
| SVPCs | Supraventricular premature complexes |
| VCs | Ventricular complexes |
| SDNN | Standard deviation of normal intervals |
| SDANN | Standard deviation of the average NN intervals |
| RMSSD | Root mean square of successive intervals |
| TI | Triangular index |
| SD | Standard deviation |
| IQR | Interquartile range |
| bpm | Beats per minute |
| ANS | Autonomic nervous system |
References
- The American Horse Council Foundation. 2023 Economic Impact Study of the U.S. Horse Industry. 2023. Available online: https://horsecouncil.org/economic-impact-study/ (accessed on 30 June 2025).
- Equine 2015: Baseline Reference of Equine Health and Management in the United States. 2016. United States Department of Agriculture. Report 1. Available online: https://www.aphis.usda.gov/sites/default/files/eq2015_rept1.pdf (accessed on 30 June 2025).
- Norris, S.L.; Little, H.A.; Ryding, J.; Raw, Z. Global donkey and mule populations: Figures and trends. PLoS ONE 2021, 16, e0247830. [Google Scholar] [CrossRef]
- Escudero, A.; González, J.R.; Benedito, J.L.; Prieto, F.R.; Ayala, I. Electrocardiographic parameters in the clinically healthy Zamorano-leones donkey. Res. Vet. Sci. 2009, 87, 458–461. [Google Scholar] [CrossRef]
- Maas, L.T.; Louie, E.W.; Finno, C.J.; Donnelly, C.G.; Stern, J.A.; Hill, A.E.; Morgan, J.M. Cardiac arrhythmia prevalence and risk factors in 24-h electrocardiograms of sedentary horses. Equine Vet. J. 2025. [Google Scholar] [CrossRef]
- Nissen, S.; Weis, R.; Krag-Andersen, E.; Hesselkilde, E.; Isaksen, J.; Carstensen, H.; Kanters, J.; Linz, D.; Sanders, P.; Hopster-Iversen, C.; et al. Electrocardiographic characteristics of trained and untrained standardbred racehorses. J. Vet. Intern. Med. 2022, 36, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chadda, K.; Matthews, G.; Marr, C.; Huang, C.; Jeevaratnam, K. Cardiac electrophysiological adaptations in the equine athlete—Restitution analysis of electrocardiographic features. PLoS ONE 2018, 13, e0194008. [Google Scholar] [CrossRef]
- Matthews, N. Donkeys—Not Just Small Horses. North Am Vet Community. 2010. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20103178201 (accessed on 18 June 2025).
- The Donkey Sanctuary. The Clinical Examination: Parameters and Important Points. Factsheet: Vets. 2013. Available online: https://www.thedonkeysanctuary.org.uk/sites/default/files/2024-08/the-donkey-clinical-examination.pdf (accessed on 18 June 2025).
- Matthews, N.S.; Taylor, T.S.; Potter, G.D. Physiologic responses during an exhaustive driving test in donkeys: Effect of conditioning. Appl. Anim. Behav. Sci. 1998, 59, 31–38. [Google Scholar] [CrossRef]
- Cruz-Aleixo, A.S.; De Oliveira, K.C.; De Oliveira Ferreira, L.V.; Quevedo, D.A.C.; Cruz, R.K.S.; Tsunemi, M.H.; Chiacchio, S.B.; Lourenco, M.L.G. Electrocardiographic and echocardiographic parameters in pega breed donkeys: A descriptive study. Animals 2023, 13, 861. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Anjum, A.D. Rectal temperature, pulse rate and breath rate in mules. Pak. J. Biol. Sci. 1998, 14, 271–273. [Google Scholar] [CrossRef]
- McLean, A.K. Comparing the physiological and biochemical parameters of mules and hinnies to horses and donkeys. In Proceedings of the International Hydra Mule and Donkey Conference, Hydra, Greece, 10–12 October 2014. [Google Scholar]
- Guccione, J.; Piantedosi, D.; Di Loria, A.; Veneziano, V.; Ciaramella, P. Long-term electrocardiography recording with holter monitoring in 15 donkeys. J. Equine Vet. Sci. 2014, 34, 302–306. [Google Scholar] [CrossRef]
- Raekallio, M. Long term ECG recording with holter monitoring in clinically healthy horses. Acta Vet. Scand. 1992, 33, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Raftery, A.G.; Cripps, P.; Senior, J.M.; McGowan, C.M. The prevalence and nature of cardiac arrhythmias in horses following general anaesthesia and surgery. Acta Vet. Scand. 2011, 53, 62. [Google Scholar] [CrossRef] [PubMed]
- Reef, V.B.; Bonagura, J.; Buhl, R.; MrGurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for management of equine athletes with cardiovascular abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Barbesgaard, L.; Buhl, R.; Meldgaard, C. Prevalence of exercise-associated arrhythmias in normal performing dressage horses. Equine Vet. J. 2010, 42, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; Meldgaard, C.; Barbesgaard, L. Cardiac arrhythmias in clinically healthy showjumping horses. Equine Vet. J. 2010, 42, 196–201. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.K.; Heleski, C.R.; Yokoyama, M.T.; Wang, W.; Doumbia, A.; Dembele, B. Improving working donkey (Equus asinus) welfare and management in Mali, West Africa. J. Vet. Behav. 2012, 7, 123–134. [Google Scholar] [CrossRef]
- Panzera, M.; Alberghina, D.; Statelli, A. Ethological and physiological parameters assessment in donkeys used in animal assisted interventions. Animals 2020, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Huangsaksri, O.; Wonghanchao, T.; Sanigavatee, K.; Poochipakorn, C.; Chanda, M. Heart rate and heart rate variability in horses undergoing hot and cold shoeing. PLoS ONE 2024, 19, e0305031. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, E.K.; Baldwin, A.; Schiltz, P.M. Heart rate variability in horses engaged in equine-assisted activities. J. Equine Vet. Sci. 2011, 31, 78–84. [Google Scholar] [CrossRef]


| Mule | Sex | Age | Breed of Dam | Use |
|---|---|---|---|---|
| Mule 1 | Molly | 3 | Quarter Horse | Show/Pleasure |
| Mule 2 | Gelding | 13 | Quarter Horse | Endurance |
| Mule 3 | Molly | 9 | Appaloosa | Show/Pleasure |
| Mule 4 | Molly | 10 | Appaloosa | Endurance |
| Mule 5 | Gelding | 20 | Quarter Horse | Gymkhana |
| Mule 6 | Molly | 10 | Appaloosa | Show/Pleasure |
| Mule 7 | Gelding | 9 | Warmblood | Show/Pleasure |
| Parameter | Mean ± SD | Median (IQR) | Max | Min | n |
|---|---|---|---|---|---|
| RMSSD (ms) | --- | 109.0 (83.0–243.0) | 657.0 | 51.0 | 7 |
| SDNN (ms) | 317.9 ± 91.9 | --- | 494.0 | 201.0 | 7 |
| SDNN Index (ms) | --- | 161.0 (145.0–201.0) | 394.0 | 129.0 | 7 |
| SDANN (ms) | 214.7 ± 54.8 | --- | 282.0 | 130.0 | 7 |
| Triangular Index (ms) | 59.0 ± 14.8 | --- | 85.0 | 42.0 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maas, L.T.; Morgan, J.M.; Case, J.; Chell, D.D.; McLean, A.K. Pilot Study: Heart Rate and Heart Rate Variability Indices in Mules Evaluated by 24-Hour Electrocardiogram. Animals 2025, 15, 2438. https://doi.org/10.3390/ani15162438
Maas LT, Morgan JM, Case J, Chell DD, McLean AK. Pilot Study: Heart Rate and Heart Rate Variability Indices in Mules Evaluated by 24-Hour Electrocardiogram. Animals. 2025; 15(16):2438. https://doi.org/10.3390/ani15162438
Chicago/Turabian StyleMaas, Lauren T., Jessica M. Morgan, Jordan Case, David D. Chell, and Amy K. McLean. 2025. "Pilot Study: Heart Rate and Heart Rate Variability Indices in Mules Evaluated by 24-Hour Electrocardiogram" Animals 15, no. 16: 2438. https://doi.org/10.3390/ani15162438
APA StyleMaas, L. T., Morgan, J. M., Case, J., Chell, D. D., & McLean, A. K. (2025). Pilot Study: Heart Rate and Heart Rate Variability Indices in Mules Evaluated by 24-Hour Electrocardiogram. Animals, 15(16), 2438. https://doi.org/10.3390/ani15162438






