1. Introduction
Poultry is the most consumed animal protein source in the world, and the trend is expected to continue increasing [
1]. The world population reached 8 billion in 2022, and is likely to reach 9 billion in 2037 [
2], which will continue to increase pressure on the global chicken-meat industry. One way to meet this increasing demand is to improve the efficiency of chicken-meat production. Precision nutrition is a strategy to improve growth efficiency, reducing the resources needed for chicken-meat production and improving the profitability of the chicken-meat industry. The concept of precision nutrition aims to meet the birds’ daily nutrient requirement according to their growth rate. Broiler production involves constant changes in nutrient requirements as they grow rapidly, so birds may not receive sufficient nutrients if they are under-fed throughout the production cycle [
3]. Consequently, a precision nutrition regime can reduce nutrient waste by adjusting the nutrient supply to more closely meet the daily requirement and prevent over-feeding of nutrient [
4]. It was reported that by implementing a precision nutrition regime, the feed efficiency of broilers increased by 4.6% [
5]. Consequently, based on this figure, the Australian poultry industry could save AUD 60 million by reducing feed production by 145,000 tons, compared with birds on a standard program of four diet phases [
5].
Kleyn [
3] indicated that blending a precise ration to meet a broiler’s daily energy and lysine requirements improved feed efficiency and reduced the coefficient of variation, as compared to a two-phase diet program. Similar results were found in pigs, where precision feeding reduced apparent digestible lysine intake by 26% without compromising growth performance compared to conventional feeding, saving USD 7.60 per pig [
6]. In addition to reduced production cost, a similar study in pigs showed that a precision nutrition program reduced nitrogen and phosphorus excretion by 38% compared to traditional phase feeding, potentially benefiting the environment [
7]. It is thought that by using synthetic amino acids, precision nutrition ensures the right balance of apparent digestible amino acids (e.g., lysine, methionine, threonine), reducing excess crude protein and, in turn, contributing to less nitrogen waste in manure.
Many studies have suggested that increasing the number of feeding phases makes feeding more efficient; however, pelleting, transporting, and storing four or more separate diets is often impractical [
5,
8]. Nevertheless, modern feed blending systems can automatically blend dietary components daily to achieve the desired nutrient profile. Thus, a protein dense concentrate diet can be formulated for day-old chicks, which can then be diluted by an energy dense concentrate on a daily basis to meet the nutritional needs. As this process requires only two concentrates to meet the daily needs of broilers, this program may now not be limited by the practicalities of feed transportation and storage of multiple diets.
Despite these potential benefits, there have not been many studies performed on precision nutrition strategies for broilers, as precision nutrition requires the use of equipment that is not at present commonly used in broiler sheds due to historically prohibitive cost; including feed blending and delivery systems, plus the cost of the extra silos required (two per shed vs. one per shed). Further, advanced tools including near-infrared spectroscopy (NIRS), are also needed to ensure the accuracy of feed formulations, but may not be affordable for non-integrated producers. Nevertheless, with the cost of technology reducing over time, this equipment is rapidly becoming more affordable for commercial use. Thus, it is timely to revisit the potential of precision nutrition regimes to improve the efficiency of chicken-meat production. To do so, as most of the current information is from studies in pigs, further study in broiler chickens is needed to confirm if precision nutrition may be an effective strategy to improve the efficiency of production.
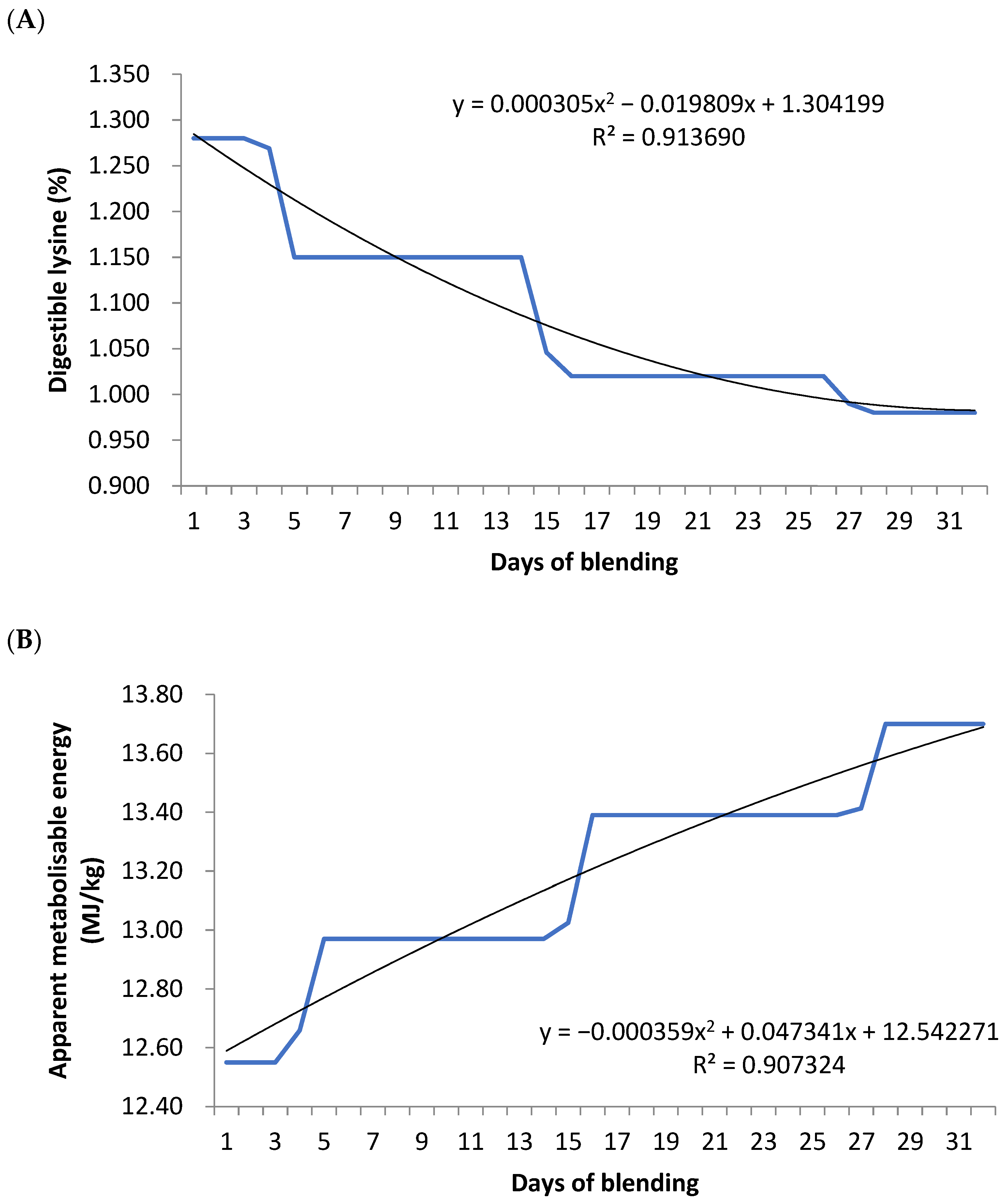
Therefore, this study explored the development and implementation of a precision feeding program that blends two dietary components to meet daily broiler energy and protein requirements via modern feeding technology, to test the hypothesis that precision nutrition will increase the efficiency and profitability of chicken-meat production. For the first time, this includes not only blending the diets but also adjusting the diet blends fed to birds based on their bodyweight to more accurately meet their requirements.
4. Discussion
Feed accounts for more than 65% of the total cost of chicken-meat production [
16]. Hence, increasing feed efficiency could increase the productivity of the poultry industry and improve economic sustainability. The present study demonstrated that precision nutrition diet programs may improve feed efficiency in the early stage of broiler production compared to the conventional phase-feeding regime, as evidenced by an improved FCR between days 14 and 21 post-hatch. It is interesting to note that the impact of the precision nutrition program on FCR was observed over the first half of the study. The grower and finisher period immediately follow the greatest change in protein concentration during the diet change (starter to grower diets and grower to finisher diets, respectively); therefore, the greatest response should be seen immediately following these periods. The daily nutrient requirement in the present study was calculated by the EFG broiler growth model. Instructively, in a previous study, Gutierrez et al. [
17] also found that blending two dietary components to meet the daily nutrient requirement as calculated by the EFG Broiler Growth Model improved weight gain and feed efficiency, especially from days 21 to 28 and 35 to 42, thereby reducing feed costs per kilogram of weight gain.
Gutierrez et al. [
17] study fed the precision nutrition diets based on the calculated nutrient requirement from the EFG Model, but did not adjust the blends based on the birds’ actual performance. In the present study, birds offered the precision nutrition treatments grew heavier than other treatments as the experiment progressed. As heavier birds have a higher FCR, weight corrected FCR was calculated for the study duration (11 to 42 days), and it was found that birds offered the adjusted precision nutrition treatment improved weight corrected FCR by 7.8% compared to the control and blended standard diet treatments. Additionally, the precision nutrition adjusted treatment finished with birds reaching the final target blend (100% low protein, high energy concentrate) five days sooner than the precision nutrition treatment. This resulted in a reduction in feed cost of 3.2 cents/kg body weight, or by 4.13% compared to the control diet (based on 2020 costings). Thus, the present study is consistent with the Gutierrez et al. [
17] findings.
Feed intake and growth rate are associated with greater body fat accumulation in broiler chickens [
18]. Considering the low economic value of broiler fat, and consumer preferences for lean meat, excessive deposition of fat presents a challenge for poultry producers and consumers alike. Birds on precision nutrition feeding programs may accumulate less fat within the body due to a reduced dietary over-supply of energy which can become stored as fat [
18]. However, in this study, birds receiving precision nutrition treatments showed no significant changes to fat pads and additionally, no significant difference in breast, thigh and drumstick weight at 28 and 42 days of age. In a similar study, Roush et al. [
19] showed that broilers fed blended diets (starter and grower, starter and finisher, and grower and finisher), similar to that of the present study’s ‘blended standard diets’ treatment, did not differ significantly in final body weight, fat pads, or breast muscle from those offered traditional 4 phase feeding programs. Entire fat pad removal from carcasses can be difficult, and thus it may be worthwhile exploring if birds offered high-protein low energy diets may exhibit reduced mRNA expression of hepatic malic enzyme (HME), acetyl coenzyme carboxylase (ACC), and fatty acid synthase (FAS); key enzymes in the de novo lipogenesis pathway in chickens [
20], however this has not yet been explored. Contrary to the present study and the Roush et al. [
19] study, Moss et al. [
21] found that carcass dressed weight increased from 2.282 to 2.502 kg (
p = 0.001), resulting in a decrease in the cost per kilogram of chicken-meat from 71.4 cents to 66.3 cents under a precision feeding program in comparison to a standard 4 phase feeding regimen at 42 days. Thus, the effect of precision nutrition programs on carcass composition is somewhat conflicting.
Improved flock uniformity may bring savings at the processing plant and is also a potential welfare indicator, associated with increased rejection rates at slaughter [
22]. It is possible that reducing excessive nutrient supply may have contributed to the observed reduction in CV (improved flock uniformity) at 14 and 42 days within the precision nutrition adjusted treatment compared to the control treatment in the current study. The number of precision feeding studies on broilers is lacking, however the effect of precision feeding for broiler breeders has gained significant recent attention. Zuidhof [
23] recorded that in comparison to conventional feeding in broiler breeders, the precision feeding station developed by his laboratory has produced 100% flock uniformity for seven of the last 10 weeks of a pilot study. It was explained in Zuidhof [
23] that the precise feeding stations (automatic weighing and tracking) can provide real-time adjustments of nutrients, preventing aggressive birds from overeating and providing equal feeding opportunities to all birds. Nevertheless, this approach is substantially different from the precision nutrition programs discussed in the present study, as it manipulates feed intake (i.e., precision feeding) and not the nutrient content of the diet (i.e., precision nutrition).
In addition to the potential improvements in efficiency and CV, precision nutrition programs also present some logistical benefits for chick performance. The poultry industry faces a logistical challenge when it starts a new batch of broilers, of how to dispose of unused feed. While it can be removed, this provides an extra cost and wastage [
24]. Thus, the withdrawal feed remaining in the silo unavoidably gets fed to the next batch of young chicks that arrive to the farm until it runs out and is replaced with the new starter feed. Precision nutrition programs avoid this logistics issue, as the concentrates are required for all stages, and so the blend percentages only need to be reset for the next flock. Thus, any leftover feed can be utilized while still meeting the next flock’s nutrient requirements.
The content of soybean meal was higher in the low protein blend than the finisher and withdrawal diets, and the content of oil was higher in the high-protein blend than the starter diet in the present study. Thus, precision nutrition diets are sensitive to the price of soybean meal and the price of oil compared to the control diet in the present study. To demonstrate this sensitivity, diet cost calculations are provided for both 2020 and updated for 2022 which saw an increased soybean meal and oil price over this period due to significant global events [
25]. However, this may be avoided partly by utilizing alternative protein and oil sources that are cheaper.
The blended standard diet treatment of the present study was included to determine if some of the benefits of blending diets could be achieved by gradually blending the four feeding phases, which may aid the adoption of the feeding strategy. We hypothesized that while not precisely meeting protein and energy requirements as well as the protein and energy blends, it may still generate benefit from eliminating sudden diet changes. It has been demonstrated in multiple animal species that a sudden diet change disrupts the gut via the microbiome [
26,
27,
28]; thus, a gradual blending of diets would provide less disruption to the gut. While we did not measure gut microbiome in the present study, this effect appears not to have been realized, as blended standard diets saw a reduction in multiple parameters including nutrient digestibility and energy utilization. Thus, blending the standard 4-phase diets may not present an alternative to the concentrate blends in the precision nutrition diets.
The apparent metabolizable energy (AME) adjusted for zero nitrogen retention (AMEn) is commonly used to evaluate the energy value of ingredients [
29]. Precision nutrition adjusted treatments showed the highest AME among dietary treatments from 25 to 27 days of age, which was significantly improved in comparison to the control. The AME was measured following the feed swap from grower to finisher diets for the control treatment. Thus, the improvement may have been attributed to either (i) more precisely meeting the energy requirement, and/or (ii) the lack of disturbance in the gut from a sudden diet change. While there is very limited research on the impact of changing feeding phases on the gut and performance of broiler chickens, there is a proven link between the gut microbiome and the maintenance of host circadian rhythms and metabolic homeostasis in several species [
30,
31]. Therefore, if the gut microbiome is disturbed under a sudden feed change, it is sensible that metabolic homeostasis would be disrupted which would disrupt the performance of the chicken [
32]. Thus, this may be another avenue by which precision nutrition may enhance broiler performance. However, as we did not see any effect from the blended standard diets treatment, blending diets to reduce impact on the gut microbiome may not be the main reason for the improvements seen.
The present study demonstrates the benefits of precision feeding regimes. However, there are some barriers to practical industry adoption. Firstly, farms usually only have one silo per shed, and so investment would need to be made in both a feed blending system and an extra silo. However, as estimated by Moss et al. [
5], the cost of the initial outlay for equipment would be recovered within a short timeframe. Secondly, while the two silos of energy concentrates do create logistical advantages as discussed above, this system is more complex and would require farm staff who are interested in learning and utilizing this technology. There does appear to be an appetite of producers for such technology, as a recent survey revealed that while broiler producers were generally unfamiliar with what technology is available in precision livestock farming for broiler production systems, they would be willing to adopt new technology given it proved to increase farm productivity and profitability [
33].