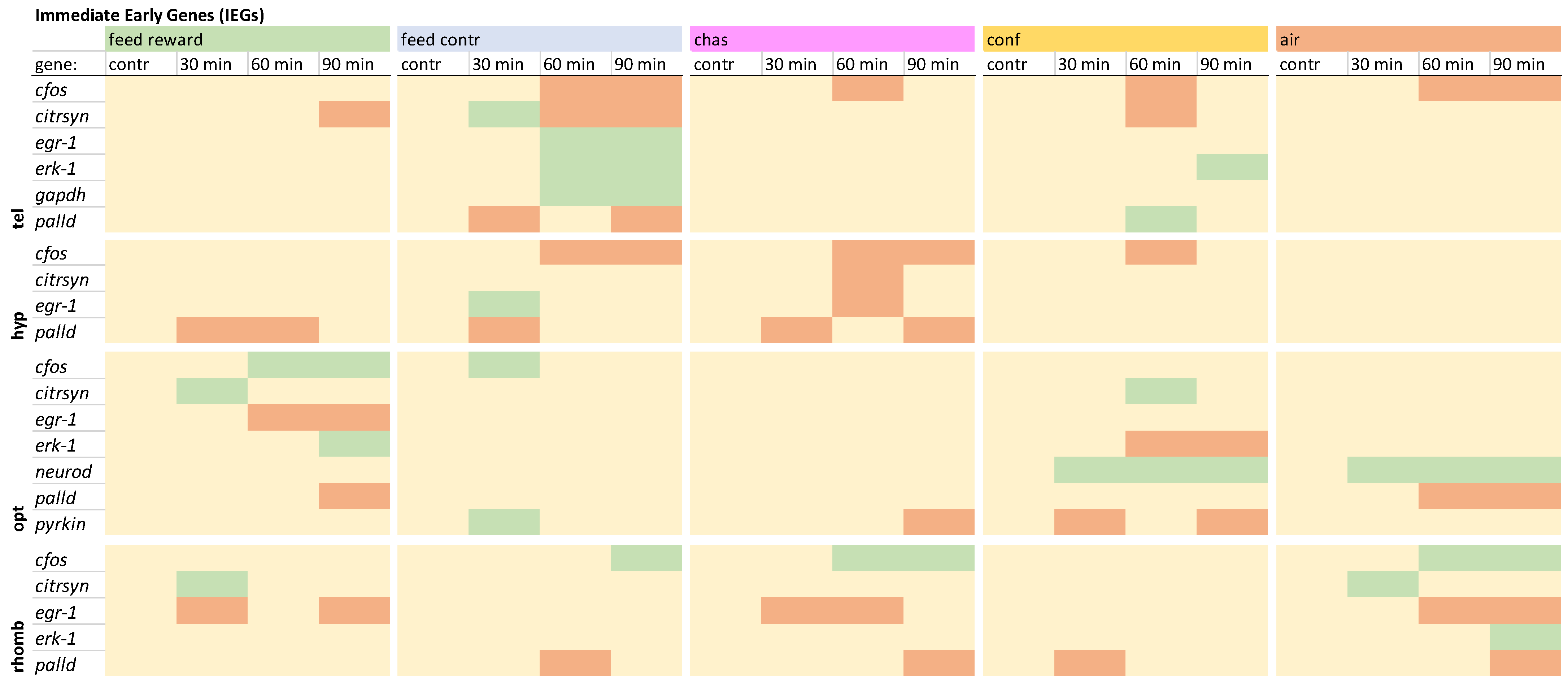
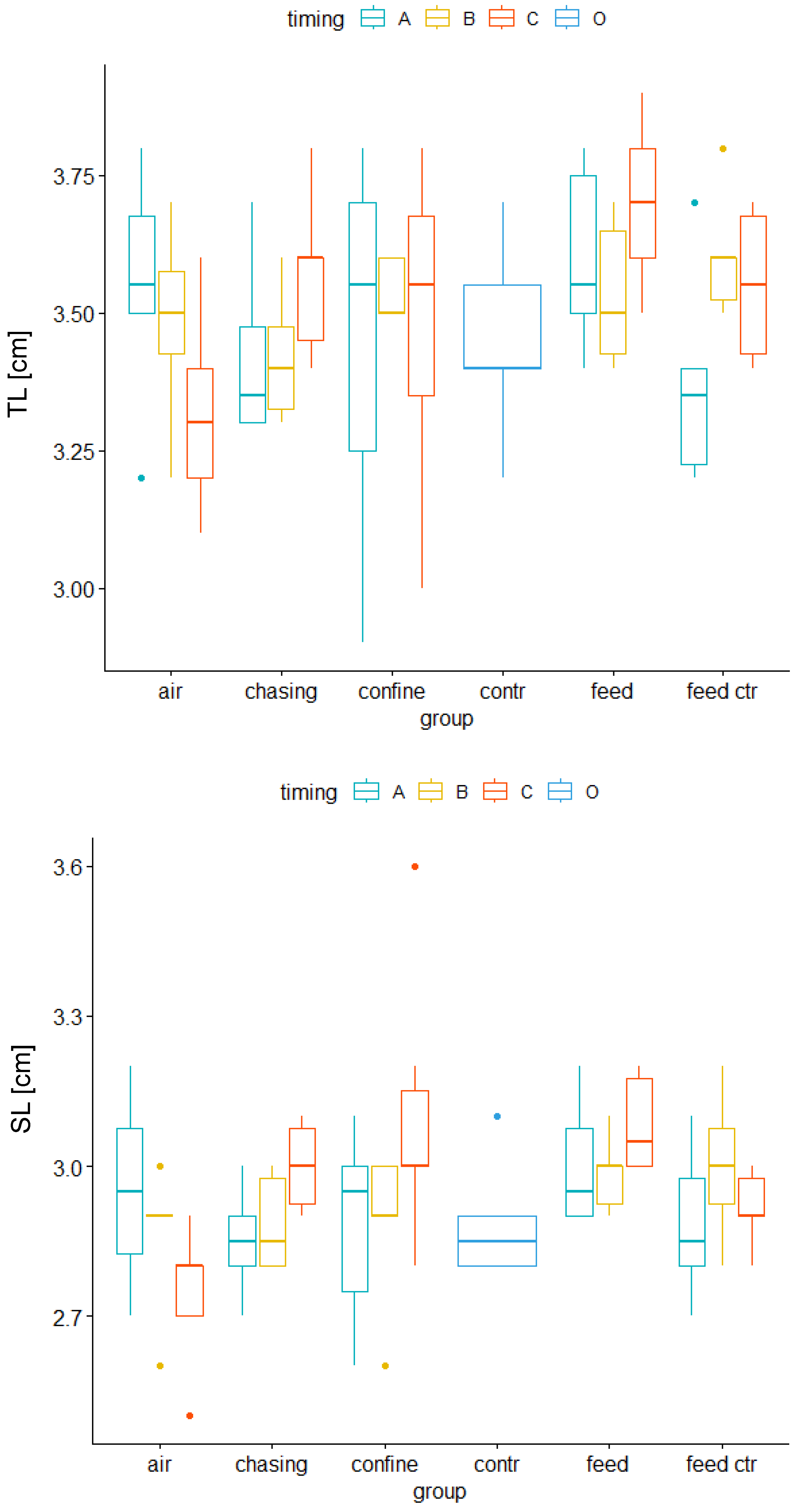
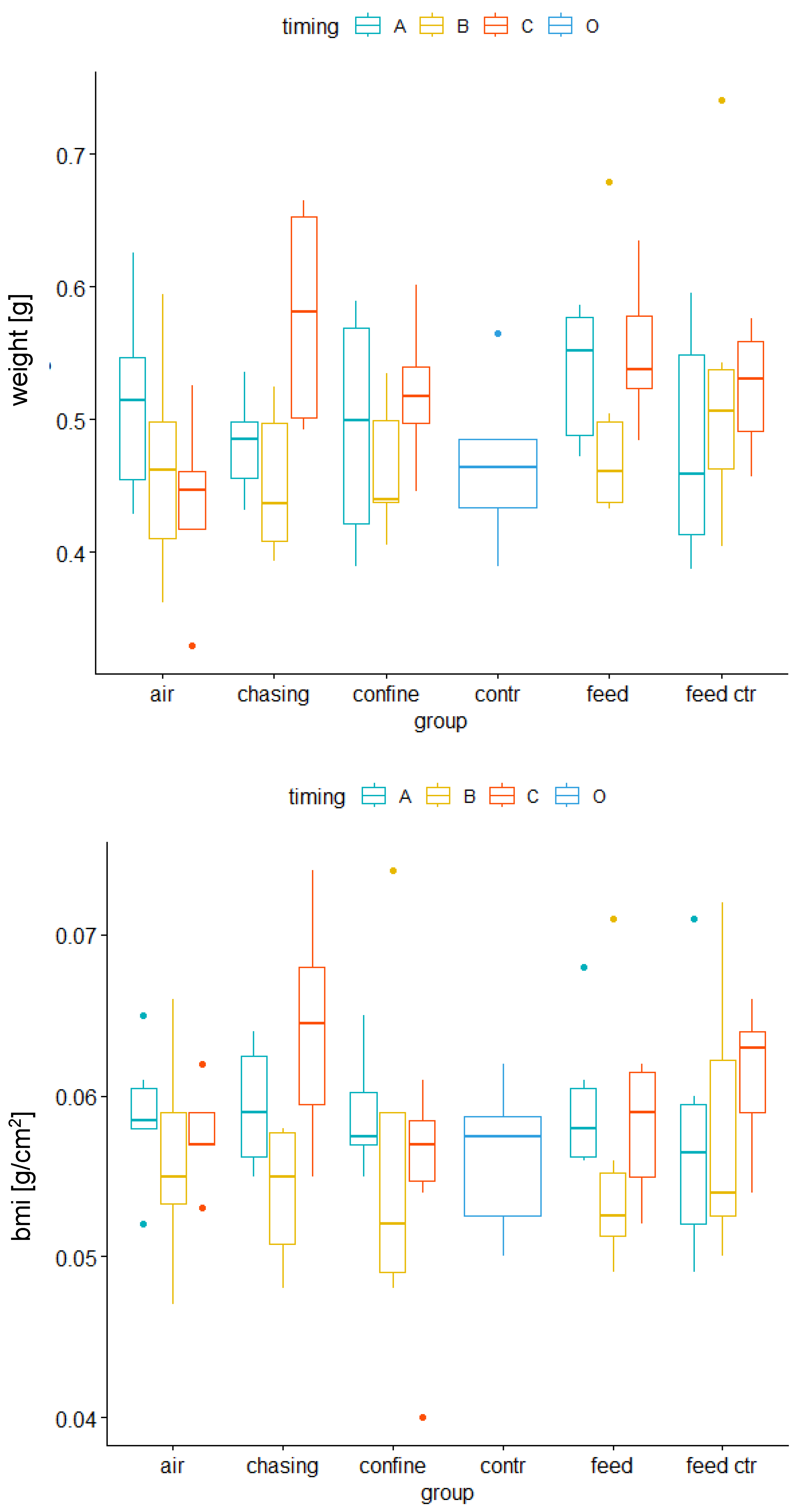
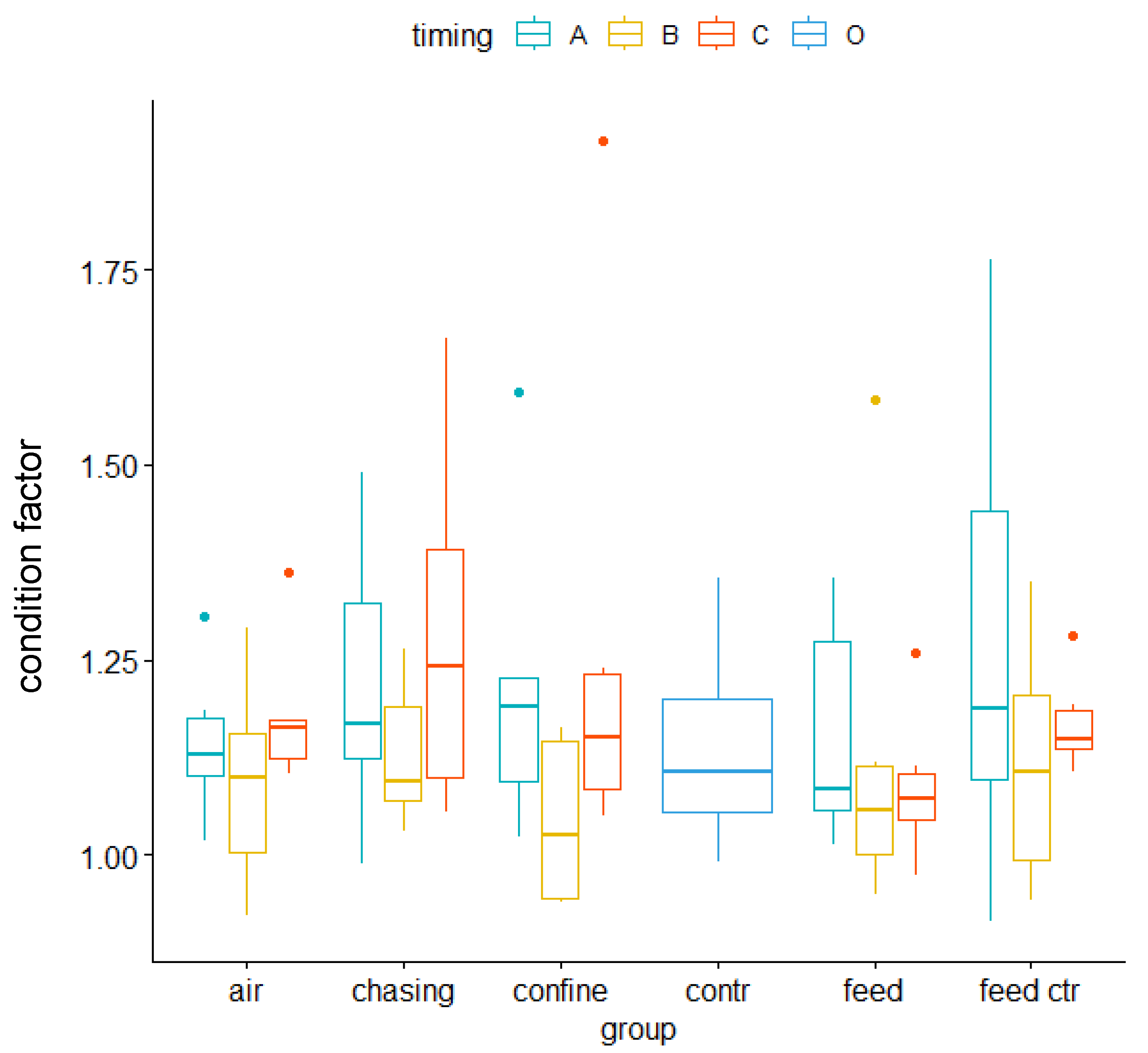
3.2. Immediate Early Genes and Metabolic Genes
The immediate early genes in the telencephalon showed different gene expression patterns for all acute stressors that had been used (
Figure 1). Only the distressed fish and the feed-control fish displayed a mutual down-regulation of
c-fos 60 min after treatment. Furthermore, the feed-control fish were the only ones showing an up-regulation of
citrsyn, egr1, erk1, and
gapdh. Moreover, the gapdh expression was not changed in the other brain parts that were investigated. In addition, chased fish showed an up-regulation of
palld. The feed-control fish showed a down-regulation of this gene in the telencephalon, and also, in the remaining brain parts, only down-regulation of
palld was observed. The detailed expression pattern visualizations for the telencephalon can be found in
Figure S1, uploaded in the repository.
In the hypothalamus, only feed-reward, feed-control, and chased fish showed decreases of
palld (
Figure 1). The expression of
egr1 displayed a difference between chased and feed-control fish, with a decreased in the chased fish and an up-regulation of this gene in the feed-control fish. In addition, a down-regulation
c-fos was absent in the feed and the air-exposure groups compared with the remaining treatment groups. A detailed visualization of expression pattern in the hypothalamus can be found in
Figure S2, uploaded in the repository.
Figure 1.
Overview of the expression profiles of early immediate genes (IEGs) in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
Figure 1.
Overview of the expression profiles of early immediate genes (IEGs) in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
In contrast to those in the telencephalon and the hypothalamus, the gene expression patterns of
c-fos in the optic tectum were increased in the feed and the feed-control groups (
Figure 1). The expression of
citrsyn was only higher in the feed-reward and confinement groups. The expression of
egr-1 was only down-regulated in the feed-rewarded fish 60 and 90 min after treatment. In addition,
erk-1 was only up-regulated in feed-rewarded fish and down-regulated in confined fish. Furthermore,
neurod was up-regulated in all chased and air-exposed fish. In addition,
pyrkin showed up-regulation in the feed-control fish but down-regulation in chased and confined animals. The detailed expression pattern visualizations can be found in
Figure S3, uploaded in the repository.
The IEGs in the rhombencephalon showed increased expression of
c-fos 60 min after chasing and air exposure, as well as 90 min after treatment in the feed-control, chasing, and air-exposure groups (
Figure 1). The expression of
citrsyn was only higher in the feed-reward and air-exposure groups. The expression of
egr-1 was not down-regulated in the feed-control or the confinement treatment. In addition,
erk-1 was only up-regulated in air-exposed fish 90 min after treatment. Finally,
palld did not show a down-regulation in the feed-rewarded fish compared with the remaining treatments. The detailed expression pattern visualizations can be found in
Figure S4, uploaded in the repository.
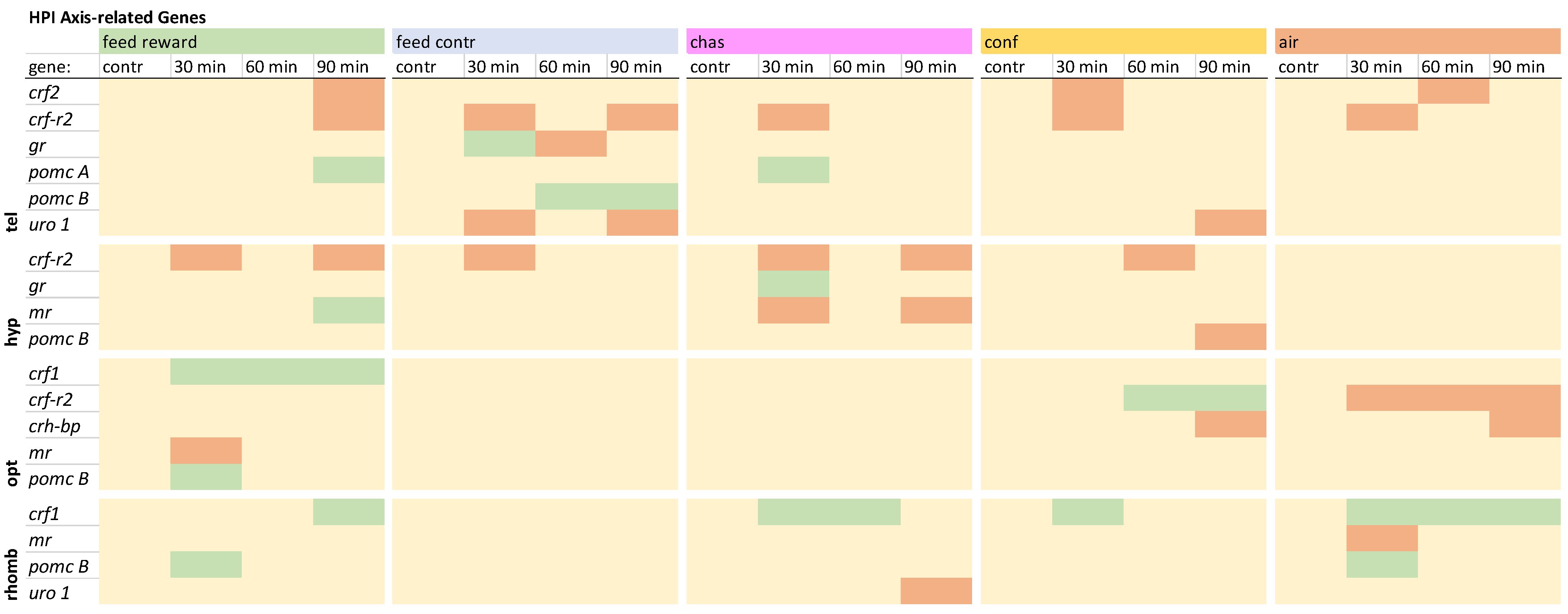
3.3. HPI Axis-Related Genes
The HPI axis-related genes in the telencephalon showed a down-regulation of
crf2 in the feed-reward, confinement, and air-exposure groups, whereas the expression of the
crf-r2 was down-regulated at different time points in all treatments (
Figure 2). Furthermore, the expression of
gr was only changed in the feed-control group. Furthermore,
pomc A was only up-regulated 30 min after chasing, whereas pomc B was up-regulated 60 and 90 min after feed-control treatment. The expression of
uro 1 was only down-regulated in the feed-control and confinement groups. The detailed visualization of the expression patterns in the telencephalon can be found in
Figure S1, uploaded in the repository.
Figure 2.
Overview of the gene expression profiles of HPI axis-related genes in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
Figure 2.
Overview of the gene expression profiles of HPI axis-related genes in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
In the hypothalamus, the expression of
crf2 was not changed, but all groups showed down-regulation of
crf-r2 except for the air-exposed fish (
Figure 2). An up-regulation of
gr was only noted for chased fish 30 min after treatment. An up-regulation of
mr was observed in feed-rewarded fish 90 min after treatment, whereas chased fish showed down-regulation of this gene. In addition,
pomc B was only down-regulated in confined fish 90 min after treatment. A detailed description of the expression pattern in the hypothalamus can be found in
Figure S2, uploaded in the repository.
The investigation of the gene expression patterns of the HPI axis-related genes in the optic tectum revealed that
crf1 was only up-regulated in all feed-rewarded fish (
Figure 2). Furthermore, the expression of
crf-r2 was down-regulated in this brain part in air-exposed fish but up-regulated in confined fish, while these two groups both showed a down-regulation of
crh-bp. While
gr changes were observed in the telencephalon and hypothalamus, the optic tectum showed only a down-regulation of
mr in the feed-rewarded fish and a
pomc B up-regulation in the optic tectum. The detailed expression pattern visualization for this brain part can be found in
Figure S3, uploaded in the repository.
The evaluation of HPI axis-related genes in the rhombencephalon revealed that the feed-control fish were the only treatment group that did not show an up-regulation of
crf1 in this brain part (
Figure 2). Changes of the
gr were also absent in the rhombencephalon, and the expression of
mr was only down-regulated 30 min after air exposure. The expression of
pomc B was up-regulated in the feed-reward and air-exposure groups 30 min after treatment. Furthermore, the expression of
uro 1 was only down-regulated in chased fish 90 min after treatment. The detailed expression pattern visualization can be found in
Figure S4, uploaded in the repository.
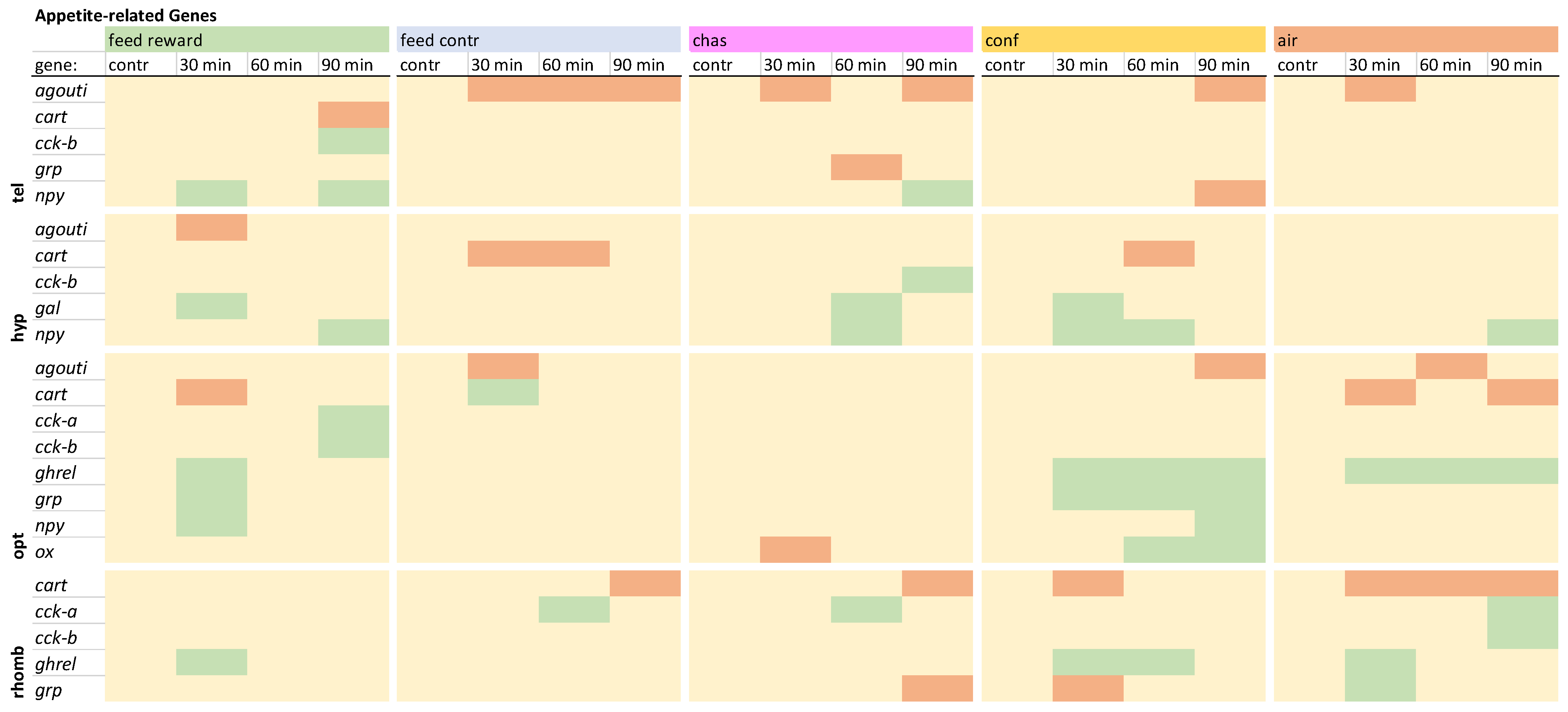
3.4. Appetite-Related Genes
The investigation of appetite-related genes in the telencephalon revealed that
agouti was not down-regulated only in the feed-rewarded fish (
Figure 3). These fish were also the only ones that showed down-regulation of
cart and up-regulation of
cck-b 90 min after treatment. The expression of
grp was only down-regulated in chased fish 60 min after treatment. Furthermore, an up-regualtion of
npy was observed in feed-rewarded and chased fish, whereas confined fish showed a down-regulation of this gene 90 min after treatment. The detailed expression pattern visualizations can be found in
Figure S1, uploaded in the repository.
Figure 3.
Overview of the gene expression profiles of appetite-related genes in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
Figure 3.
Overview of the gene expression profiles of appetite-related genes in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
In the hypothalamus,
agouti was only down-regulated in feed-rewarded fish 30 min after treatment (
Figure 3). The expression of
cart was only down-regulated in some of the feed-control and confinement groups. Only chased fish showed an up-regulation of
cck-b 90 min after treatment. An up-regulation of
gal was absent in the feed-control and the air-exposure groups in this brain part. The expression of
grp was not changed in any of the treatments, but the expression of
npy was increased in all treatments at different time points except for the feed-control fish. The detailed expression pattern visualizations can be found in
Figure S2, uploaded in the repository.
The investigation of the gene expression patterns of the appetite-related genes in the optic tectum revealed that the expression of
agouti was not down-regulated in the feed-reward and chased groups (
Figure 3). The expression of
cart was down-regulated in feed-rewarded and air-exposed fish but up-regulated in feed-control fish 30 min after treatment.
Cck-a and
-b were only up-regulated in feed-rewarded fish 90 min after treatment.
Ghrel and
grp showed similar expression patterns in feed-rewarded and confined fish. In addition, the expression of
npy was only increased in these two treatment groups. Finally, the expression of
ox was found to be changed only in the optic tectum, where it was increased in confined fish and decreased in chased animals. The detailed visualization and description of the expression patterns for this brain part can be found in
Figure S3, uploaded in the repository.
The appetite-related genes in the rhombencephalon showed no signficant changes of
agouti, although changes of this gene have been observed in the other three brain parts. In addition, no down-regulation of
cart was observed in the feed-rewarded fish, but was noted in all remaining treatment groups (
Figure 3). The expression of
cck-a was up-regulated in the feed-control fish and chased fish 60 min after treatment and in the air-exposed fish 90 min after treatment, whereas the expression of
cck-b was only increased in air-exposed fish. The expression of
ghrel was not increased in the feed-control fish and the chased fish. In addition, the expression of
grp was down-regulated in chased and confined fish at different time points and up-regulated in air-exposed fish 30 min after treatment. The detailed expression pattern visualizations can be found in
Figure S4, uploaded in the repository.
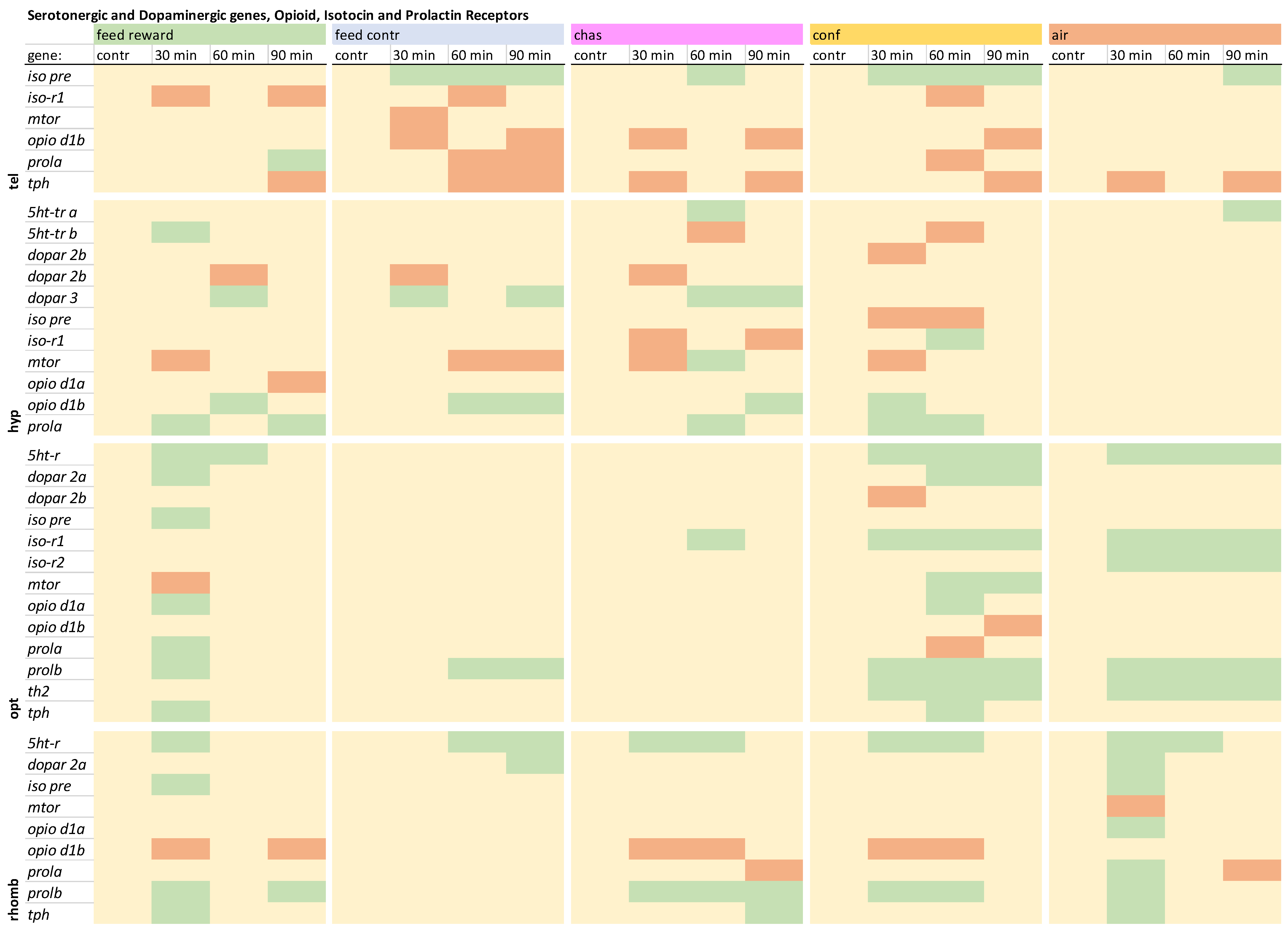
3.5. The Gene Expression Patterns of the Serotonergic and Dopaminergic Genes, Opioid, Isotocin, and Prolactin Receptors
The remaining genes that were investigated in the telencephalon showed that
iso pre was not increased in the feed-rewarded fish (
Figure 4), whereas the
iso-r1 showed down-regulation only in the feed-rewarded, the feed-control, and the confined fish at different time points. The expression of
mtor was only decreased in feed-control fish 30 min after treatment. The
opio d1b expression was not decreased in the feed-rewarded fish or the air-exposed animals. The
prola showed increased expression in the feed-rewarded fish 90 min after treatment and down-regulation in feed-control and confined fish 60 and/or 90 min after treatment. Finally, the
tph expression was down-regulated in all treatments, but at different time points. The detailed expression pattern visualizations can be found in
Figure S1, uploaded in the repository.
Figure 4.
Overview of the gene expression profiles of the serotonergic and dopaminergic genes, opioid, isotocin and the prolactin receptors in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
Figure 4.
Overview of the gene expression profiles of the serotonergic and dopaminergic genes, opioid, isotocin and the prolactin receptors in the telencephalon (tel), hypothalamus (hyp), optic tectum (opt), and rhombencephalon (rhomb) of male zebrafish 0, 30, 60, and 90 min after the treatment, whereby feed-rewarding, feed-control treatment, chasing, confinement, and air exposure were used as stressors. N = 6 per treatment. The significance value p < 0.05 was calculated from the Bayes models performed on the log2 values of the normalized gene expression values; yellow = not changed, red = down-regulated, green = up-regulated.
In contrast to the telencephalon, the expression of
5ht-tr a and
b was changed in the hypothalamus, displaying increased
5ht-tr a expression in chased and air-exposed fish and increased
5ht-tr b expression in the feed-reward group (
Figure 4). The expression of
5ht-tr b was decreased only in the chased and confined fish 60 min after treatment. Changes of the dopamine receptors have also not been observed in the telencephalon, but in the hypothalamus, confined fish showed a decreased expression of
dopar 2a 60 min after treatment. In addition, the expression of
dopar 2b was decreased in feed-reward, feed-control, and chased fish 30 and/or 60 min after treatment. The expression of
dopar 3 was increased in these treatment group at different time points, but not in confined or air-exposed fish. Furthermore, the expression of
iso pre was only decreased in confined fish, which also showed an increased
iso-r1 expression 60 min after treatment. The expression of
mtor was down-regulated in all treatments at different time points except for air-exposed fish. In addition, only chased fish showed an increased
mtor expression 60 min after treatment. The
opio d1a expression was only decreased in the hypothalamus of the feed-rewarded fish, whereas the expression of
opio d1b was found to be increased in feed-rewarded, chased, and confined fish at different time points after treatment. Finally, the
prola expression was not increased in the feed-control and air-exposure groups but was increased in the remaining treatment groups at different time point after treatment. The detailed expression pattern visualizations can be found in
Figure S2, uploaded in the repository.
The gene expression patterns of the remaining genes in the optic tectum showed increased
5ht-r expression in feed-rewarded, confined, and air-exposed fish (
Figure 4). The expression of
dopar 2a was increased in feed-rewarded and confined fish, but the expression of
dopar 2b only down-regulated in confined fish 30 min after treatment.
Iso pre was only up-regulated in feed-rewarded fish 30 min after treatment. The expression of the
iso-r1 was only up-regulated in distressed fish, and only all air-exposed fish showed an up-regulation of
iso-r2. In addition, the expression of
mtor was down-regulated in feed-rewarded fish 30 min after treatment and up-regulated in confined fish 60 and 90 min after treatment. Furthermore, the expression of
opio d1a was found to be up-regulated in feed-rewarded and confined fish, whereas the expression of
opio d1b was down-regulated only in confined fish 90 min after treatment.
An increased
prola expression was observed in feed-reward fish 30 min after treatment and a down-regulation of this gene in confined fish 60 min after treatment. In contrast,
prolb was up-regulated in this brain part in all treatments groups at different time points after treatment except for the chased fish. Changes of the
th2 expression were only observed in the optic tectum, and only the confined and air-exposed fish showed increases of its gene expression. In contrast to the telencephalon, which showed down-regulation of
tph, the optic tectum displayed up-regulation of this gene in the feed-rewarded and confined fish. The detailed expression pattern visualizations and descriptions can be found in
Figure S3, uploaded in the repository.
The expression of
5ht-r in the rhombencephalon showed increased values in all treatments but at different time points after treatment (
Figure 4). The expression of
dopar 2a was increased only in the feed-control and air-exposure groups. Furthermore, the expression of
iso pre was increased in the feed-reward and air-exposure groups. The expression of
mtor and
opio d1a was changed only in air-exposed fish 30 min after treatment. In contrast, the expression of
opio d1b was down-regulated in feed-rewarded, chased, and confined fish at different time points after treatment. The expression of
prola was down-regulated only in chased fish 90 min after treatment and up-regulated in air-exposed animals 30 min after treatment. In contrast, all treatment groups showed up-regulation of
prolb in the rhombencephalon at different time points after treatment except for the feed-control fish. Finally, up-regulation of
tph was not observed in the feed-control and confinement groups. The detailed expression pattern visualizations can be found in
Figure S4, uploaded in the repository.
3.6. Principal Component Analyses Revealing Gene Expression Patterns
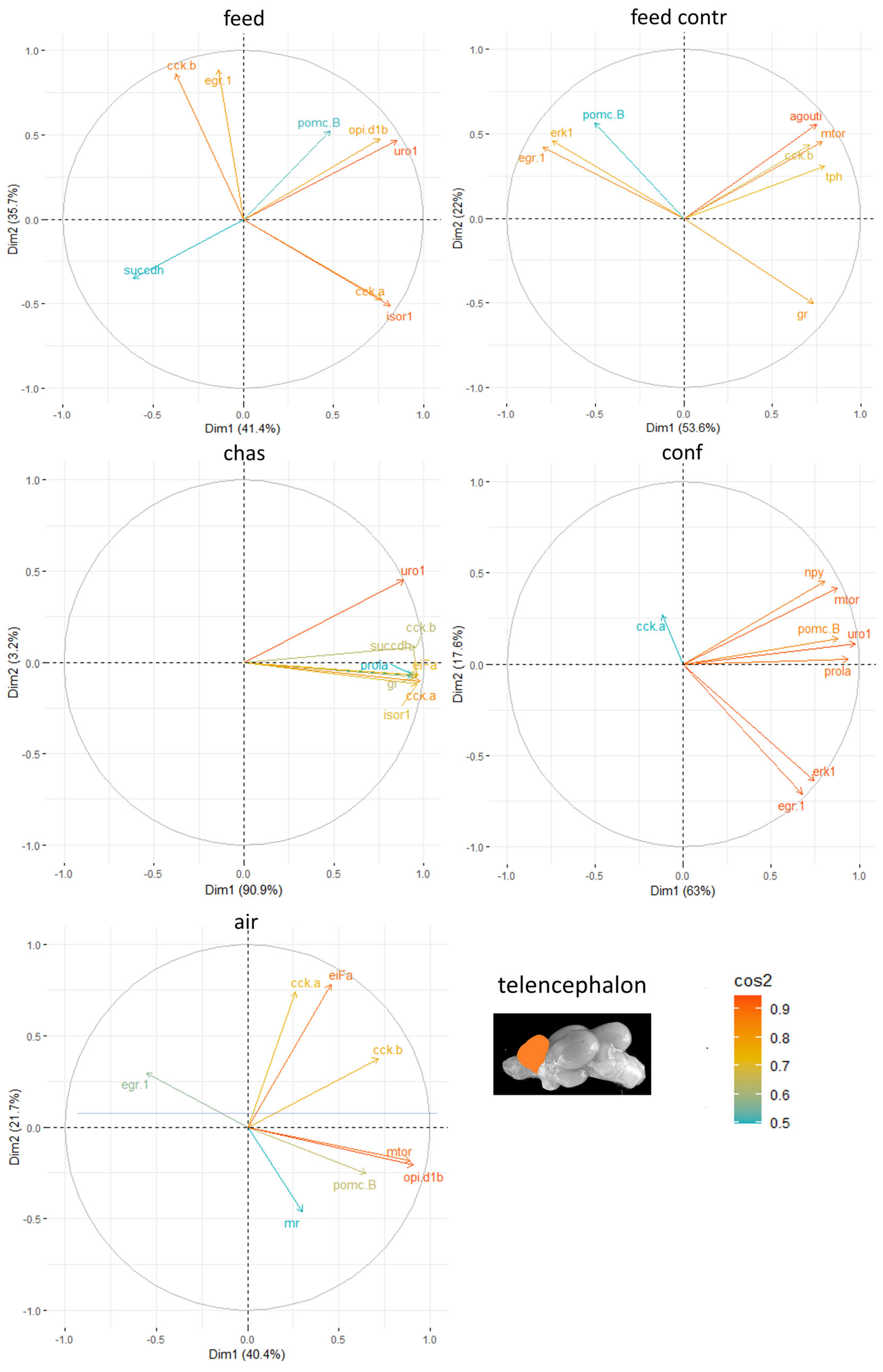
The principal component analyses (PCA) for all genes and brain parts have been uploaded in the repository. Based on these results, the two most important genes for each brain regulation pathway were selected and used for additional PCA. For the genes in the telencephalon,
uro 1 and
mtor were identified as genes with a strong relevance for the expression patterns in this brain part (
Figure 5). In contrast, the contribution of
pomc B to the outcome of gene expressions in the telencephalon was considerably low, and genes such as
cck-a and
cck-b repeatedly are among the most contributing genes in this brain part.
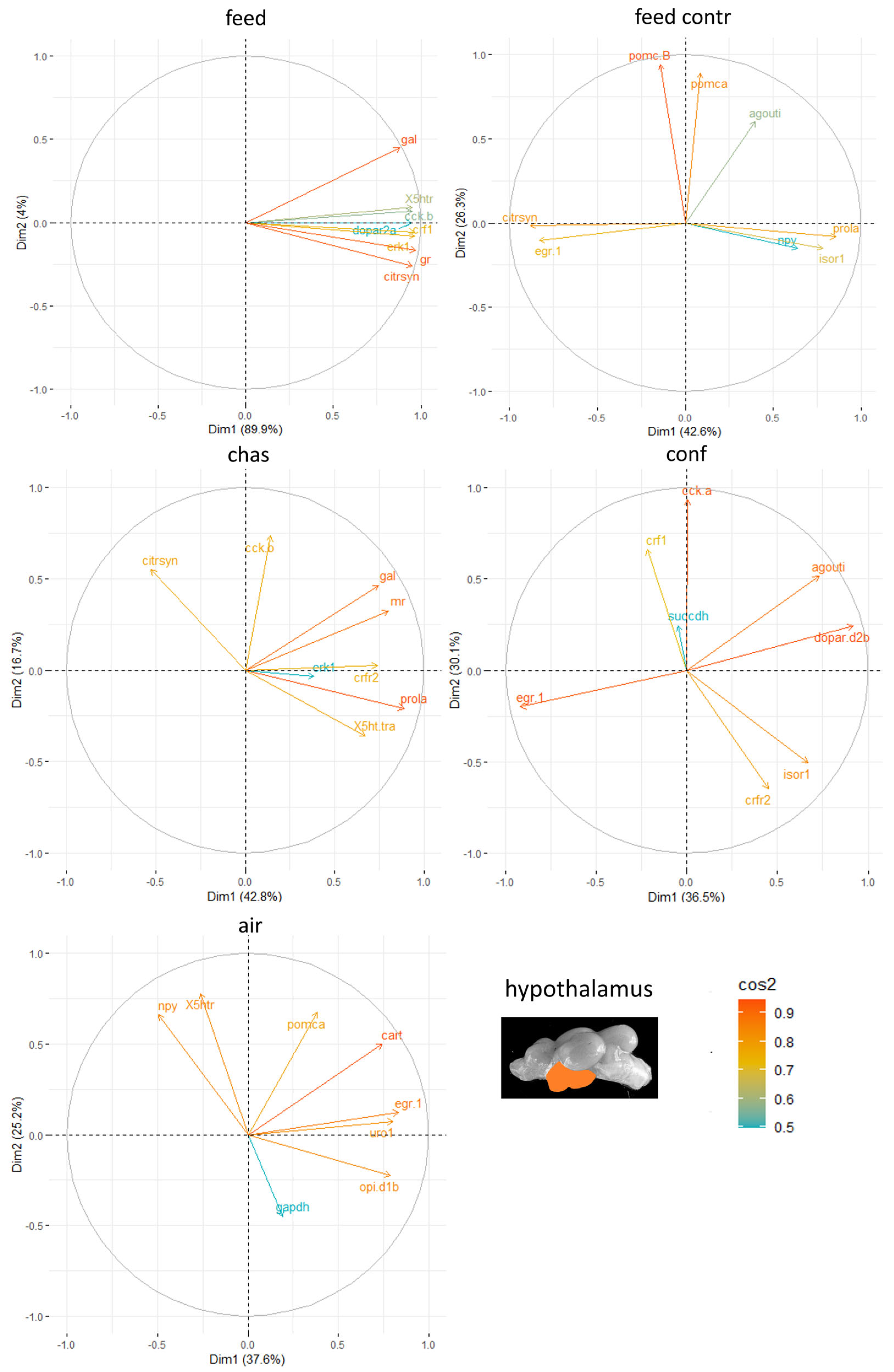
Moreover, the genes with the highest influence in the hypothalamus of the male zebrafish were appetite-related genes, such as
agouti and
gal, but the different stressors resulted in different genes contributing to the stress responses in this brain part (
Figure 6).
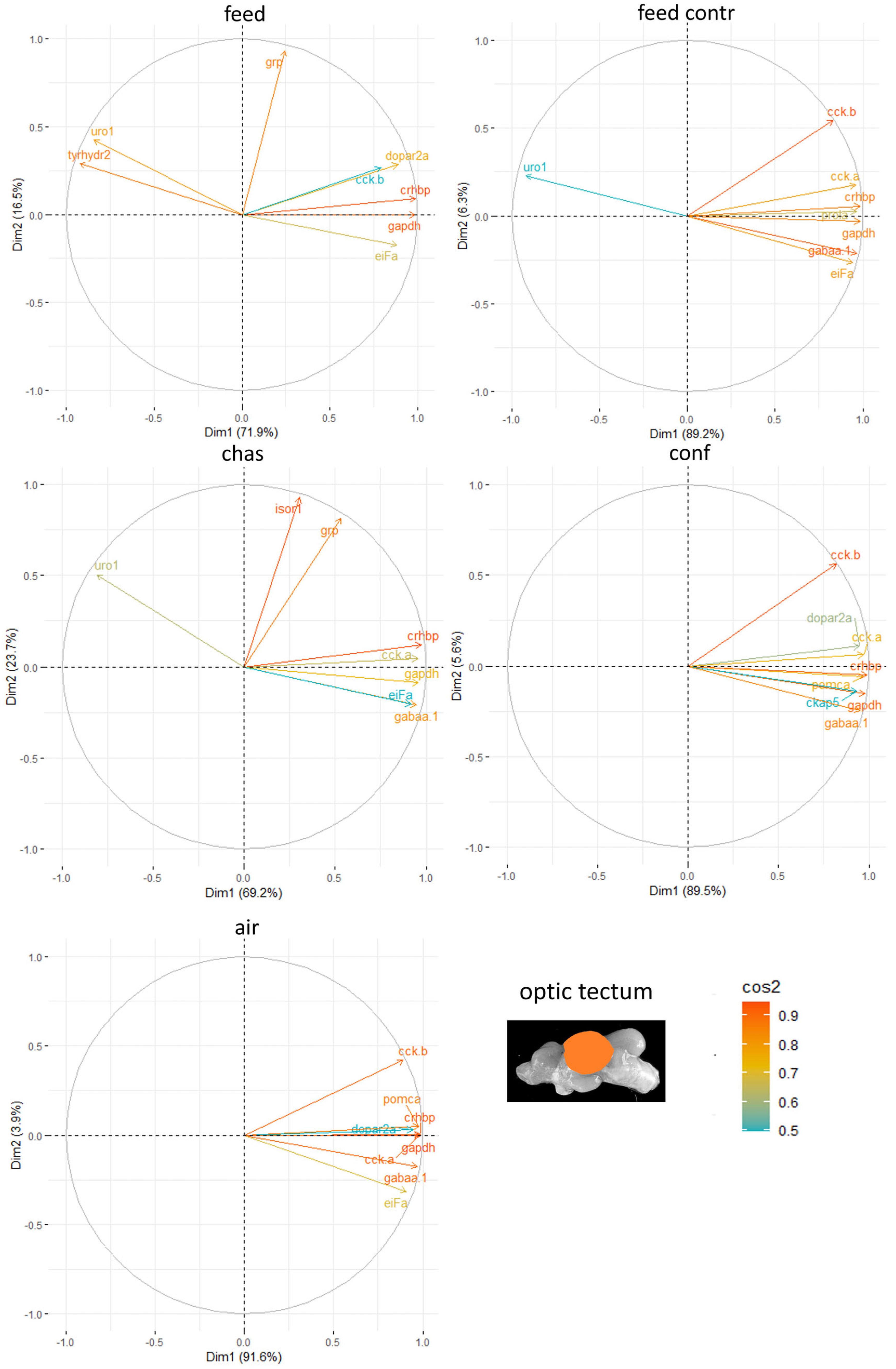
In the optic tectum of males,
crh-bp often had the strongest ability to explain the variability in the data sets obtained from the analysis of gene expression patterns after application of the different stressors (
Figure 7). The other genes with strong contributions to the outcome in this brain part varied for each stressor.
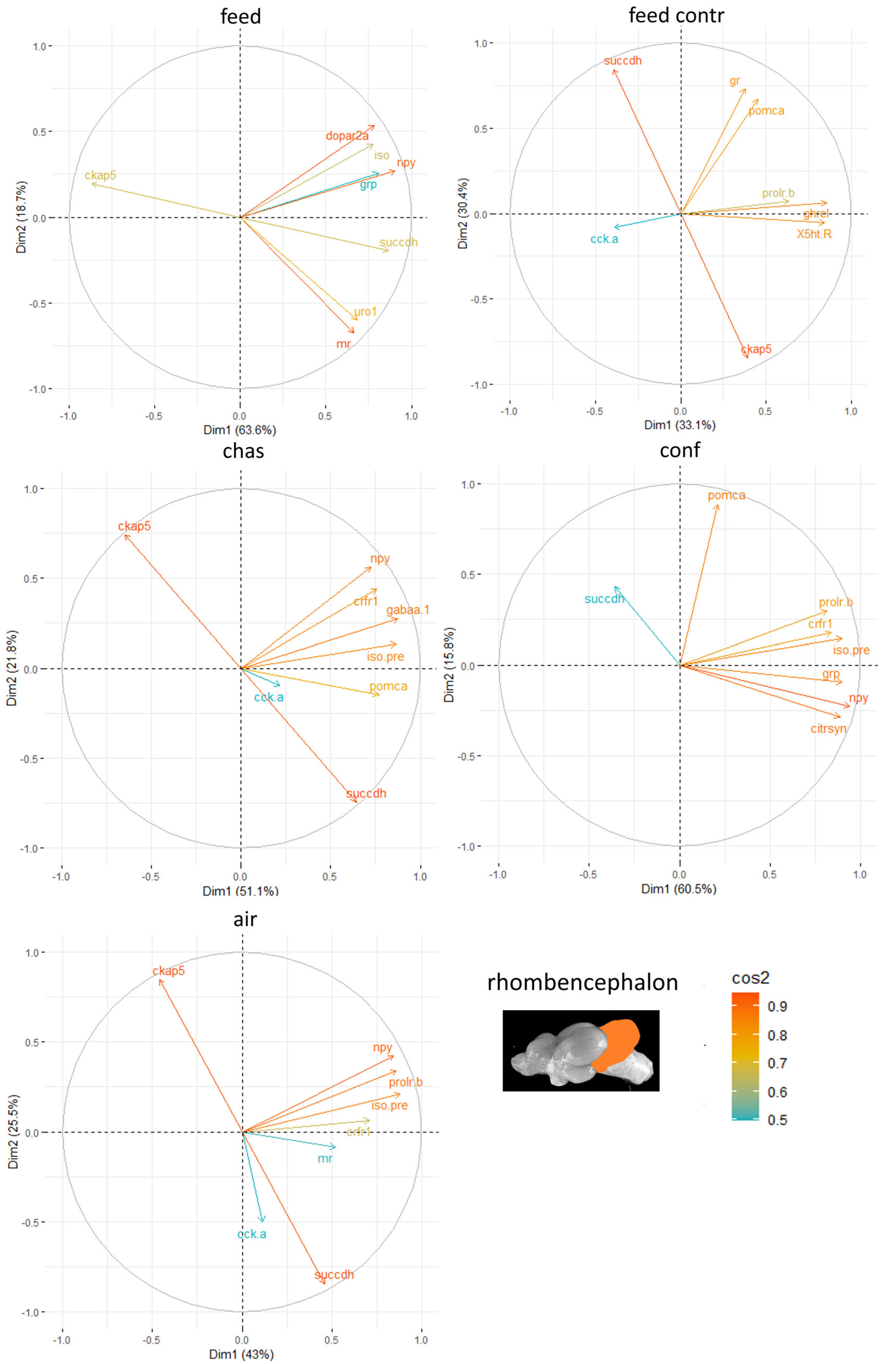
Finally, the rhombencephalon showed the strongest influence of the genes
ckap-5 and
succdh (
Figure 8).
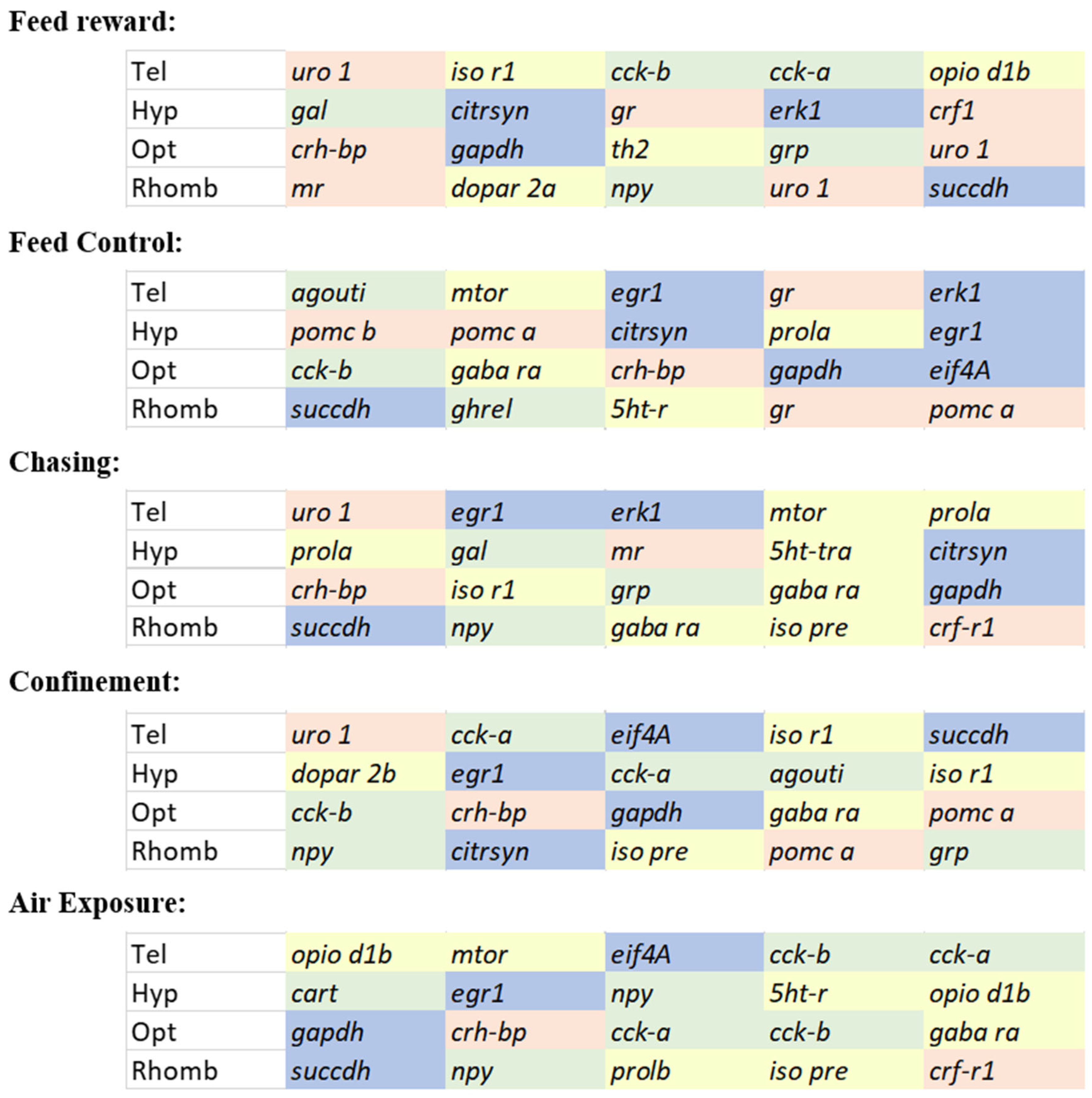
Selecting the optimal set of genes from these analyses for each brain part and each treatment separately resulted in an overview of genes that mostly contributed to the gene expression patterns in
Figure 9. This figure revealed that a mixture of genes contributed to the stress responses the most. The most frequently mentioned IEGs across all treatments and all brain parts were
succdh, gapdh, and
egr-1. Similarly, the most frequently mentioned HPI axis-related genes were
uro-1 and
crh-bp. The appetite genes with the highest frequency were
cck-a and
-b and
npy. From the remaining regulatory pathways in the brain, only
gaba ra was mentioned as frequently as the genes belonging to the IEGs, the HPI axis, or the appetite-regulating genes. For future investigations of gene expression patterns in the brain of stressed zebrafish, it is, therefore, recommended to use the most frequently contributing genes mentioned here to allow an optimal assessment of the early stress responses in this species.